Introduction
Morusin which has been isolated from the root bark
of Morus alba L. (Moraceae), known and read as SangBekPi in
Republic of Korea, possesses various biological activities
including antimicrobial, scavenging against superoxide anion
radical and anti-inflammatory activities (1–3). Previous
studies have indicated that morusin suppresses cancer cell growth,
and induces cell death in cervical cancer, hepatoma, glioma and
prostate cancer (4–9). The anticancer activity of morusin is
mediated by inhibiting signal transducer and activator of
transcription 3 (STAT3) and/or nuclear factor (NF)-κB pathways,
which results in apoptosis (5–7).
Breast cancer is the most common type of cancer, and
the third leading cause of cancer-associated mortality, in females
worldwide with >522,000 mortalities in 2012 (15% of female
mortalities) (10,11). Mortality rates for breast cancer are
lowest in Eastern Asia; however, the incidence of female breast
cancer has been continuously increasing in the Republic of Korea
(12). Although different types of
treatment including surgery, radiotherapy, chemotherapy, targeted
therapy and hormonal therapy are available for patients with
primary breast cancer, there are still numerous limitations in
breast cancer treatment due to the complex disease factors
(13). Therefore, advances in
scientific knowledge and the underlying molecular mechanisms
associated with breast cancer may help to reduce the incidence rate
of breast cancer.
Malignant tumors usually possess the capability to
avoid apoptosis and other death signals, which leads to therapeutic
resistance (14,15). The modulation of apoptosis-associated
proteins including Bcl-2-associated-x protein (Bax) and Survivin in
tumors is the most common strategy of evading apoptosis. The
pro-apoptotic protein Bax is classified as a multi-domain protein
of the B-cell lymphoma 2 (Bcl-2) family that regulates
mitochondrial signaling by mitochondrial outer membrane
permeabilization (MOMP). Bax requires the heterodimer with Bcl-2
antagonist/killer 1 (Bak) in order to be responsible for
mitochondrial dysfunction and MOMP (16). Inactivating mutations of Bax occur in
numerous types of human cancer, including breast cancer, leading to
the uncontrolled growth of tumors (17–19). As an
anti-apoptotic protein, Survivin regulates apoptosis and the cell
cycle. Survivin has been demonstrated to increase the drug
resistance in cancers via caspase-dependent mechanisms. However,
the inhibition of Survivin in tumor cells induces apoptotic cell
death (20–23). The expression of Survivin is also
modulated in the majority of human cancer types including breast
cancer (20). Thus, induction of
apoptosis through targeting anti- and/or pro-apoptotic proteins has
recently been regarded as a potentially effective strategy for
cancer treatment. In the present study, the anti-cancer activity of
morusin was investigated in multiple human breast cancer cell
lines.
Materials and methods
Cell culture and reagents
Human breast cancer MCF-7, MDA-MB-231, MDA-MB-157
and MDA-MB-453 cell lines and a human normal mammary epithelial
MCF10A cell line were purchased from the American Type Culture
Collection (ATCC; Manassas, VA, USA). MDA-MB-231 cells were
cultured in Dulbecco's modified Eagle's medium and the other breast
cancer cell lines were cultured in RPMI-1640 supplemented with 10%
fetal bovine serum (HyClone; GE HealthCare Life Sciences, Logan,
UT, USA) and 1% antibiotic-antimycotic solution containing
penicillin G (10,000 U/ml), streptomycin sulfate (10 mg/ml) and
amphotericin B (25 µg/ml; cat. no. 30-004-CI; Mediatech; Corning,
Tewksbury, MA, USA). MCF10A cells were cultured in Mammary
Epithelial Basal medium with the supplements provided in the MEGM™
BulletKit™ (Lonza, Basel, Switzerland) according to the ATTC
culture method (24). All cells were
cultured in a humidified incubator with 5% CO2 at 37°C
and the viability of cultured cells was monitored using a LUNA-FL
Automated cell counter (Logos Biosystems, Inc., Anyang, Republic of
Korea). Morusin was purchased from Biopurify Phytochemicals Ltd.
(Chengdu, China) and dissolved in dimethylsulfoxide. MTT was
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Cell viability assays
Cells (3–5×103 cells/well) were seeded
into 96-well plates 1 day prior to treatment. Following treatment
for the indicated time periods (24, 28 and 72 h) with 5–30 µM
morusin, cell viability was evaluated using MTT assays as
previously described (25). Cell
viability values are presented as a percentage as compared with the
mock-treated control.
Western blot analysis
Whole cell lysates were prepared using cell lysis
buffer (cat. no. 9803) from Cell Signaling Technology, Inc.
(Danvers, MA, USA), and western blotting was performed as
previously described (25).
Subsequent to performing the SDS-PAGE and transfer, the membranes
were incubated in 5% skim milk in PBST (PBS with 0.1% Tween-20
(Sigma-Aldrich; Merck KGaA) at room temperature for 1 h for
blocking, and then incubated with each primary antibody in 5% skim
milk or 5% bovine serum albumin in PBST with gentle agitation
overnight at 4°C. Incubations with the secondary antibodies were
performed in 5% skim milk in PBST at room temperature for 1 h.
Primary antibodies directed against Bax (SC-493), Bcl-2 (SC-492),
Bcl-extra large (Bcl-xL) (SC-7195), cleaved caspase-3 (cs9661s),
Survivin (SC-17779), poly ADP-ribose polymerase (PARP) (cs9546)
(all dilution, 1:1,000), and cleaved caspase-8 (cs9496) and 9
(SC-22182) (both dilution, 1:3,000) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) or Cell Signaling Technology,
Inc. (Danvers, MA, USA) and β-actin (A5441) (dilution, 1:10,000)
from Sigma-Aldrich (Merck KGaA), and horseradish
peroxidase-conjugated anti-mouse Immunoglobulin G (IgG) (cs7076) or
anti-rabbit IgG (cs7074) secondary antibodies (dilution, 1:5,000)
from Cell Signaling Technology, Inc. were used for immunoblotting.
Subsequently, the membranes were detected using a chemiluminescence
system (Daeil Lab Service Co., Ltd., Seoul, Korea). β-actin was
used as an internal control.
Apoptosis analysis
Cells (1–2×103 cells/well) in 6-well
plates were treated with morusin at 37°C for 2 days. Apoptosis was
observed using staining with Annexin V-FITC and propidium iodide
(PI; BioVision, Inc., Milpitas, CA, USA) and compared with the
mock-treated control group. The cells were subsequently analyzed
using a BD FACSCalibur™ flow cytometer and BD CellQuest™ (BD
Biosciences, Franklin Lakes, NJ, USA) as described (25).
Statistical analysis
Statistical analysis was performed using Excel 2013
(Microsoft, Redmond, WA, USA) and SigmaPlot v.13 (Systat Software,
Inc., San Jose, CA, USA). Data are presented as the mean ± standard
error of the mean from ≥3 independent experiments performed in
triplicate or more and analyzed for statistical significance using
the unpaired Student's t-test. P<0.05 was considered to indicate
a statistically significant difference.
Results
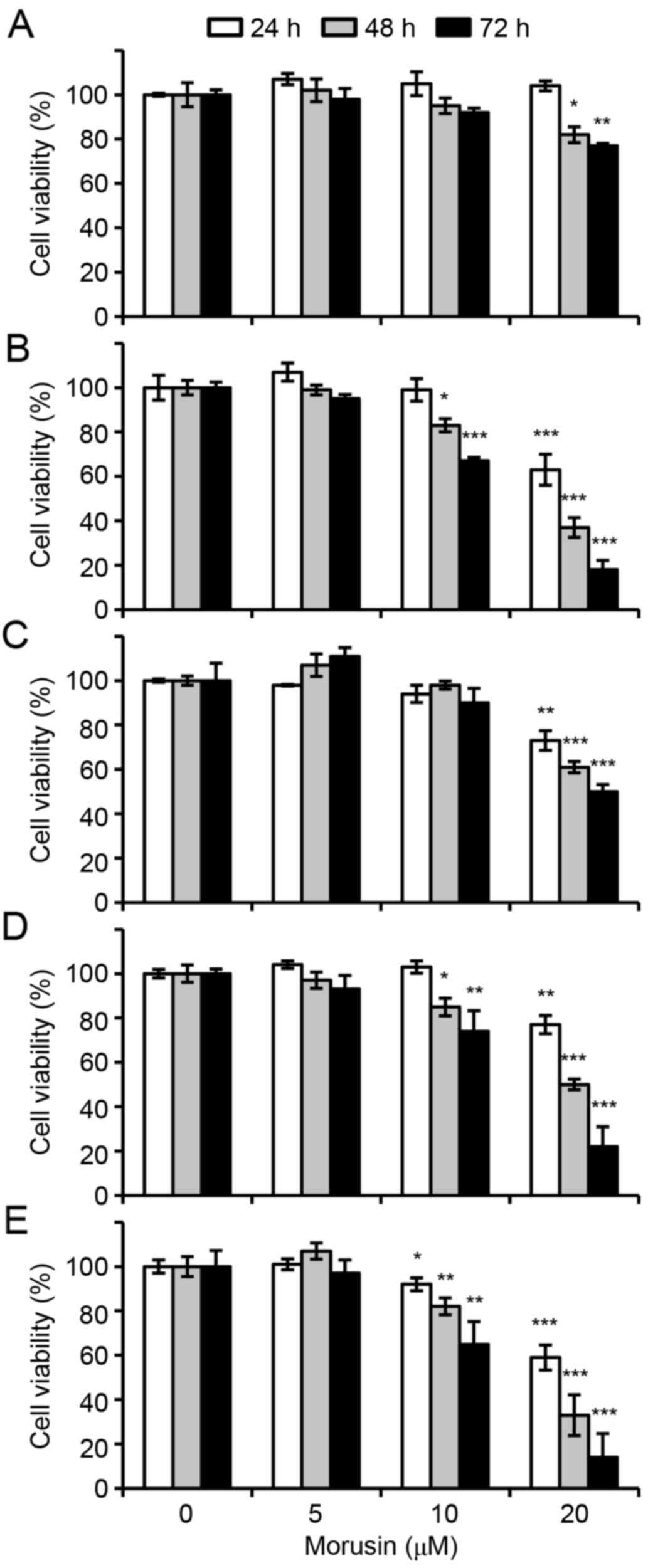
Morusin suppresses cell viability in
human breast cancer cells
In order to investigate the possible therapeutic
effect of morusin in breast cancer, the effects of morusin on the
viability of human breast cancer cells was evaluated. Human breast
cancer cell lines MCF-7, MDA-MB-157, MDA-MB-231 and MDA-MB-453, and
a normal immortalized breast epithelial cell line MCF10A were
treated with morusin for 24, 48 and 72 h, and cell viability was
measured using MTT assays. As illustrated in Fig. 1, morusin significantly suppressed cell
viability in a dose- or/and a time-dependent manner in all breast
cancer cell lines treated with 20 µM morusin. However, notably
morusin exhibited a lesser effect on normal mammary epithelial
MCF10A cells, which was not significant until 48 h of treatment.
Furthermore, IC50 values indicated that morusin is a
more potent cytotoxic reagent in breast cancer cells compared with
that in normal cells (Table I). These
results suggested that morusin is a potentially effective
therapeutic agent for breast cancer.
 | Table I.IC50 values for morusin in
different breast cancer cell lines. |
Table I.
IC50 values for morusin in
different breast cancer cell lines.
|
| IC50
(µM) |
|---|
|
|
|
|---|
| Cell line | 24 h | 48 h | 72 h |
|---|
| MCF10A | 117.24±3.24 | 45.51±4.60 | 33.05±1.38 |
| MCF-7 |
40.46±0.67 | 16.98±1.82 | 13.53±0.15 |
| MDA-MB-157 |
44.22±1.44 | 24.40±0.77 | 23.79±0.95 |
| MDA-MB-231 |
18.89±1.16 | 10.79±0.29 | 10.84±1.61 |
| MDA-MB-453 |
26.57±0.32 | 14.23±0.11 | 11.99±0.92 |
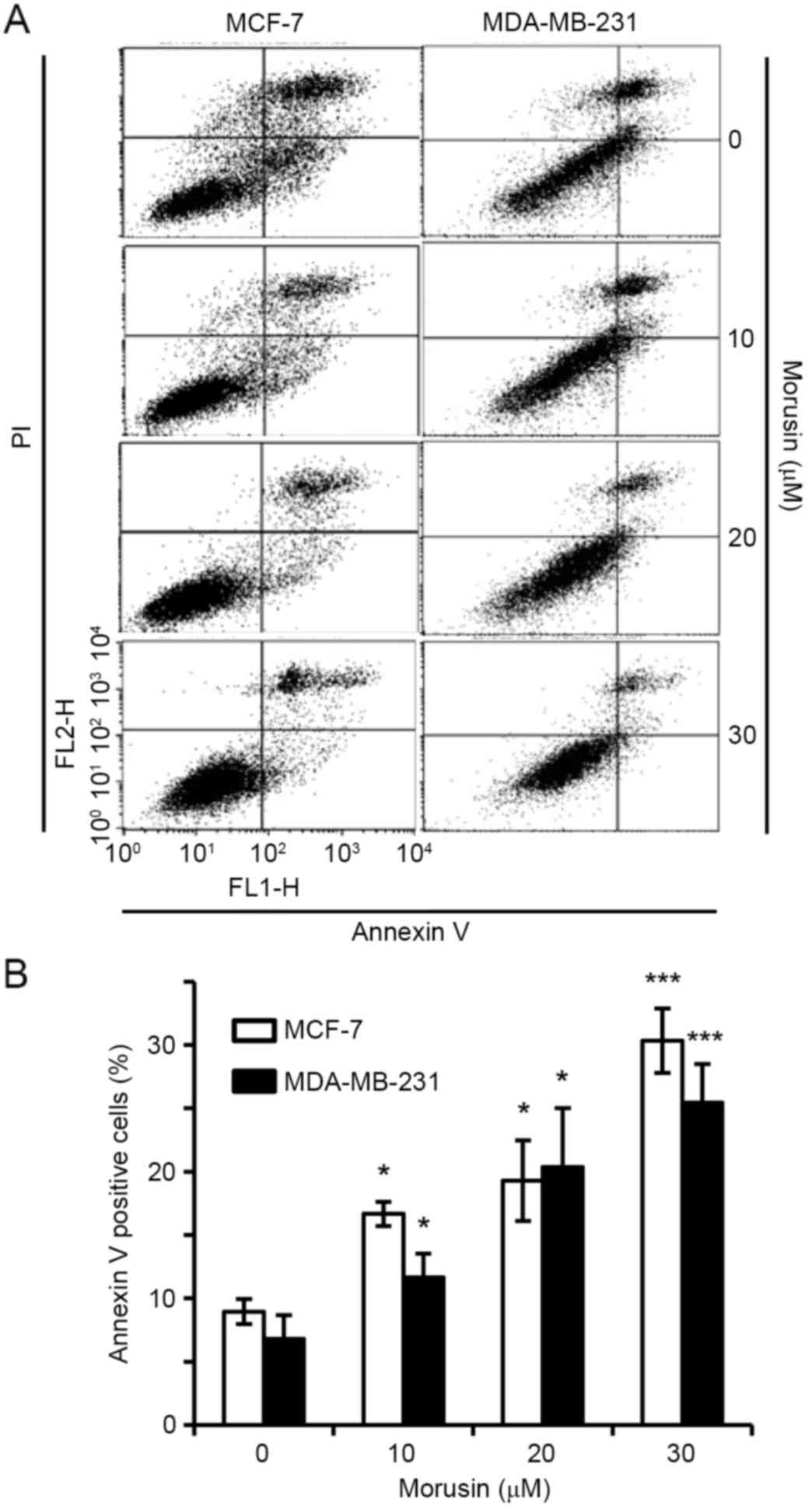
Morusin induces apoptosis in human
breast cancer cells
To investigate whether a decrease in cell viability
by morusin is due to the induction of apoptosis, the effect of
morusin on apoptosis in human breast cancer MCF-7 and MDA-MB-231
cells was evaluated using Annexin V/propidium iodide (PI) double
staining. As illustrated in Fig. 2,
10 µM morusin significantly increased the Annexin V-positive cell
population in a dose-dependent manner in MDA-MB-231 and MCF-7
cells, which is indicative of apoptosis. Furthermore, it was
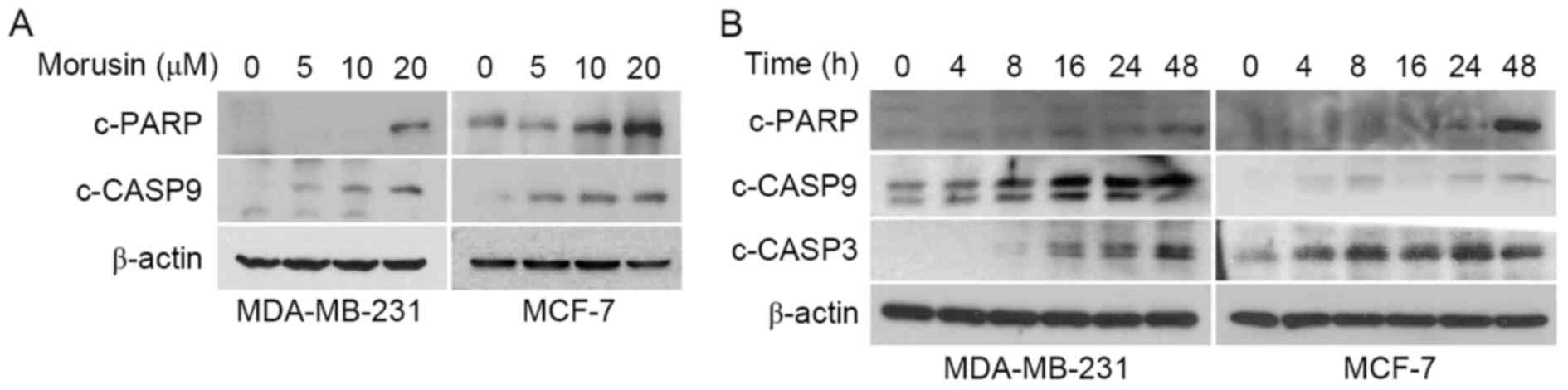
consistently demonstrated that morusin increased the expression of
apoptotic marker proteins, cleaved caspase 9, cleaved caspase 3 and
PARP cleavage, in a dose- (Fig. 3A)
and time- (Fig. 3B) dependent manner.
Together these results indicate that morusin induces apoptosis in
human breast cancer cells.
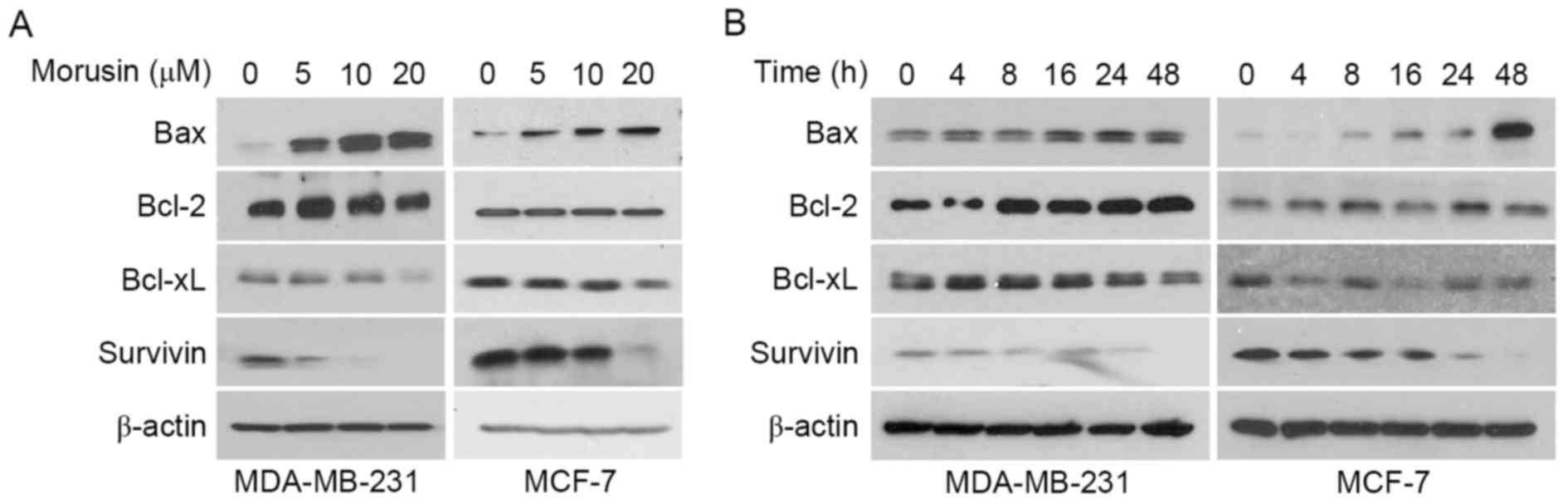
Morusin decreases Survivin and
increases Bax in human breast cancer cells
Previous studies have reported that morusin induces
apoptosis by modulating STAT3 and/or NF-κB in various types of
cancer cells, which are well-known transcription factors that
regulate various genes involved in cellular proliferation, survival
and apoptosis (5–7). Thus, the effect of morusin on the
regulation of apoptosis-associated protein expression was
determined in MDA-MB-231 and MCF-7 cells. As illustrated Fig. 4, morusin induced Bax and reduced
Survivin expression in a dose- (Fig.
4A) and time- (Fig. 4A) dependent
manner, while no effect on the expression of Bcl-2 and Bcl-xL was
observed in human breast cancer cells. These results indicate that
morusin induces apoptosis by regulating the pro-apoptotic protein
Bax and the anti-apoptotic protein Survivin in human breast cancer
cells.
Discussion
Breast cancer is the most common type of cancer and
the third leading cause of cancer-associated mortality for women
worldwide with >522,000 mortalities in 2012 (11). In the Republic of Korea, the incidence
of breast cancer in women has been continuously increasing
(12). Various therapeutic strategies
for breast cancer have been developed to date. However, the overall
treatment efficiency for malignant and metastatic breast cancer
remains stagnant. Recent studies have identified effective
phytochemicals for the treatment of cancer, including advanced
stage breast cancer (26–28). Therefore, the potential of morusin as
a therapeutic agent for breast cancer was evaluated in the present
study. Cytotoxicity assays were used to verify whether morusin
could be a therapeutic agent for breast cancer, and revealed that
morusin suppressed cell viability in human breast cancer cells with
an IC50 of 18–45 µM. However, morusin exhibited little
toxic effect in normal breast MCF10A cells with an IC50
of >118 µM following 24 h morusin treatment, which demonstrated
similar results following treatment for 48 and 72 h. Although it
was revealed that morusin induced apoptosis in breast cancer cells,
the apoptotic rate was lower compared with the cytotoxicity rate
for the same concentration of morusin. These results indicate that
morusin may induce other types of cell death, including necrosis
and autophagy in breast cancer cells.
Apoptosis is an evolutionarily conserved essential
process for development and tissue homeostasis (29). However, an analysis of the evidence
suggests that the upregulation of anti-apoptotic proteins in cancer
is considered one of the strategies of evading apoptosis (as
reviewed in 30). For this reason, scientists have developed
anti-cancer drugs targeting these apoptosis-associated proteins. In
the present study, it was demonstrated that morusin is a candidate
for anti-cancer agent to treat breast cancer by suppression of the
anti-apoptotic protein Survivin (Fig.
4). Furthermore, morusin increased pro-apoptotic protein Bax
expression in breast cancer cells, indicating its ability at
targeting anti- and pro-apoptotic signals that may increase
anticancer activity. Previous studies have reported that morusin
inhibits proliferation of cancer cell by suppressing STAT3 signal
(5–7).
Inhibition of STAT3 subsequently affects the expression of its
downstream targets, including Bax and Survivin (31,32). In
the present study, morusin was revealed to modulate the expression
of Bax and Survivin in human breast cancer cells, suggesting that
STAT3 may be involved in this regulation.
In conclusion, the results of the present study
indicate that morusin can induce apoptosis by decreasing Survivin
and increasing Bax protein expression in human breast cancer cells.
These results indicate that morusin may be a potentially effective
anticancer agent for the treatment of patients with breast
cancer.
Acknowledgements
The present study was supported by the National
Research Foundation of Korea (grant nos. 2007-0054931,
NRF-2013R1A1A2007263 and NRF-2014R1A1A2057918).
References
|
1
|
Bellik Y, Boukraâ L, Alzahrani HA,
Bakhotmah BA, Abdellah F, Hammoudi SM and Iguer-Ouada M: Molecular
mechanism underlying anti-inflammatory and anti-allergic activities
of phytochemicals: An update. Molecules. 18:322–353. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fukai T, Satoh K, Nomura T and Sakagami H:
Antinephritis and radical scavenging activity of prenylflavonoids.
Fitoterapia. 74:720–724. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sohn HY, Son KH, Kwon CS, Kwon GS and Kang
SS: Antimicrobial and cytotoxic activity of 18 prenylated
flavonoids isolated from medicinal plants: Morus alba L., Morus
mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora
flavescens Ait and Echinosophora koreensis Nakai. Phytomedicine.
11:666–672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo H, Liu C, Yang L, Dong L, Wang L, Wang
Q, Li H, Zhang J, Lin P and Wang X: Morusin inhibits glioblastoma
stem cell growth in vitro and in vivo through stemness attenuation,
adipocyte transdifferentiation, and apoptosis induction. Mol
Carcinog. 55:77–89. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee JC, Won SJ, Chao CL, Wu FL, Liu HS,
Ling P, Lin CN and Su CL: Morusin induces apoptosis and suppresses
NF-kappaB activity in human colorectal cancer HT-29 cells. Biochem
Biophys Res Commun. 372:236–242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lim SL, Park SY, Kang S, Park D, Kim SH,
Um JY, Jang HJ, Lee JH, Jeong CH, Jang JH, et al: Morusin induces
cell death through inactivating STAT3 signaling in prostate cancer
cells. Am J Cancer Res. 5:289–299. 2015.PubMed/NCBI
|
|
7
|
Lin WL, Lai DY, Lee YJ, Chen NF and Tseng
TH: Antitumor progression potential of morusin suppressing STAT3
and NFκB in human hepatoma SK-Hep1 cells. Toxicol Lett.
232:490–498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wan LZ, Ma B and Zhang YQ: Preparation of
morusin from Ramulus mori and its effects on mice with transplanted
H22 hepatocarcinoma. Biofactors. 40:636–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Guo H, Yang L, Dong L, Lin C,
Zhang J, Lin P and Wang X: Morusin inhibits human cervical cancer
stem cell growth and migration through attenuation of NF-κB
activity and apoptosis induction. Mol Cell Biochem. 379:7–18. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim Z, Min SY, Yoon CS, Lee HJ, Lee JS,
Youn HJ, Park HK, Noh DY and Hur MH; Korean Breast Cancer Society,
: The basic facts of korean breast cancer in 2011: Results of a
nationwide survey and breast cancer registry database. J Breast
Cancer. 17:99–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thompson A, Brennan K, Cox A, Gee J,
Harcourt D, Harris A, Harvie M, Holen I, Howell A, Nicholson R, et
al: Evaluation of the current knowledge limitations in breast
cancer research: A gap analysis. Breast Cancer Res. 10:R262008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH
and Lee JS: Prediction of cancer incidence and mortality in Korea,
2014. Cancer Res Treat. 46:124–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park SK, Sakoda LC, Kang D, Chokkalingam
AP, Lee E, Shin HR, Ahn YO, Shin MH, Lee CW, Lee DH, et al: Rising
prostate cancer rates in South Korea. Prostate. 66:1285–1291. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei MC, Zong WX, Cheng EH, Lindsten T,
Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB and
Korsmeyer SJ: Proapoptotic BAX and BAK: A requisite gateway to
mitochondrial dysfunction and death. Science. 292:727–730. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bae SI, Park JG, Kim YI and Kim WH:
Genetic alterations in gastric cancer cell lines and their original
tissues. Int J Cancer. 87:512–516. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meijerink JP, Mensink EJ, Wang K, Sedlak
TW, Slöetjes AW, de Witte T, Waksman G and Korsmeyer SJ:
Hematopoietic malignancies demonstrate loss-of-function mutations
of BAX. Blood. 91:2991–2997. 1998.PubMed/NCBI
|
|
19
|
Rampino N, Yamamoto H, Ionov Y, Li Y,
Sawai H, Reed JC and Perucho M: Somatic frameshift mutations in the
BAX gene in colon cancers of the microsatellite mutator phenotype.
Science. 275:967–969. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Altieri DC: Survivin, versatile modulation
of cell division and apoptosis in cancer. Oncogene. 22:8581–8589.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Altieri DC: Validating survivin as a
cancer therapeutic target. Nat Rev Cancer. 3:46–54. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li F, Ambrosini G, Chu EY, Plescia J,
Tognin S, Marchisio PC and Altieri DC: Control of apoptosis and
mitotic spindle checkpoint by survivin. Nature. 396:580–584. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
https://www.atcc.org/Products/All/CRL-10317.aspx#culturemethod
|
|
25
|
Park SY, Lim SL, Jang HJ, Lee JH, Um JY,
Kim SH, Ahn KS and Lee SG: Embelin induces apoptosis in human
glioma cells through inactivating NF-κB. J Pharmacol Sci.
121:192–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dandawate PR, Subramaniam D, Jensen RA and
Anant S: Targeting cancer stem cells and signaling pathways by
phytochemicals: Novel approach for breast cancer therapy. Semin
Cancer Biol. 40–41. 192–208. 2016.PubMed/NCBI
|
|
27
|
Kotecha R, Takami A and Espinoza JL:
Dietary phytochemicals and cancer chemoprevention: A review of the
clinical evidence. Oncotarget. 7:52517–52529. 2016.PubMed/NCBI
|
|
28
|
Shankar E, Kanwal R, Candamo M and Gupta
S: Dietary phytochemicals as epigenetic modifiers in cancer:
Promise and challenges. Semin Cancer Biol. 40–41. 82–99.
2016.PubMed/NCBI
|
|
29
|
Du Toit A: Cell death: Balance through a
bivalent regulator. Nat Rev Mol Cell Biol. 14:5462013. View Article : Google Scholar
|
|
30
|
Mohammad RM, Muqbil I, Lowe L, Yedjou C,
Hsu HY, Lin LT, Siegelin MD, Fimognari C, Kumar NB, Dou QP, et al:
Broad targeting of resistance to apoptosis in cancer. Semin Cancer
Biol. 35 Suppl:S78–S103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gritsko T, Williams A, Turkson J, Kaneko
S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, et al:
Persistent activation of stat3 signaling induces survivin gene
expression and confers resistance to apoptosis in human breast
cancer cells. Clin Cancer Res. 12:11–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nielsen M, Kaestel CG, Eriksen KW,
Woetmann A, Stokkedal T, Kaltoft K, Geisler C, Röpke C and Odum N:
Inhibition of constitutively activated Stat3 correlates with
altered Bcl-2/Bax expression and induction of apoptosis in mycosis
fungoides tumor cells. Leukemia. 13:735–738. 1999. View Article : Google Scholar : PubMed/NCBI
|