Introduction
Pancreatic metastases are rare. Metastases are
reported to account for 2–5% of all malignant lesions occurring in
the pancreas (1–5). Studies have reported that people of ~60
years old are at high risk of developing pancreatic metastases
(1–3).
Metastases to the pancreas may occur a significant amount of time
subsequent to diagnosis and management of the primary tumor
(3). Primary tumors that may give
rise to pancreatic metastases include renal cell carcinoma (RCC),
non-small cell lung carcinoma, melanoma, sarcoma and colorectal
carcinoma. Previous studies have indicated that the kidney may be
more common than the lung as a primary tumor site of metastases to
the pancreas (1,4). Symptoms of pancreatic metastases are
often not apparent, and these lesions are thus detected
incidentally by physical examination in the majority of cases
(6). Abdominal computed tomography
(CT) scan and magnetic resonance imaging (MRI) examinations can be
used in the differential diagnosis of pancreatic lesions (7). Pathological analysis is the gold
standard in the diagnosis of pancreatic metastases (8). However, occasionally it is difficult to
diagnose pancreatic metastasis based on pathology alone (9).
In recent years, pancreatic metastases have been
observed with increasing frequency, particularly at high-volume
pancreatic surgery centers, due to increased utilization of
advanced imaging methods, including ultrasonography, CT, MRI and
endoscopic ultrasonography (EUS) (10). However, the treatment for pancreatic
metastases remains controversial. The majority of patients with
pancreatic metastases are in the advanced stages of primary disease
(11); however, the surgical
resection of pancreatic metastases may still confer a survival
benefit, based on a number of small, retrospective case studies, as
previously reviewed (5).
To the best of our knowledge, the association
between genetic mutations and pancreatic metastases is not
well-reported. The present study aimed to report our clinical
experience regarding the diagnosis and detection of mutations in
cases of pancreatic metastases, and to review the relevant
literature, in order to expand the knowledge pertaining to this
disease.
Patients and methods
Patients
A retrospective analysis of the history and
clinicopathological features of patients with pancreatic metastases
was performed. Between January 2013 and July 2016, 394 cases of
pancreatic-occupying lesions were documented in the database of The
First Affiliated Hospital of Soochow University (Suzhou, China), of
which 4 cases were diagnosed as pancreatic metastases.
A number of different variables were reviewed for
each case, including age, symptoms, tumor focality and size, tumor
location, tumor pathological types, type of primary tumor, latency
period between primary tumor and metastases, and data regarding
peri-pancreatic infiltration, lymph node metastases,
extra-pancreatic metastases, mutations, and treatment methods.
The study was approved by the ethics committee of
The First Affiliated Hospital of Soochow University, and all 4
patients provided written informed consent.
Diagnosis
The following methods were used in the diagnosis of
pancreatic metastases: CT, pathological staining,
immunohistochemistry and mutation detection. For the
immunohistochemical analyses, adjacent non-tumor pancreatic tissues
were used as the normal control group, and are shown for Cases 1, 2
and 3. Case 4 underwent EUS-guided fine-needle aspiration (FNA),
which did not include non-tumor tissues.
Literature search strategy
Studies were identified by searching PubMed
(https://www.ncbi.nlm.nih.gov/pubmed),
EMBASE (https://www.embase.com) and the Cochrane
Library (http://www.cochranelibrary.com). The search term was
‘pancreatic metastases’. No language limitation was applied. When a
study reporting the same patient cohort was included in several
publications, only the most recent or complete study was
selected.
Pathological hematoxylin and eosin
staining and immunohistochemistry
Samples were fixed with 10% formalin overnight at
room temperature (GE Healthcare Life Sciences, Logan, UT, USA) and
embedded in paraffin (GE Healthcare Life Sciences) and sectioned.
Serial sections (4-µm) were stained with hematoxylin-eosin (Muto
Pure Chemicals Co. Ltd, Tokyo, Japan) or subjected to
immunohistological staining. Immunohistological staining:
Subsequent to placing in blocking reagent (3% bovine serum albumin;
Roche Diagnostics, Basel, Switzerland) for 15 min at room
temperature, the sections were incubated with primary mouse
monoclonal anti-Ki-67 (dilution, 1:500; cat. no., ab6526; Abcam,
Cambridge, MA, USA) or mouse polyclonal anti-cytokeratin (dilution,
1:500; cat. no., ab9377; Abcam) antibody overnight at 4°C, followed
by incubation with horseradish peroxidase-conjugated polyclonal
goat anti-mouse or anti-rabbit IgG secondary antibody (dilution,
1:500; cat. no., ab97200; Abcam) for 2 h at 4°C. The signal was
visualized by treatment with 3,3′-diaminobenzidene for 5–10 min at
room temperature (Sangon Biotech Co., Ltd, Shanghai, China).
Detection of mutations
Samples were fixed in formalin and embedded in
paraffin, then sectioned as aforementioned. Mutations to genes
including KRAS, epidermal growth factor receptor (EGFR), von
Hippel-Lindau tumor suppressor (VHL) and vascular epidermal growth
factor receptor (VEGFR), were detected by Suzhou MicroDiag
Biomedicine Company Co., Ltd. (Suzhou, China) using an
Taqman-Amplification refractory mutation system (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Results
Of the 394 cases of pancreatic-occupying lesions, 4
patients (1.02%) were diagnosed with pancreatic metastases between
January 2013 and July 2016 (Table I),
including 3 males and 1 female. At the time of pancreatic
metastasis diagnosis, the mean age of the patients was 52.5 years
(range, 43–70 years).
 | Table I.General characteristics and genetic
mutations of the four cases of PM. |
Table I.
General characteristics and genetic
mutations of the four cases of PM.
| Patient no. | Sex | Age, years | Symptoms | Focality and size
of PM | Location of PM in
pancreas | Pathological
diagnosis of PM | Primary tumor | Latency period
between primary tumor and PM | Peri-pancreatic
infiltration | Lymph node
metastases | Treatment
method | Mutations
detected | Molecular targeted
drug treatment |
|---|
| 1 | M | 43 | Negative | Unifocal, 7 cm | Tail | Poorly
differentiated carcinoma | Lung large cell
carcinoma | Synchronous | Colon/spleen area
infiltration | Negative | Surgery (distal
pancreatectomy) | KRAS
Gly12(−); EGFR E19Del(+); EGFR Ins(−) | No |
| 2 | M | 48 | Negative | Unifocal, 2 cm | Head | Metastatic clear
cell carcinoma | Renal clear cell
carcinoma | 17 months | Negative | Negative | Surgery
(pancreaticodu-odenectomy)+drug (sunitinib) | VHL(+);
EGFR G719 X(+); VEGFR2 increase | Yes |
| 3 | F | 49 | Abdominal
discomfort | Unifocal, 5 cm | Head | Moderately
differentiated adenocarcinoma | Gastric
carcinoma | Synchronous | Negative | Positive | Surgery (pancreatic
tumor enucleation) | None | No |
| 4 | M | 70 | Abdominal
discomfort | Multifocal | Head, neck, body,
tail | Metastatic clear
cell carcinoma | Renal clear cell
carcinoma | 24 months | Negative | Negative | Drug
(sunitinib) | VHL(+);
EGFR E19 Del(+); VEGFR2 increase | Yes |
In 2 cases, the pancreatic metastases were
synchronous with the primary tumor, whereas the other 2 cases were
metachronous, with latency periods of 17 and 24 months,
respectively, between the primary tumor and the metastases. The
primary tumors were lung cancer in 1 case, renal cell cancer in 2
cases, and gastric cancer in 1 case. Regarding the focality of the
pancreatic metastases, 3 patients exhibited unifocal lesions (1 in
the tail and 2 in the head of the pancreas), and 1 had multifocal
lesions, with 3 lesions located in the pancreatic head, 4 in the
body, and 2 in the tail of the pancreas.
Only 2 patients were diagnosed based on abdominal
discomfort, whereas the other 2 were diagnosed during follow-up
after primary tumor resection. Upon diagnosis, 3 patients were
treated by various metastasectomy methods, including distal
pancreatectomy (Case 1), pancreaticoduodenectomy (Case 2), and
pancreatic tumor enucleation (Case 3). Case 4 underwent EUS-FNA and
pathological diagnosis. Mutation detection was performed in 3
patients to obtain precise mutation information. The detection of
mutations in Case 1 revealed KRAS Gly12(−), EGFR E19Del(+)
and EGFR INS(−), whereas Case 2 and Case 4 exhibited VHL(+)
and VEGFR2 increase.
All 4 patients were diagnosed with pancreatic
metastases by CT imaging or MRI, and confirmed by pathological
staining and immunohistochemistry (Figs.
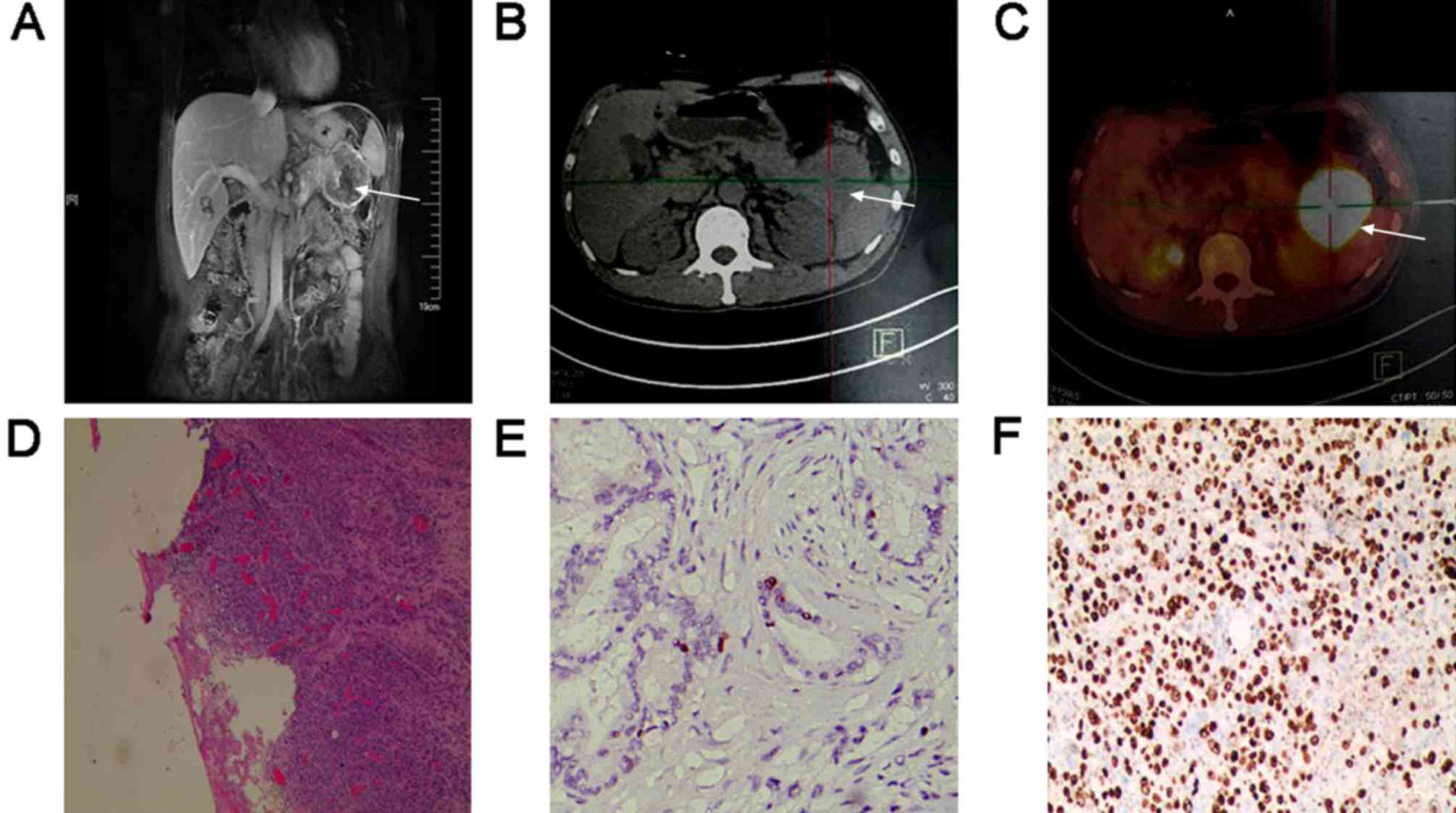
1–4). MRI revealed pancreatic
metastasis as a unifocal hypo-signal lesion in the tail of the
pancreas on T1WI image, hematoxylin and eosin staining indicated
poorly-differentiated carcinoma, and immunohistochemical staining
showed Ki-67 expression (70%) in pancreatic metastasis tissues
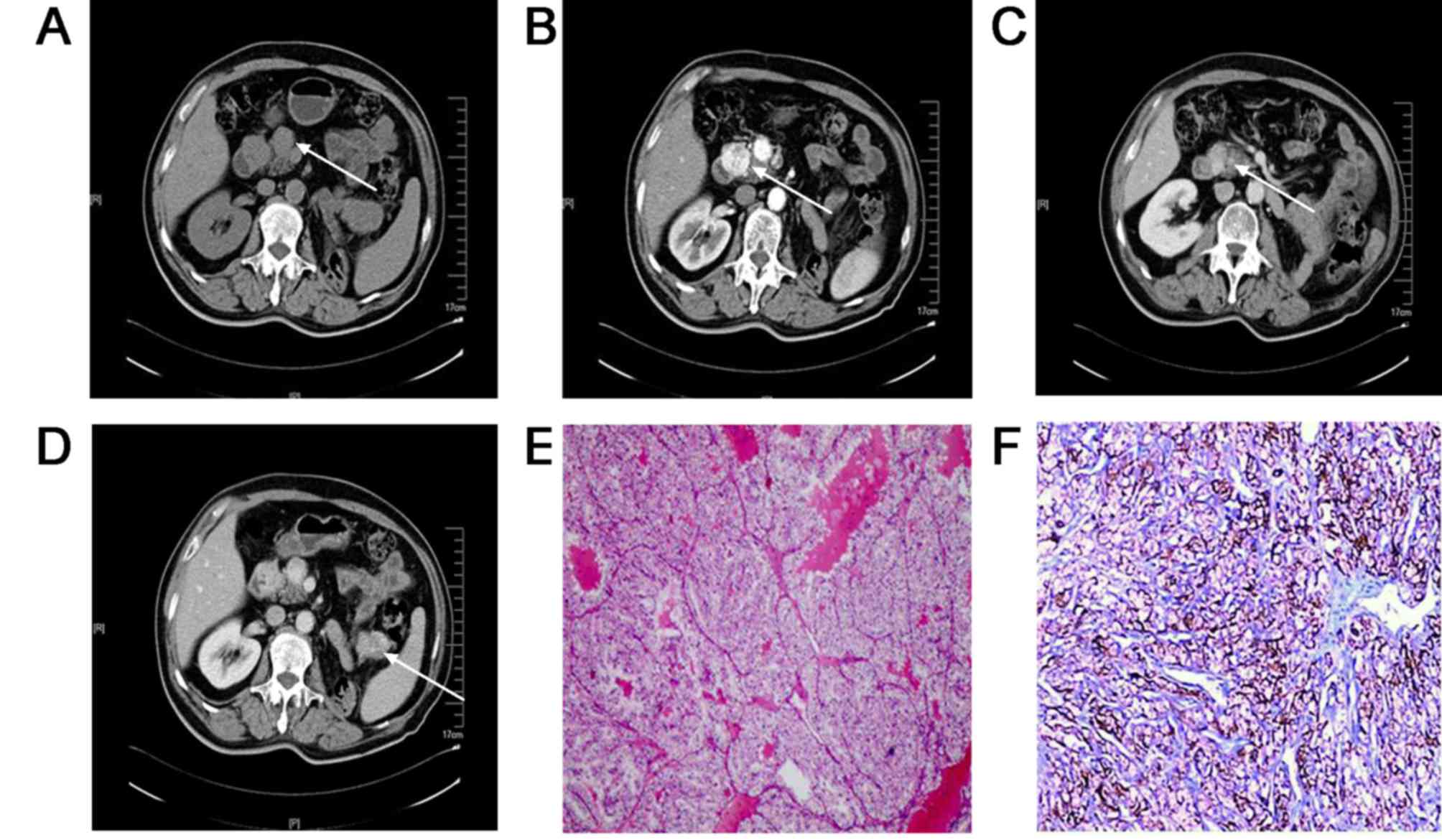
(Fig. 1). As shown in Fig. 2, CT showed pancreatic metastasis as a
unifocal hypervascular lesion in the head of the pancreas,
hematoxylin and eosin staining indicated metastatic clear cell
carcinoma, and immunohistochemical staining showed positive
expression of CK protein in pancreatic metastasis tissues. CT
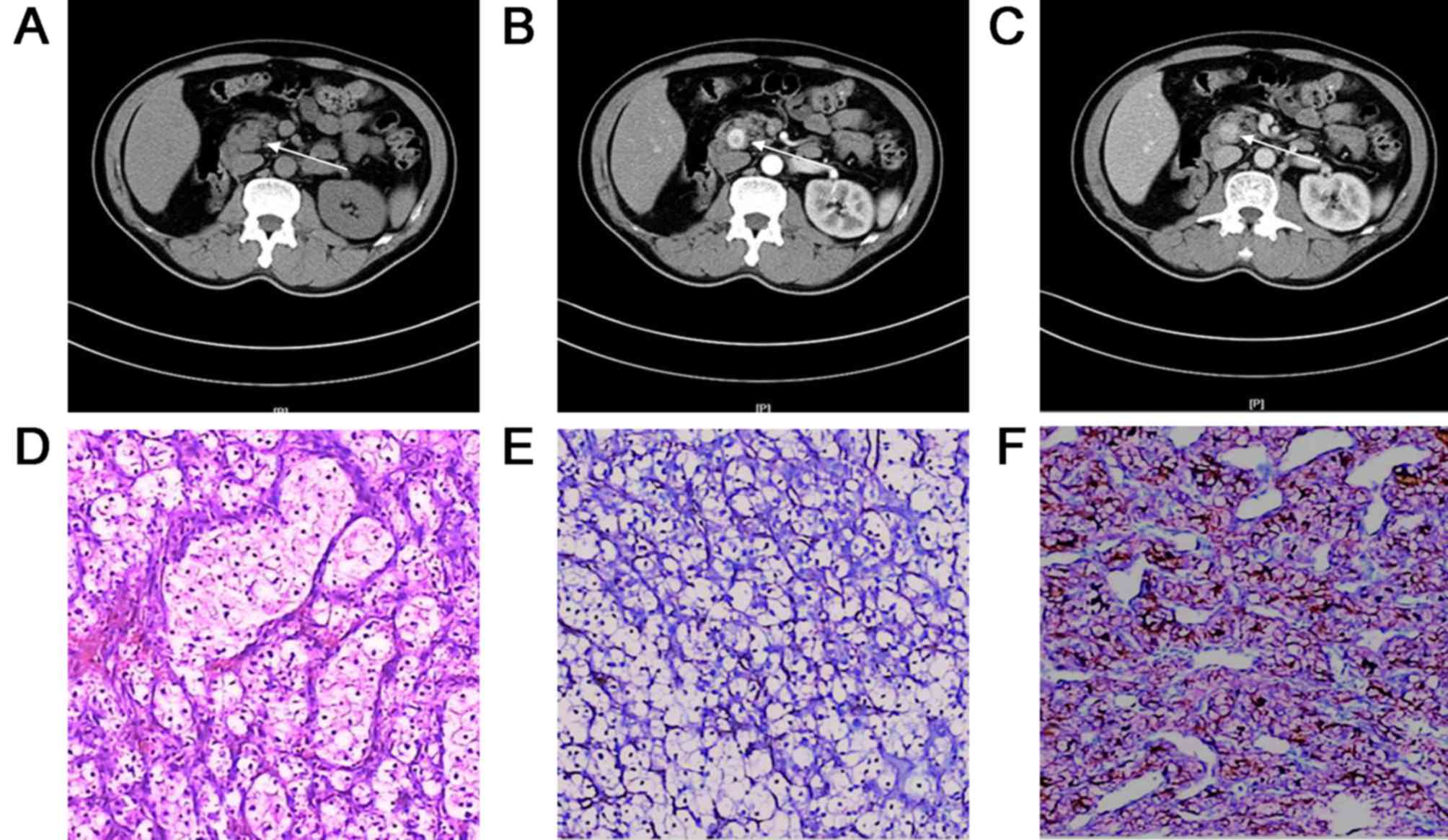
showed pancreatic metastasis presented as unifocal lesion in the
head of the pancreas, hematoxylin and eosin staining showed
moderately-differentiated adenocarcinoma, and immunohistochemical
staining showed Ki-67 expression (15%) in pancreatic metastasis
tissues (Fig. 3). As shown in
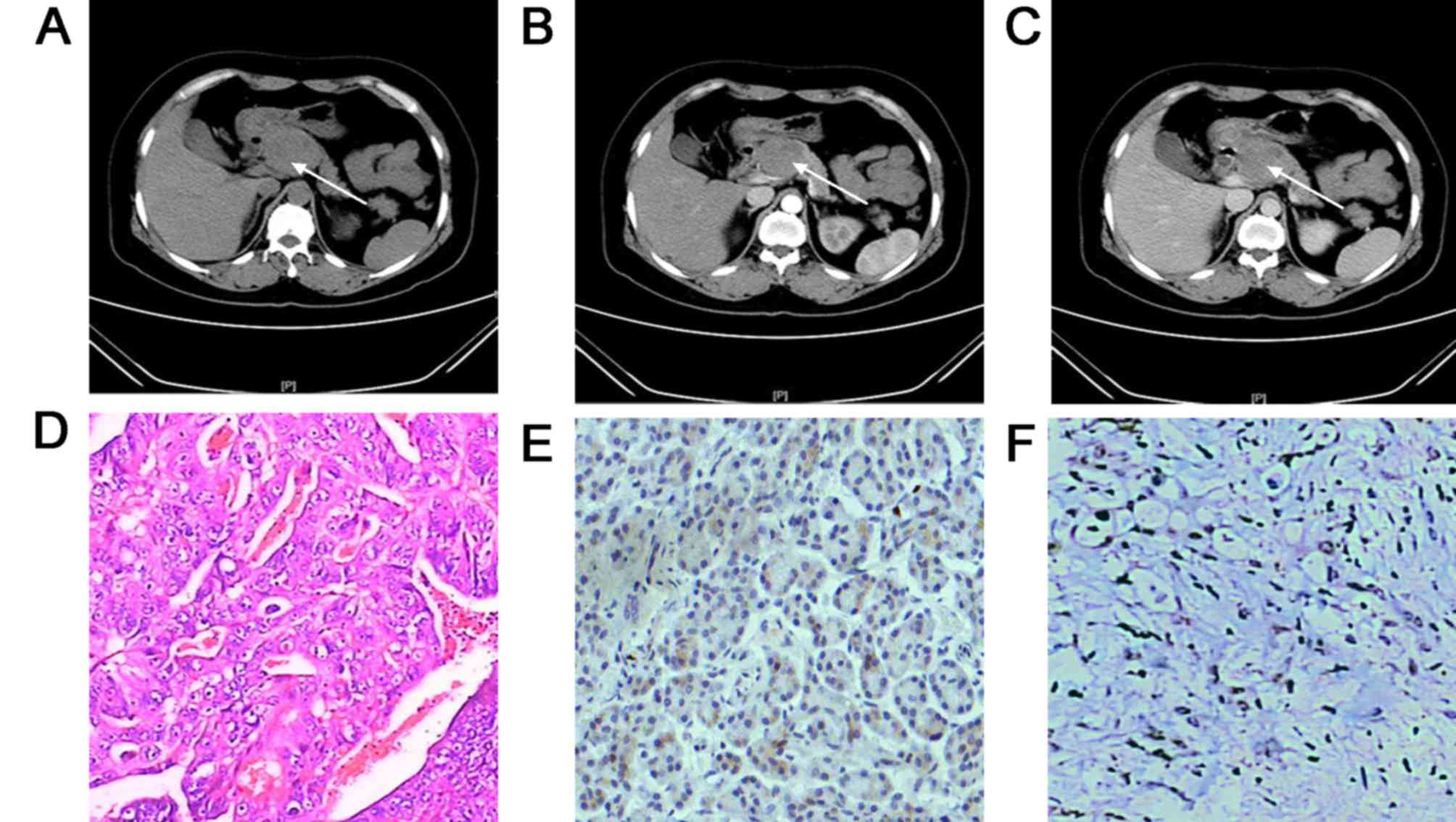
Fig. 4, CT showed pancreatic
metastases as multifocal lesions in the pancreas, hematoxylin and
eosin staining showed metastatic clear cell carcinoma, and
immunohistochemical staining showed positive expression of CK
protein in pancreatic metastasis tissues. Molecular targeted drugs,
according to the mutation information, were prescribed for 2
patients (Cases 2 and 4; sunitinib, 50 mg, orally, once per day,
4/2 dosage regimen). Sunitinib is a small-molecule inhibitor of
several receptor tyrosine kinases relevant to tumor angiogenesis,
including the vascular endothelial growth factor (VEGF) receptor
(12,13). Chemotherapy was administered to 1
patient [Case 3; day 1: oxaliplatin 150 mg + CF 0.5 iv +
fluorouracil (5-FU) 0.5 iv, followed by a 46 h continuous 5-FU 3.0
civ (iv, intravenous infusion; civ, central venous infusion)]. The
follow-up periods were 0.5, 3, 2.8 and 0.6 year for Cases 1–4,
respectively. All four patients are currently alive.
Discussion
Although metastases in the liver and lungs are
well-described, the pancreas is an unusual site for metastasis. Out
of all cases of pancreatic-occupying lesions, the reported
incidence of pancreatic metastasis is 2–5% (1–5,7,14). In the
present study, pancreatic metastases represented 1.02% of all cases
of malignancies encountered in the pancreas. However, in a report
of 190 autopsy cases by Adsay et al (1), 42% patients (81/190) exhibited
metastases in the pancreas. This number is significantly higher
than the generally accepted incidence rate (6). Therefore, the proportion of patients
affected by pancreatic metastases is speculated to be significantly
higher based on the longer lifespans and improved sensitivity of
diagnostic medical examinations. As pancreatic metastases almost
always occur in elderly patients and those with later-stage
malignancies, certain authors have suggested that the prognosis for
pancreatic metastasis is better than primary pancreatic cancer
(4,15). Research should be conducted to develop
improved diagnostic and treatment techniques for pancreatic
metastases.
In the present study, 3 of the 4 patients were male.
However, in previously published reports, the distribution was
equal between the two sexes (6,15).
Pancreatic metastases can occur in any part of the pancreas. Jarufe
et al reported that 11 patients (84.6%) had tumors in the
head of pancreas, whereas 2 exhibited lesions in the body and tail
of the pancreas (14). The majority
of pancreatic metastases are unifocal (63.6%) (7), while multifocal presentation of
pancreatic metastases accounts for 20 to 45% (7,16), and
diffuse-type accounts for ~9% (7). As
shown in a previous study of 399 patients with pancreatic
metastases, the mean age at the time of diagnosis is 61.7 years
(6). Following treatment of the
primary tumor, pancreatic metastases have an unpredictable latency
period; they may be synchronous or metachronous with the primary
tumors. El Hajj et al (17)
reported a case of RCC in which pancreatic metastasis was detected
29 years after diagnosis of the primary tumor. In another study,
the median time between diagnosis of the primary tumor and of the
initial pancreatic metastasis was 9 years (18).
Metastatic pancreatic lesions often differ from
primary pancreatic lesions; 90% of primary pancreatic tumors are of
ductal origin, whereas metastatic pancreatic lesions are often of
epithelial or hematopoietic origin. Data from Adsay et al
(1) showed that pancreatic
metastases, including those arising from lung cancer, RCC and
digestive cancers, were predominantly of epithelial origin (65 of
81 cases). RCC metastasis to the pancreas has been extensively
reported in the literature, and this is considered the most common
origin of pancreatic metastatic lesions (18–22). A
recent literature review (18),
including 582 patients with pancreatic metastases, showed that the
five most common primary tumors are RCC, lung carcinoma, colorectal
cancer, melanoma, and breast carcinoma, representing 80% of all
cases. However, Adsay et al (1) and Tsitouridis et al (7) reported that the most common origin of
metastases in the pancreas was primary lung cancer. This
controversy is likely due to the different compositions between the
samples. Furthermore, a study from Japan indicated stomach cancer
as the most common origin of pancreatic metastases, suggesting
population-based differences (23).
A proportion of patients with pancreatic metastases
may experience certain non-specific symptoms, including abdominal
discomfort, back pain, nausea and weight loss (24). However, the majority of patients with
pancreatic metastases (50–83%) do not exhibit any obvious symptoms,
and the patient's admission to the diagnosis may appear several
years after primary tumor resection (25). Metastases are usually diagnosed during
follow-up studies. Therefore, in patients with pancreatic tumors
and history of carcinoma, pancreatic metastasis should always be
considered.
Tumor markers, including carcinoembryonic antigen
(CEA) and carbohydrate antigen 19–9 (CA19-9), do not aid in the
diagnosis of pancreatic metastases, although CA19-9 is a relatively
sensitive indicator for primary pancreatic cancer (10). Abdominal CT can be used in the
differential diagnosis of pancreatic lesions. Pancreatic metastases
are usually hypervascular, whereas primary pancreatic
adenocarcinoma is typically hypovascular (with the exception of
endocrine pancreatic tumors, which appear as hypervascular
lesions). Furthermore, compared with primary pancreatic cancer,
pancreatic metastases exhibit less vascular invasion and
peri-pancreatic lymphatic metastasis (7). Positron-emission tomography (PET)/CT may
be advantageous in the detection of distant metastases (26). Among the 4 patients in the present
study, only the patient in Case 1 underwent PET/CT. Due to its
higher cost, PET/CT is not routinely used as an examination prior
to surgery in clinical practice in China (7). However, as metastatic lesions may be
small, it can be difficult to detect them without PET/CT (26).
Pathological analysis is the gold standard in the
diagnosis of pancreatic metastases. FNA may be useful in
pathological diagnosis when a specimen is difficult to remove
surgically. EUS-FNA is a minimally invasive and accurate method of
sampling lesions of the pancreas (8,27); it has
been shown to have an accuracy of 89% in diagnosing pancreatic
metastases (28).
With the popularity of gene-targeted therapy, the
detection of individual tumor mutations is being utilized more
widely in clinical settings. The detection of mutations can provide
precise pharmacogenomics-related information for individual tumor
patients (29). According to the
detected mutation information, doctors can classify tumors
precisely, select appropriate drug treatments, and provide
individualized therapy (29).
Occasionally, difficulty arises in determining the
primary tumor that originated the pancreatic metastasis based
solely on pathological and immunohistochemistry results; however,
this difficulty may be overcome with the combination of mutation
information (30). Studies have shown
that thyroid transcription factor 1 (TTF-1) and napsin A are
specific markers for primary lung adenocarcinoma (31,32), and
that cytokeratin 20 (CK20) and caudal type homeobox 2 (CDX2)
positivity supported the diagnose of primary pancreatic cancer
(30); however, the positivity rate
of TTF-1 and Napsin A is low. Krasinskas et al (30) reported that KRAS G12C
mutations, TTF-1 and napsin A are associated with primary lung
adenocarcinoma, whereas KRAS G12R mutations, CK20 and CDX2
are more common in pancreatic adenocarcinoma. Combining gene
mutation information and immunohistochemical results can improve
the accuracy of primary tumor diagnosis. Research has demonstrated
that activating KRAS mutations are present in the majority
of pancreatic ductal adenocarcinomas (~90%) and less frequently in
lung adenocarcinomas (13–23%) (33,34).
EGFR gene mutations are present in 30–48% of lung
adenocarcinomas and less frequently in pancreatic adenocarcinomas
(2.3%) (35). VHL gene
mutations or inactivation are present in 80% of clear cell RCCs,
and may promote excessive expression of VEGF, platelet-derived
growth factors (PDGF), and transforming growth factor-α (36). Tumors are the result of accumulation
of multiple mutations and their interactions (33). Oncogenic gain-of-function mutations
cause excessive stimulation of the cell cycle, while
loss-of-function mutations in tumor suppressor genes can result in
a loss of control over this excessive activity (36). The detection of mutations in
pancreatic metastases can help to confirm the primary tumor
(35). In the present study, 3
patients underwent the detection of mutations. Although the
immunohistochemical analysis results of Case 1 indicated TTF-1(−)
and napsin A(−) expression, the combination of the mutation
results, KRAS(−) and EGFR(+), and cell morphology led
to a diagnosis of the primary tumor as a large cell lung carcinoma.
Mutation detection in Cases 2 and 4 indicated VHL(+),
confirming the diagnosis of the primary tumor as RCC. The detection
of typical mutation profiles can aid in the diagnosis of pancreatic
metastases; and combining mutation information and pathology, it
may be easier to confirm the diagnosis of the primary tumor.
Mutation information also has clinical value with
regard to drug selection and effective prediction of pancreatic
metastases. Although distant metastasis is considered a late
manifestation of primary cancer, radical resection of metastases in
the pancreas is the current standard treatment for pancreatic
metastases in patients with no contraindication to surgery, and can
improve patient survival (11,24,37,38).
However, for patients with pancreatic metastases and
extra-pancreatic metastases, radical resection cannot be
implemented.
Sunitinib is used as molecularly targeted drug for
advanced metastatic clear cell RCC. The primary targets of
sunitinib are VEGF receptors (VEGFRs) and PDGF receptors. In the
present study, the expression of VEGFR was detected in 2 patients,
which indicated that molecularly targeted drug treatment with
sunitinib would be effective. Niess et al (39) compared the outcomes of patients with
extra-pancreatic metastases at the time of resection, and reported
a median survival time of 11 months in 8 patients with multi-organ
disease. Medioni et al (13)
performed a retrospective review of patients treated with sunitinib
for metastatic RCC, and reported a median survival time of 20
months. Although the compositions of the two study samples were
different, we speculate that using the molecularly targeted drug
sunitinib based on mutation information may prolong the survival
time of patients with unresectable pancreatic metastases and
extra-pancreatic metastases. However, this hypothesis requires
confirmation through further study.
In conclusion, pancreatic metastasis is rare, and
its morbidity appears to be significantly increasing with the
extension of human lifespan and improved sensitivity of diagnostic
examinations. The majority of pancreatic metastases develop from
RCC, and also commonly from lung carcinoma, colorectal cancer,
melanoma and breast carcinoma. Metastases can occur several months
or several years after surgery. The diagnosis of pancreatic
metastases predominantly relies on CT, pathology and
immunohistochemistry. The detection of mutations has clinical value
as an auxiliary test to aid diagnosis and therapy selection for
pancreatic metastases. Using molecularly targeted drugs based on
mutation information may prolong the survival time of patients with
unresectable pancreatic metastases and extra-pancreatic
metastases.
Acknowledgements
This project was supported by the Project of Key
Research and Development of Jiangsu Province of China (grant no.
BE2016673), the Project of Suzhou People's Livelihood Science and
Technology (grant nos. SS201531 and SS201632), and Jiangsu
Provincial Medical Youth Talent (grant no. QNRC2016734).
References
|
1
|
Adsay NV, Andea A, Basturk O, Kilinc N,
Nassar H and Cheng JD: Secondary tumors of the pancreas: An
analysis of a surgical and autopsy database and review of the
literature. Virchows Arch. 444:527–535. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zerbi A, Ortolano E, Balzano G, Borri A,
Beneduce AA and Di Carlo V: Pancreatic metastasis from renal cell
carcinoma: Which patients benefit from surgical resection? Ann Surg
Oncol. 15:1161–1168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roland CF and van Heerden JA:
Nonpancreatic primary tumors with metastasis to the pancreas. Surg
Gynecol Obstet. 168:345–347. 1989.PubMed/NCBI
|
|
4
|
Faure JP, Tuech JJ, Richer JP, Pessaux P,
Arnaud JP and Carretier M: Pancreatic metastasis of renal cell
carcinoma: Presentation, treatment and survival. J Urol. 165:20–22.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crippa S, Angelini C, Mussi C, Bonardi C,
Romano F, Sartori P, Uggeri F and Bovo G: Surgical treatment of
metastatic tumors to the pancreas: A single center experience and
review of the literature. World J Surg. 30:1536–1542. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adler H, Redmond CE, Heneghan HM, Swan N,
Maguire D, Traynor O, Hoti E, Geoghegan JG and Conlon KC:
Pancreatectomy for metastatic disease: A systematic review. Eur J
Surg Oncol. 40:379–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsitouridis I, Diamantopoulou A,
Michaelides M, Arvanity M and Papaioannou S: Pancreatic metastases:
CT and MRI findings. Diagn Interv Radiol. 16:45–51. 2010.PubMed/NCBI
|
|
8
|
Mallery JS, Centeno BA, Hahn PF, Chang Y,
Warshaw AL and Brugge WR: Pancreatic tissue sampling guided by EUS,
CT/US, and surgery: A comparison of sensitivity and specificity.
Gastrointest Endosc. 56:218–224. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chu PG, Chung L, Weiss LM and Lau SK:
Determining the site of origin of mucinous adenocarcinoma: An
immunohistochemical study of 175 cases. Am J Surg Pathol.
35:1830–1836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zerbi A and Pecorelli N: Pancreatic
metastases: An increasing clinical entity. World J Gastrointest
Surg. 2:255–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reddy S and Wolfgang CL: The role of
surgery in the management of isolated metastases to the pancreas.
Lancet Oncol. 10:287–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Radulovic S and Bjelogrlic SK: Sunitinib,
sorafenib and mTOR inhibitors in renal cancer. J Buon. 12 Suppl
1:S151–S162. 2007.PubMed/NCBI
|
|
13
|
Medioni J, Choueiri TK, Zinzindohoue F,
Cho D, Fournier L and Oudard S: Response of renal cell carcinoma
pancreatic metastasis to sunitinib treatment: A retrospective
analysis. J Urol. 181:2470–2475. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jarufe N, McMaster P, Mayer AD, Mirza DF,
Buckels JA, Orug T, Tekin K and Bramhall SR: Surgical treatment of
metastases to the pancreas. Surgeon. 3:79–83. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Markinez I, Jiménez R, Ruiz I, Villarreal
E, Lizarazu A, Borda N, Arteaga X, Medrano MÁ, Guisasola E,
Beguiristain A and Enríquez-Navascués JM: Pancreatic metastases due
to renal carcinoma. Our cases and a literature review. Cir Esp.
91:90–95. 2013.(In Spanish).
|
|
16
|
Machado NO and Chopra P: Pancreatic
metastasis from renal carcinoma managed by Whipple resection. A
case report and literature review of metastatic pattern, surgical
management and outcome. JOP. 10:413–418. 2009.PubMed/NCBI
|
|
17
|
El Hajj II, LeBlanc JK, Sherman S,
Al-Haddad MA, Cote GA, McHenry L and DeWitt JM: Endoscopic
ultrasound-guided biopsy of pancreatic metastases: A large
single-center experience. Pancreas. 42:524–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith AL, Odronic SI, Springer BS and
Reynolds JP: Solid tumor metastases to the pancreas diagnosed by
FNA: A single-institution experience and review of the literature.
Cancer Cytopathol. 123:347–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thompson LD and Heffess CS: Renal cell
carcinoma to the pancreas in surgical pathology material. Cancer.
89:1076–1088. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hiotis SP, Klimstra DS, Conlon KC and
Brennan MF: Results after pancreatic resection for metastatic
lesions. Ann Surg Oncol. 9:675–679. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klein KA, Stephens DH and Welch TJ: CT
characteristics of metastatic disease of the pancreas.
Radiographics. 18:369–378. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Le Borgne J, Partensky C, Glemain P, Dupas
B and de Kerviller B: Pancreaticoduodenectomy for metastatic
ampullary and pancreatic tumors. Hepatogastroenterology.
47:540–544. 2000.PubMed/NCBI
|
|
23
|
Nakamura E, Shimizu M, Itoh T and Manabe
T: Secondary tumors of the pancreas: Clinicopathological study of
103 autopsy cases of Japanese patients. Pathol Int. 51:686–690.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sweeney AD, Fisher WE, Wu MF, Hilsenbeck
SG and Brunicardi FC: Value of pancreatic resection for cancer
metastatic to the pancreas. J Surg Res. 160:268–276. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ahmed S, Johnson PT, Hruban R and Fishman
EK: Metastatic disease to the pancreas: Pathologic spectrum and CT
patterns. Abdom Imaging. 38:144–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song SW, Cheng JF, Liu N and Zhao TH:
Diagnosis and treatment of pancreatic metastases in 22 patients: A
retrospective study. World J Surg Oncol. 12:2992014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen L and Brainard JA: Pancreatic
metastasis from papillary thyroid carcinoma diagnosed by endoscopic
ultrasound-guided fine needle aspiration: A case report. Acta
Cytol. 54:640–644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ardengh JC, Lopes CV, Kemp R, Venco F, de
Lima-Filho ER and dos Santos JS: Accuracy of endoscopic
ultrasound-guided fine-needle aspiration in the suspicion of
pancreatic metastases. Bmc Gastroenterol. 13:632013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saiz M, Alvarez-Cubero MJ,
Martinez-González LJ, Alvarez JC, Lorente M and Lorente JA: The
identification of individuals through genetic testing and DNA
profiling banks. Rev Derecho Genoma Hum. 157–168. 2014.PubMed/NCBI
|
|
30
|
Krasinskas AM, Chiosea SI, Pal T and Dacic
S: KRAS mutational analysis and immunohistochemical studies can
help distinguish pancreatic metastases from primary lung
adenocarcinomas. Mod Pathol. 27:262–270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu J, Chu PG, Jiang Z and Lau SK: Napsin A
expression in primary mucin-producing adenocarcinomas of the lung:
An immunohistochemical study. Am J Clin Pathol. 139:160–166. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stoll LM, Johnson MW, Gabrielson E, Askin
F, Clark DP and Li QK: The utility of napsin-A in the
identification of primary and metastatic lung adenocarcinoma among
cytologically poorly differentiated carcinomas. Cancer Cytopathol.
118:441–449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hruban RH, van Mansfeld AD, Offerhaus GJ,
van Weering DH, Allison DC, Goodman SN, Kensler TW, Bose KK,
Cameron JL and Bos JL: K-ras oncogene activation in adenocarcinoma
of the human pancreas. A study of 82 carcinomas using a combination
of mutant-enriched polymerase chain reaction analysis and
allele-specific oligonucleotide hybridization. Am J Pathol.
143:545–554. 1993.PubMed/NCBI
|
|
34
|
Dacic S, Shuai Y, Yousem S, Ohori P and
Nikiforova M: Clinicopathological predictors of EGFR/KRAS
mutational status in primary lung adenocarcinomas. Mod Pathol.
23:159–168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oliveira-Cunha M, Hadfield KD, Siriwardena
AK and Newman W: EGFR and KRAS mutational analysis and their
correlation to survival in pancreatic and periampullary cancer.
Pancreas. 41:428–434. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Patard JJ, Rioux-Leclercq N, Masson D,
Zerrouki S, Jouan F, Collet N, Dubourg C, Lobel B, Denis M and
Fergelot P: Absence of VHL gene alteration and high VEGF expression
are associated with tumour aggressiveness and poor survival of
renal-cell carcinoma. Br J Cancer. 101:1417–1424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reddy S, Edil BH, Cameron JL, Pawlik TM,
Herman JM, Gilson MM, Campbell KA, Schulick RD, Ahuja N and
Wolfgang CL: Pancreatic resection of isolated metastases from
nonpancreatic primary cancers. Ann Surg Oncol. 15:3199–3206. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Strobel O, Hackert T, Hartwig W, Bergmann
F, Hinz U, Wente MN, Fritz S, Schneider L, Büchler MW and Werner J:
Survival data justifies resection for pancreatic metastases. Ann
Surg Oncol. 16:3340–3349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Niess H, Conrad C, Kleespies A, Haas F,
Bao Q, Jauch KW, Graeb C and Bruns CJ: Surgery for metastasis to
the pancreas: Is it safe and effective? J Surg Oncol. 107:859–864.
2013. View Article : Google Scholar : PubMed/NCBI
|