Introduction
Lung cancer is the primary cause of
cancer-associated mortality in males and females worldwide
(1). Lung cancer was estimated to be
responsible for ~1.6 million mortalities worldwide in 2012 and ~20%
of cancer-associated mortality (1,2). The
majority of lung cancer cases (~90%) are non-small cell lung cancer
(NSCLC), which comprises a number of subtypes driven by the
activation of various oncogenes (3,4).
Typically, ~50% of NSCLC cases are diagnosed at an advanced stage
(5,6).
For the majority of patients with NSCLC, chemotherapy represents
the mainstay of treatment and prognosis remains poor (5–7).
Therefore, improved understanding of the molecular pathogenesis of
NSCLC and the identification of novel therapeutic targets for NSCLC
may facilitate the early detection and improved survival,
respectively, of patients with NSCLC.
Glutamate ionotropic receptor AMPA type subunit 2
(GluA2) encodes an α-amino-3-hydroxy-5-methyl-4-isoxa-zolepropionic
acid (AMPA) glutamate receptor, which acts as an excitatory
neurotransmitter at numerous synapses in the central nervous system
(8,9).
AMPA type subunit 2 (GluA2), one of the four subunits of AMPA
receptors, is a potential novel marker for poor prognosis in
patients with human lung cancer, in which it is typically
deregulated (10). However, glutamate
signaling pathways have also been identified in various
non-excitable tissues, in addition to being implicated in several
diseases, including cancer (11,12).
Binding of glutamate to its receptors activates Src family kinases
and downstream signaling pathways, which stimulates proliferation,
apoptosis resistance, migration and invasion in different cancer
cell lines, including hepatocellular carcinoma, clear cell renal
carcinoma and glioma cells (11–13). Choi
et al (14) demonstrated that
the expression of GluA2 is associated with an improved prognosis of
patients with advanced serous papillary ovarian adenocarcinoma.
Herner et al (15) revealed
that glutamate-mediated GluA2 activation increased the invasion and
migration of pancreatic cancer cells via activation of the
classical mitogen-activated protein kinase signaling pathway.
Although GluA2 serves an important role in the development and
progression of various types of cancer, limited research is
available regarding its effect on the proliferation of NSCLC cells.
Therefore, the effects of GluA2 in lung cancer cells, in addition
to the molecular mechanisms underlying these effects, requires
additional study.
The present study aimed to explore the mechanism
underlying GluA2 deregulation in NSCLC. The effects of
GluA2-knockdown were investigated in in vitro experiments,
including an apoptosis assay. GluA2 was revealed to induce
apoptosis via caspase-dependent and cellular tumor antigen p53
(p53) signaling pathways in human A549 NSCLC cells. The results of
the current study may aid in the development of novel treatments
for NSCLC.
Materials and methods
Reagents
Antibodies directed against B-cell lymphoma-2
(Bcl-2; cat. no., D198628) and Bcl-2-associated X protein (Bax;
cat. no., D120073) were purchased from Sangong Biotech Co., Ltd.
(Shanghai, China). Antibodies directed against GluA2 (cat. no.,
13607), Blc-2-associated death promoter (Bad; cat. no., 9292),
phosphorylated (p)-Bad (cat. no., 9291) and p53 (cat. no., 9282),
and β-actin (cat. no., 3700) were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The anti-X-linked inhibitor of
apoptosis protein (XIAP; cat. no., 2042) antibody was obtained from
Cell Signaling Technology, Inc., the anti-p21Cip1/Waf1
antibody (cat. no., 621134) was purchased from BD Pharmingen (San
Diego, CA, USA) and the anti-p16INK4a antibody (cat.
no., sc-73434) was obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Other chemicals were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The GluA2-shRNA
plasmid kit (cat. no., C01001) was purchased from GeneChem Co.,
Ltd. (Shanghai, China).
RNA interference
Cells were transfected using
Lipofectamine® 2000 reagent (Thermo Fisher Scientific,
Inc., Waltham, MA, USA), according to the manufacturer's protocol.
Two sequence-validated and knockdown-warranted GluA2-siRNA were
used together to improve the efficacy of knockdown (siRNA 1,
ATATTGGAGTGAAGTGGACAA and siRNA 2, GGAGCTCTCCTTAGCTTGATT; Shanghai
GenePharma Co., Ltd., Shanghai, China). Commercial
6-carboxyfluorescein-tagged, negative control (NC) scrambled siRNAs
(5′-UCCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUGACACGUUCGGAGAATT-3′;
Shanghai GenePharma Co., Ltd.) were used together as a control for
efficiency and non-specific side effects.
A549 cell culture
The lung cancer A549 cell line was provided by the
Department of Thoracic Surgery of the Second Affiliated Hospital of
China Medical University (Shenyang, China) and cultured in
RPMI-1640 medium containing 10% (v/v) heat-inactivated fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.), 50 U/ml penicillin
and 100 µg/ml streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C in an incubator containing humidified air with 5%
(v/v) CO2.
Cell Counting Kit-8 (CCK-8) cell
proliferation assay
A549 cells were transfected with the GluA2-siRNAs or
scrambled siRNAs. A total of 24 h after transfection, cells were
transferred into 96-well plates at a density of 2×103
cells/well. Cell viability was assessed using a CCK-8 (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) assay at days 1, 2
and 4 after transfection.
Acridine orange/ethidium bromide
(AO/EB) fluorescence staining
A549 cells were transfected with the GluA2-siRNAs or
scrambled siRNA. The cells were incubated with AO/EB solution (cat.
no., CA1140; Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) for 5 min at room temperature, according to a
previously described method (16).
Cellular morphological changes were subsequently examined using
fluorescence microscopy; live cells were stained green, apoptotic
cells, yellow and necrotic cells, red. The percentage of apoptotic
cells was calculated using the following formula: Apoptotic rate
(%)=(number of apoptotic cells/total number of cells)x100, as
previously described (17,18).
Western blot analysis
A549 cells were transfected with the chosen
GluA2-siRNA or scrambled siRNA. Cell lysates were prepared for
western blotting 48 h after transfection, in order to determine the
efficiency of gene expression ablation. The amount of protein
obtained was quantified using a bicinchoninic acid protein assay
kit (Beyotime Institute of Biotechnology, Shanghai, China). Protein
samples (60 µg/lane) were separated on a 10% gel via SDS-PAGE and
blotted onto nitrocellulose membranes. The blots were incubated
with the previously described antibodies specific for Bcl-2
(dilution, 1:1,000), Bax (dilution, 1:1,000), Bad (dilution,
1:500), p-Bad (dilution, 1:500), GluA2 (dilution, 1:1,000), XIAP
(dilution, 1:500), p53 (dilution, 1:500), p21Cip1/Waf1
(dilution, 1:500), p16INK4a (dilution, 1:500) and
β-actin (dilution, 1:1,000) overnight at 4°C, then at 37°C for 2 h.
Subsequent to washing in PBS and incubating with secondary
antibodies (cat. no., v926-32211 for rabbit and v926-32210 for
mouse; dilution, 1:10,000; LI-COR Biosciences, Lincoln, NE, USA) at
room temperature for 1 h, the blots were visualized using an
enhanced chemiluminescence reagent (GE Healthcare Life Sciences,
Shanghai, China) and normalized to β-actin using the software from
an Odyssey imaging system (version 3.0; LI-COR Biosciences).
Caspase-3 activity assay
A total of 5×103 cells were seeded into
96-well cell culture plates. Following transfection with
GluA2-siRNA or scrambled siRNA for 48 h, apoptotic rates were
measured based on the activation of caspase-3 using a Caspase-3
Activity Assay kit (Beyotime Institute of Biotechnology), according
to the manufacturer's protocol.
Statistical analysis
Data were obtained from ≥3 independent experiments.
All statistical analysis was performed in SPSS 19.0 (IBM SPSS,
Armonk, NY, USA) and results were illustrated using GraphPad Prism
5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Results are
presented as the mean ± standard deviation. Differences between two
groups were evaluated using an unpaired Student's t-test;
differences between multiple groups were evaluated with an ANOVA
followed by Tukey's multiple comparison test. P<0.05 was
considered to indicate a statistically significant difference.
Results
GluA2 knockdown suppresses the
proliferation of A549 cells
Two GluA2 shRNAs were designed and tested. The first
shRNA did not produce efficient depletion of GluA2 (data not
shown). However, successful suppression of GluA2 using the second
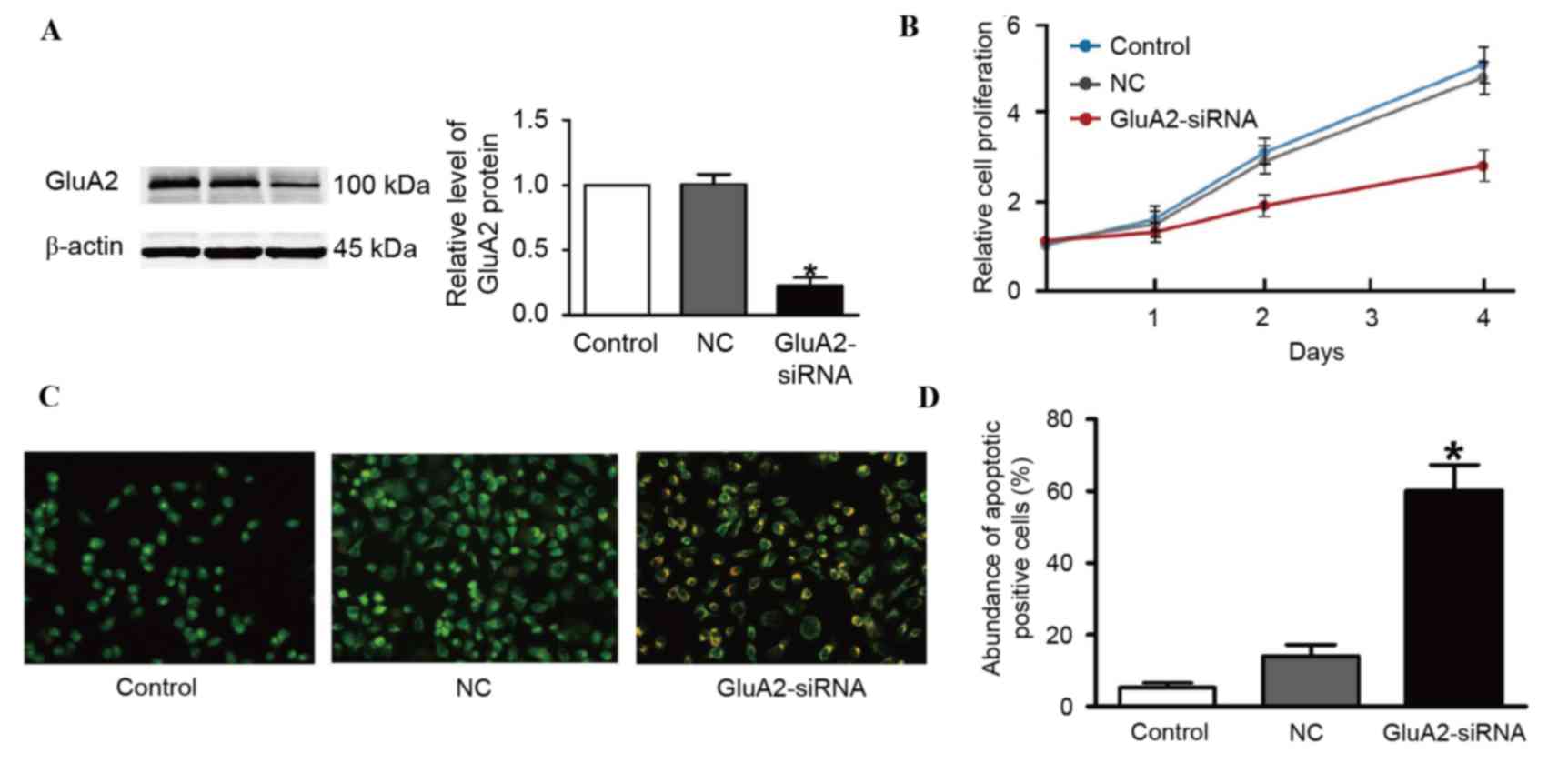
shRNA was verified using western blotting (Fig. 1A). The second shRNA was thus used for
the following experiments. It was identified that GluA2 depletion
markedly inhibited A549 cell proliferation compared to an
untransfected control (Fig. 1B).
Silencing GluA2 expression induces the
apoptosis of A549 cells
To assess whether increased apoptosis was associated
with the decrease in cell proliferation observed following GluA2
knockdown, AO/EB staining was used to detect apoptotic cells. The
results from fluorescence microscopy analysis are illustrated in
Fig. 1C and D. A total of three cell
stages were recognized as follows: Live cells (control cells),
apoptotic cells (NC cells) and necrotic cells
(GluA2-shRNA-transfected cells). GluA2 knockdown significantly
increased the amount of apoptotic cells compared with the control
group (P<0.05; Fig. 1D).
Silencing GluA2 expression activates
caspase-dependent proapoptotic signaling pathways in A549
cells
To explore the mechanisms by which GluA2 knockdown
induces apoptosis in A549 cells, the levels of downstream proteins
of the GluA2 apoptotic pathway were measured by western blotting,
including Bax, Bcl-2, Bad, p-Bad and XIAP. Fig. 2 demonstrates that silencing of GluA2
significantly upregulated Bad and Bax expression, and significantly
downregulated p-Bad and Bcl-2 expression (all P<0.05 vs. the
control group; Fig. 2A and B).
Knockdown of GluA2 significantly decreased expression of XIAP
protein (P<0.05 vs. the control group; Fig. 2C), which is an essential antiapoptotic
protein. In addition, relative caspase-3 activity was significantly
increased by 2.2-fold following to GluA2-shRNA transfection
(P<0.05 vs. the control group; Fig.
2D).
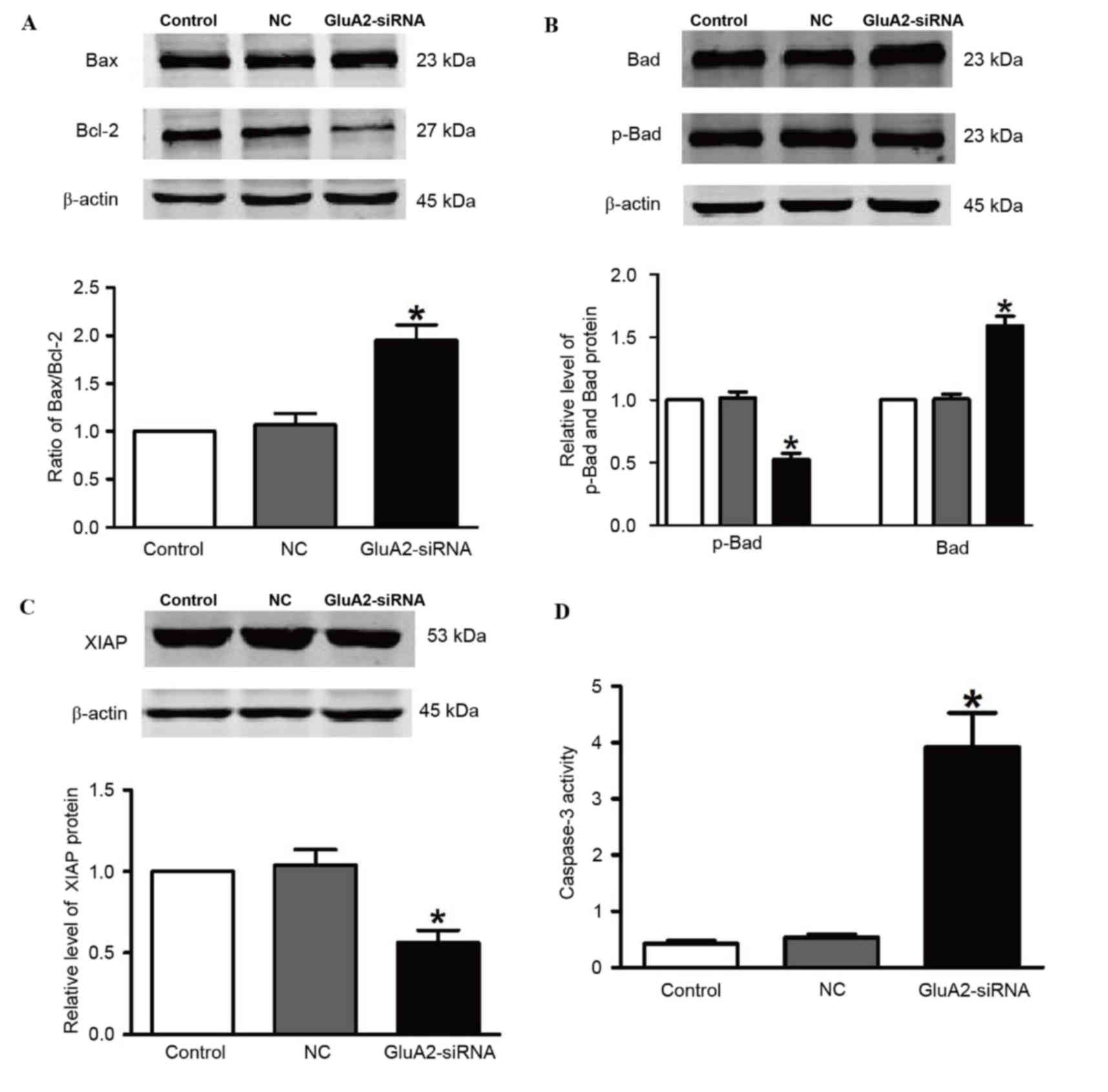 | Figure 2.Silencing GluA2 expression alters
p-Bad, Bad, Bax, Bcl-2 and XIAP expression, and increases caspase-3
activity. Western blotting was used to detect (A) Bax and Bcl-2
(increased and decreased, respectively), (B) p-Bad and Bad
(decreased and increased, respectively), and (C) XIAP (decreased)
expression in A549 cells transfected with GluA2-siRNA. (D)
Caspase-3 activity was increased by GluA2-siRNA treatment.
*P<0.05 vs. the control group. GluA2, glutamate ionotropic
receptor AMPA type subunit 2; Bcl-2, B-cell lymphoma-2; Bax,
Bcl-2-associated X protein; Bad, Bcl-2-associated death promoter;
p-, phosphorylated; XIAP, X-linked inhibitor of apoptosis protein;
siRNA, small interfering RNA; NC, negative control. |
Silencing GluA2 expression decreases
the expression of p53, p21Cip1/Waf1and
p16INK4a in A549 cells
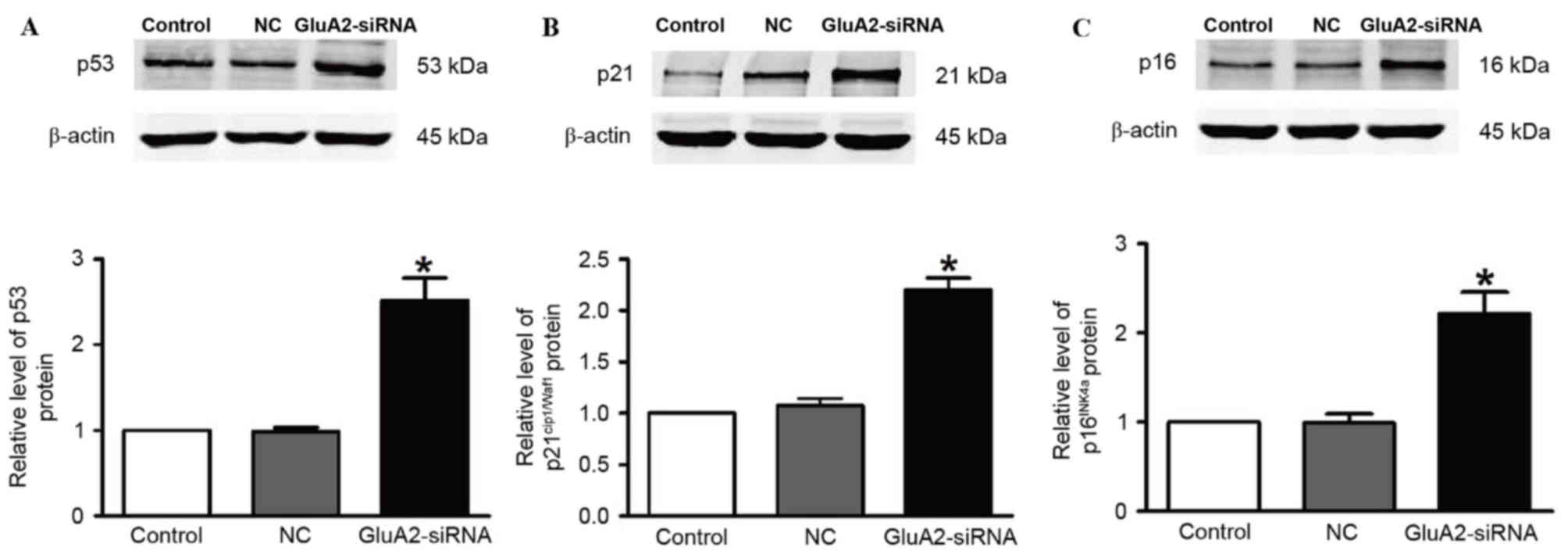
The expression of p53 (Fig. 3A), p21Cip1/Waf1 (Fig. 3B) and p16INK4a (Fig. 3C) proteins was revealed to be
significantly upregulated in A549 cells following GluA2-shRNA
transfection (all P<0.05). These observations indicate that the
p53 signaling pathway serves an important role in the GluA2-induced
apoptosis of A549 cells.
Discussion
In the present study, knockdown of GluA2 was
revealed to have a potent antiproliferative effect on A549 cells by
inducing apoptosis and reducing proliferation in vitro. The
results of the present study also identified that activation of
caspase-3-dependent and p53-dependent signaling pathways are
important mechanisms for these effects of GluA2. Therefore, the
present study has elucidated the signaling pathway by which GluA2
affects lung cancer.
AMPA receptors are a major class of glutamatergic
receptors, which serve essential roles in normal neuronal activity,
including synaptic plasticity, synaptic scaling, learning and
memory, in addition to synaptogenesis and the formation of neuronal
circuitry (19,20). AMPA receptors are heterotetrameric
proteins composed of GluA1-4 subunits (21,22). The
predominant AMPA receptor in the adult cerebral neocortex and
hippocampus is GluA2 (23,24). GluA2 is also unique, as its inclusion
into the heterotetramer renders AMPA receptors significantly less
permeable to Ca2+ (25).
AMPA receptor-mediated signals may be associated with
carcinogenesis due to their regulation of the differentiation,
proliferation and migration of embryonic stem cells (26,27). This
hypothesis was confirmed in a number tumor types, including
astrocytoma, glioblastoma, breast carcinoma, lung carcinoma, colon
adenocarcinoma and prostate carcinoma (28–31).
However, the mechanism by which GluA2 contributes to human lung
carcinogenesis remains poorly understood. In the present study, the
ratio of Bcl-2/Bax significantly increased in the
GluA2-siRNA-transfected group compared with the control group,
suggesting that the knockdown of GluA2 induced apoptosis in the
A549 cells. In addition, Bad, a member of the Bcl-2 family that
normally binds to the Bcl-2/Bcl-extra larger (Bcl-xL) complex and
triggers apoptosis, was significantly increased in this group.
p-Bad dissociates from the Bcl-2/Bcl-xL complex, resulting in the
suppression of apoptosis (32).
However, p-Bad was significantly decreased in the
GluA2-siRNA-transfected group compared with the control group in
the present study. Levels of XIAP were also significantly decreased
following knockdown of GluA2. XIAP interacts with caspase-3 to
inhibit its activation, which normally leads to cell death
(33). Caspase-3 activity was
significantly decreased in the GluA2-siRNA-transfected group in the
current study. These results indicate that the caspase-3 signaling
pathway serves an important role in the GluA2-induced apoptosis of
lung cancer A549 cells.
The p53 signaling pathway serves an important role
in apoptosis in lung cancer (34,35).
Mutations to p53 protein are common in lung carcinoma (36); p53 has a antitumorigenic effect in
NSCLC (37). The present study
demonstrated that caspase-3 activity in lung cancer cells
significantly increased following silencing of GluA2. It was
identified that GluA2 silencing was able to induce apoptosis in
A549 cells, and this effect appeared to be conferred by the
significant increase in p53, p21Cip1/Waf1 and
p16INK4a expression observed following GluA2-knockdown.
This indicates that the
p53/p21Cip1/Waf1/p16INK4a signaling pathway
is associated with the GluA2-induced apoptosis of lung cancer A549
cells.
The present results demonstrated that the silencing
of GluA2 expression may inhibit the proliferation, and promote the
apoptosis of lung cancer A549 cells. Therefore, administration of
an AMPA receptor antagonist may inhibit tumor growth. The AMPA
receptor antagonist talampanel has been demonstrated to be well
tolerated, but exerted no antitumorigenic activity as a single
agent, in a phase II clinical trial of patients with recurrent
glioma (38). Additional studies are
required to evaluate the potential benefit of AMPA inhibitors in
the treatment of lung cancer, particularly the types that do not
respond well to current chemotherapy regimens.
In conclusion, the results of the present study
indicate that the silencing of GluA2 may inhibit the proliferation,
and promote the apoptosis of lung cancer A549 cells. These effects
were determined to be associated with the caspase-3 and
p53/p21Cip1/Waf1/p16INK4a signaling pathways.
These results suggest that GluA2 may be a potential novel
therapeutic target for the treatment of lung cancer.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sullivan I and Planchard D: ALK inhibitors
in non-small cell lung cancer: The latest evidence and
developments. Ther Adv Med Oncol. 8:32–47. 2016.PubMed/NCBI
|
|
3
|
Ettinger DS, Akerley W, Bepler G, Blum MG,
Chang A, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Ganti AK, et
al: Non-small cell lung cancer. J Natl Compr Canc Netw. 8:740–801.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Larsen JE, Cascone T, Gerber DE, Heymach
JV and Minna JD: Targeted therapies for lung cancer: Clinical
experience and novel agents. Cancer J. 17:512–527. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reck M, Popat S, Reinmuth N, De Ruysscher
D, Kerr KM and Peters S; ESMO Guidelines Working Group, :
Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 25 Suppl 3:iii27–iii39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bordi P, Del Re M, Danesi R and Tiseo M:
Circulating DNA in diagnosis and monitoring EGFR gene mutations in
advanced non-small cell lung cancer. Transl Lung Cancer Res.
4:584–597. 2015.PubMed/NCBI
|
|
7
|
Hall RD, Le TM, Haggstrom DE and Gentzler
RD: Angiogenesis inhibition as a therapeutic strategy in non-small
cell lung cancer (NSCLC). Transl Lung Cancer Res. 4:515–523.
2015.PubMed/NCBI
|
|
8
|
Priya A, Johar K, Nair B and Wong-Riley
MT: Nuclear respiratory factor 2 regulates the transcription of
AMPA receptor subunit GluA2 (Gria2). Biochim Biophys Acta.
1843:3018–3028. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Isaac JT, Ashby MC and McBain CJ: The role
of the GluR2 subunit in AMPA receptor function and synaptic
plasticity. Neuron. 54:859–871. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stepulak A, Luksch H, Gebhardt C,
Uckermann O, Marzahn J, Sifringer M, Rzeski W, Staufner C, Brocke
KS, Turski L and Ikonomidou C: Expression of glutamate receptor
subunits in human cancers. Histochem Cell Biol. 132:435–445. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nedergaard M, Takano T and Hansen AJ:
Beyond the role of glutamate as a neurotransmitter. Nat Rev
Neurosci. 3:748–755. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu H, Takano N, Xiang L, Gilkes DM, Luo W
and Semenza GL: Hypoxia-inducible factors enhance glutamate
signaling in cancer cells. Oncotarget. 5:8853–8868. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Groot JF, Piao Y, Lu L, Fuller GN and
Yung WK: Knockdown of GluR1 expression by RNA interference inhibits
glioma proliferation. J Neurooncol. 88:121–133. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi CH, Choi JJ, Park YA, Lee YY, Song
SY, Sung CO, Song T, Kim MK, Kim TJ, Lee JW, et al: Identification
of differentially expressed genes according to chemosensitivity in
advanced ovarian serous adenocarcinomas: Expression of GRIA2
predicts better survival. Br J Cancer. 107:91–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Herner A, Sauliunaite D, Michalski CW,
Erkan M, De Oliveira T, Abiatari I, Kong B, Esposito I, Friess H
and Kleeff J: Glutamate increases pancreatic cancer cell invasion
and migration via AMPA receptor activation and Kras-MAPK signaling.
Int J Cancer. 129:2349–2359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mcgahon AJ, Martin SJ, Bissonnette RP,
Mahboubi A, Shi Y, Mogil RJ, Nishioka WK and Green DR: The end of
the (Cell) line: Methods for the study of apoptosis in vitro.
Method Cell Biol. 46:153–185. 1995. View Article : Google Scholar
|
|
17
|
Ni B, Bai FF, Wei Y, Liu MJ, Feng ZX,
Xiong QY, Hua LZ and Shao GQ: Apoptosis induced by lipid-associated
membrane proteins from Mycoplasma hyopneumoniae in a porcine lung
epithelial cell line with the involvement of caspase 3 and the MAPK
pathway. Genet Mol Res. 14:11429–11443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He Y, Chen W, Hu Y, Luo B, Wu L, Qiao Y,
Mo Q, Xu R, Zhou Y, Ren Z, et al: E. adenophorum induces cell cycle
and apoptosis of renal cells through mitochondrial pathway and
caspase activation in Saanen Goat. PLoS One. 10:e01385042015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Borges K and Dingledine R: AMPA receptors:
Molecular and functional diversity. Prog Brain Res. 116:153–170.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Henley JM and Wilkinson KA: AMPA receptor
trafficking and the mechanisms underlying synaptic plasticity and
cognitive aging. Dialogues Clin Neurosci. 15:11–27. 2013.PubMed/NCBI
|
|
21
|
Serulle Y, Arancio O and Ziff EB: A role
for cGMP-dependent protein kinase II in AMPA receptor trafficking
and synaptic plasticity. Channels. 2:230–232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Priya A, Johar K, Nair B and Wong-Riley
MT: Specificity protein 4 (Sp4) regulates the transcription of AMPA
receptor subunit GluA2 (Gria2). Biochim Biophys Acta.
1843:1196–1206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Craig AM, Blackstone CD, Huganir RL and
Banker G: The distribution of glutamate receptors in cultured rat
hippocampal neurons: Postsynaptic clustering of AMPA-selective
subunits. Neuron. 10:1055–1068. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wenthold RJ, Petralia RS, J II Blahos and
Niedzielski AS: Evidence for multiple AMPA receptor complexes in
hippocampal CA1/CA2 neurons. J Neurosci. 16:1982–1989.
1996.PubMed/NCBI
|
|
25
|
Sommer B, Köhler M, Sprengel R and Seeburg
PH: RNA editing in brain controls a determinant of ion flow in
glutamate-gated channels. Cell. 67:11–19. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ikonomidou C, Bosch F, Miksa M, Bittigau
P, Vöckler J, Dikranian K, Tenkova TI, Stefovska V, Turski L and
Olney JW: Blockade of NMDA receptors and apoptotic
neurodegeneration in the developing brain. Science. 283:70–74.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Joo JY, Kim BW, Lee JS, Park JY, Kim S,
Yun YJ, Lee SH, Lee SH, Rhim H and Son H: Activation of NMDA
receptors increases proliferation and differentiation of
hippocampal neural progenitor cells. J Cell Sci. 120:1358–1370.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoshioka A, Ikegaki N, Williams M and
Pleasure D: Expression of N-methyl-D-aspartate (NMDA) and non-NMDA
glutamate receptor genes in neuroblastoma, medulloblastoma and
other cells lines. J Neurosci Res. 46:164–178. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takeda M, Haga M, Yamada H, Kinoshita M,
Otsuka M, Tsuboi S and Moriyama Y: Ionotropic glutamate receptors
expressed in human retinoblastoma Y79 cells. Neurosci Lett.
294:97–100. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rzeski W, Ikonomidou C and Turski L:
Glutamate antagonists limit tumor growth. Biochem Pharmacol.
64:1195–1200. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ishiuchi S, Yoshida Y, Sugawara K, Aihara
M, Ohtani T, Watanabe T, Saito N, Tsuzuki K, Okado H, Miwa A, et
al: Ca2+-permeable AMPA receptors regulate growth of human
glioblastoma via Akt activation. J Neurosci. 27:7987–8001. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin HI, Lee YJ, Chen BF, Tsai MC, Lu JL,
Chou CJ and Jow GM: Involvement of Bcl-2 family, cytochrome c and
caspase 3 in induction of apoptosis by beauvericin in human
non-small cell lung cancer cells. Cancer Lett. 230:248–259. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Paulsen M, Ussat S, Jakob M, Scherer G,
Lepenies I, Schütze S, Kabelitz D and Adam-Klages S: Interaction
with XIAP prevents full caspase-3/−7 activation in proliferating
human T lymphocytes. Eur J Immunol. 38:1979–1987. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng J, Zhang S, Wu K, Wang B, Wong JY,
Jiang H, Xu R, Ying L, Huang H, Zheng X, et al: Combined effects of
suberoylanilide hydroxamic acid and cisplatin on radiation
sensitivity and cancer cell invasion in non-small-cell lung cancer.
Mol Cancer Ther. 15:842–853. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rajagopalan P, Alahmari KA, Elbessoumy AA,
Balasubramaniam M, Suresh R, Shariff ME and Chandramoorthy HC:
Biological evaluation of 2-arylidene-4, 7-dimethyl indan-1-one
(FXY-1): A novel Akt inhibitor with potent activity in lung cancer.
Cancer Chemother Pharmacol. 77:393–404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smardova J, Liskova K, Ravcukova B,
Malcikova J, Hausnerova J, Svitakova M, Hrabalkova R, Zlamalikova
L, Stano-Kozubik K, Blahakova I, et al: Complex analysis of the p53
tumor suppressor in lung carcinoma. Oncol Rep. 35:1859–1867.
2016.PubMed/NCBI
|
|
37
|
Zhang G, An Y, Lu X, Zhong H, Zhu Y, Wu Y,
Ma F, Yang J, Liu Y, Zhou Z, et al: A Novel naphthalimide compound
restores p53 function in non-small-cell lung cancer by reorganizing
the bak-Bcl-xl complex and triggering transcriptional regulation. J
Biol Chem. 291:4211–4225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Iwamoto FM, Kreisl TN, Kim L, Duic JP,
Butman JA, Albert PS and Fine HA: Phase 2 trial of talampanel, a
glutamate receptor inhibitor, for adults with recurrent malignant
gliomas. Cancer. 116:1776–1782. 2010. View Article : Google Scholar : PubMed/NCBI
|