Introduction
Triple-negative breast cancer (TNBC; estrogen
receptor-negative, progesterone receptor-negative and
Her-2-negative) remains challenging to treat due to the innate
aggressive biological characteristics and the lack of effective
therapies (1,2). TNBCs represent ~15% of all breast cancer
cases and are often accompanied by a higher frequency of p53 gene
mutations (2,3). The tumor suppressor gene p53 serves a
critical role in conferring cancer cell sensitivity to DNA-damaging
agents (3). Failure of p53 signaling
leads to resistance to chemotherapeutics (3–5). p73 is a
member of the p53 gene family. Under certain conditions, p73 is
able to replace the p53 function in response to DNA damage,
activate the transcription of p53-responsive genes and inhibit cell
growth in a p53-like manner by inducing cell cycle arrest and
apoptosis (6–8). Therefore, the identification of
anticancer drugs able to activate p73 and target p53 downstream
genes may provide a chemotherapeutic approach for the treatment of
p53-deficient types of cancer.
Curcuma is a traditional Chinese herbal
medicine, which may be subgrouped into Curcuma aeruginosa
Roxb and Curcuma wenyujin Y. H. Chen et C. Ling
(Curcuma wenyujin). Curcuma is traditionally used to
treat various ailments, including cacochylia, traumatic hematoma,
parasitic infection and tumorous diseases (9). The essential oils of Curcuma
wenyujin exhibit antitumor, anti-inflammatory, antioxidant and
antimicrobial characteristics with low cytotoxicity and are
embodied in the official Pharmacopoeia of the P.R. China as an
anticancer and antiviral remedy (10,11). A
total of six volatile compounds (curdione, curcumol, germacrone,
curzerene, 1,8-cineole and β-elemene) have been successfully
isolated from the essential oil of Curcuma wenyujin
(12). As one of the major components
of the essential oil, curcumol,
C15H24O2-(3s-(3a,
3aa,5a,6a,8ab))-octahydro-3-methyl-8-methylene-5-(1-methylethyl)-6h-3a,
6-epoxyazulen-6-ol (13), has been
reported to be capable of blocking the proliferation of various
types of human tumor cells, including lung, prostate and ovarian
cancer cell lines in vitro and inducing apoptosis via a
caspase-independent mitochondrial pathway in ASTC-a-1 cells
(14–17). However, the molecular mechanisms
underlying curcumol-induced anti-proliferative effects are still
unknown. In addition, little is currently understood regarding the
anticancer effect of this compound on breast cancer cells. The
present study investigated the anticancer properties of curcumol
in vitro and in vivo using human p53 mutant TNBC
MDA-MB-231 cells and BALB/c nu/nu mice. The data revealed that
curcumol inhibits the proliferation and xenograft growth of
MDA-MB-231 cells, and triggers cell apoptosis via a p53-independent
pathway involved in the upregulation of p73 and the activation of
the pro-apoptotic genes p53 upregulated modulator of apoptosis
(PUMA) and B-cell lymphoma-2 (Bcl-2) antagonistic killer (Bak).
These findings demonstrate the potential of curcumol as a
therapeutic agent towards TNBC.
Materials and methods
Animals, cell lines and materials
In total of 24 female 4-week-old BALB/c nu/nu (nude)
mice (~15 g) were purchased from the Experimental Animal Centre of
the Shanghai Institutes for Biological Sciences (Shanghai, China)
and raised in SPF conditions; the experimental protocol of the
present study was approved by the Guilin Medical University Ethics
Committee for Animal Experimentation (Guilin, China). The
MDA-MB-231 and MCF-7 cell lines was donated by Dr P. Wedegeartner
(Thomas Jefferson University, Philadelphia, PT, USA), but
originally obtained from the American Type Culture Collection
(Manassas, VA, USA).
Curcumol (purity >99% by high performance liquid
chromatography, lot no. P02-03) was purchased from Shanghai Aihui
Bio-Tech Co., Ltd. (Shanghai, China) and dissolved in ethanol at a
concentration of 100 mg/ml. MTT, propidium iodide (PI), RNase A,
DAPI and adriamycin were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Annexin V-fluorescein isothiocyanate (FITC)
Apoptosis Detection kit was purchased from BD Biosciences (San
Jose, CA, USA). Rabbit anti-p53 (cat. no., sc-53394), -p21 (cat.
no., sc-397), -p73 (cat. no., sc-7975) and poly (ADP-ribose)
polymerase (PARP)-1 polyclonal antibodies (cat. no., sc-7150), and
mouse anti-BAK (cat. no., sc-1035), PUMA (cat. no., sc-374223) and
Actin (cat. no., sc-8432) monoclonal antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) and used at a
dilution of 1:1,000. Goat anti-rabbit (cat. no., W4011) and
anti-mouse (cat. no., W4021) immunoglobulin G (H+L)-horseradish
peroxidase-conjugated secondary antibodies were purchased from
Promega Corporation (Madison, WI, USA) and used at a dilution of
1:5,000. An Enhanced Chemiluminescence Detection kit was obtained
from Pierce (Thermo Fisher Scientific, Inc., Waltham, MA, USA). All
cell culture plates were purchased from Corning Incorporated
(Corning, NY, USA).
Cell culture
The TNBC MDA-MB 231 cell line was cultured in
Dulbecco's modified Eagle's medium (HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 100 µg/ml streptomycin and
100 U/ml penicillin (HyClone; GE Healthcare Life Sciences) at 37°C
in a humidified atmosphere containing 5% CO2. When cells
were ~50% subconfluent, the cells were treated with 12.5–800 µg/ml
of curcumol (stock 100 mg/ml dissolved in ethanol). Untreated
MDA-MB-231 cells served as a control and were cultured following a
similar protocol in medium supplemented with equivalent volumes of
ethanol (vehicle).
MTT assay for cell viability
An MTT assay (Sigma-Aldrich; Merck KGaA) was used to
assess cellular viability reflected by the metabolic activity of
the cells. The cells were seeded on 96-well plates at a density of
4,000 cells/well. Following an incubation at 37°C overnight, the
cells were exposed to curcumol at concentrations of 12.5, 25, 50,
100, 200, 400 and 800 µg/ml for 48 and 72 h. Ethanol served as the
control. Subsequent to the indicated time points, 10 µl MTT (5
mg/ml dissolved in PBS, pH 7.4) was added to each well and
incubated at 37°C for 4 h. Following incubation, the culture medium
was aspirated and the plates were dried by inversion at room
temperature for ~15 min. The formazan crystals were then dissolved
using dimethyl sulfoxide (100 µl for each well) and the absorbance
was measured at 510 nm using an iMark microplate absorbance reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). All assays were
performed in quadruplicate and each experiment was conducted five
times. Cell inhibition was calculated using the following formula:
Inhibition rate (%)=[1-(OD test/OD control)]x100. Optical density
(OD) represents the absorbance value. The half maximal inhibitory
concentration (IC50) was calculated using SPSS version
20.0 (IBM SPSS, Armonk, NY, USA).
Xenograft model assay
BALB/c nu/nu mice were randomly assigned into three
groups with eight mice per group, and maintained in the
specific-pathogen-free Animal Facility at Guilin Medical
University. A total of 1×107 TNBC MDA-MB-231 cells were
suspended in 0.2 ml PBS and injected subcutaneously into the right
flank of the nude mice. The mice were intraperitoneally injected
with curcumol (100 or 200 µg/kg) every 48 h after developing
measurable tumors. The tumor growth was monitored every 3 days and
the mice were sacrificed by cervical dislocation on the 21st day
subsequent to i.p. injection. Tumors were then dissected and
weighed.
Cell cycle analysis
TNBC MDA-MB-231 cells were seeded in 6 cm (diameter)
dishes, synchronized by serum deprivation at 37°C for 96 h. Cells
were then exposed to 25 or 100 µg/ml curcumol for 20 and 24 h,
respectively, at 37°C. Cells treated with an equal volume of
ethanol at 37°C were used as a control. At the designated time
points (20 and 24 h after exposure to curcumol), adherent cells
were harvested by trypsinization and floating cells were collected
by centrifugation (3,000 × g, 5 min, room temperature). Cells were
fixed in 70% ethanol overnight at 4°C and then treated with a
staining buffer (PBS containing 1 mg/ml PI and 10 mg/ml RNase A) at
37°C in the dark for 30 min. The DNA content of the cells was
determined by analyzing 10,000 ungated cells using
FACSAria™ III flow cytometer (BD Biosciences, San Jose,
CA, USA) and FACSDiva™ software version 6.1.3 (BD
Biosciences). The experiment was performed in triplicate.
Morphological analyses
TNBC MDA-MB-231 cells were seeded onto 6-well plates
containing microscope coverslips at a density of 4×105
cells/well. Following an overnight incubation at 37°C, cells were
treated with 50, 100 or 200 µg/ml curcumol or with the matched
ethanol vehicle and incubated for an additional 48 h at 37°C. Cells
were then fixed in 0.4% PBS-buffered paraformaldehyde solution for
15 min, stained with DAPI for 5 min at room temperature and mounted
on microscope slides. The morphology of the cell nuclei was
observed at an excitation wavelength of 350 nm using a laser
confocal fluorescence microscope (LSM710 Meta; Carl Zeiss AG,
Oberkochen, Germany). The percentage of apoptotic cells (cells with
nuclear condensation and/or fragmentation) was evaluated by
randomly counting ≥10 fields for each condition.
Annexin V/PI-staining
MDA-MB-231 cells were seeded onto 6-well plates at a
density of 4×105 cells/well. After 24 h, cells were
treated with 50, 100 and 200 µg/ml curcumol. Following 18, 24 or 36
h exposure to curcumol, the cells were collected and analyzed using
the Annexin V-FITC Apoptosis Detection kit (BD Biosciences)
according to the manufacturer's instructions. Briefly, the cells
were trypsinized, washed with PBS and resuspended in 100 µl buffer
solution (10 mM Hepes/NaOH at pH 7.4, 140 mM NaCl, 2.5 mM
CaCl2) at a final concentration of 1×106
cells/ml. The cells were incubated with 5 µl Annexin V-FITC for 15
min at room temperature without light exposure. Subsequent to the
addition of 5 µl PI, flow cytometry was immediately performed using
the FACSAria™ III system. A total of 10,000 ungated
events were acquired for each sample; the data were analyzed with
FACSDiva® (version 6.1.3; BD Biosciences).
Protein extraction and
immunoblotting
Cells were treated with 25 or 100 µg/ml curcumol or
vehicle for 24 h. Following incubation at 37°C for 24 h, floating
and adherent cells were collected and lysed for 30 min on ice in
radioimmunoprecipitation assay buffer (150 mM sodium chloride, 1.0%
NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-pH 8.0) added
with 1 mM phenylmethylsulfonyl fluoride, 10 µg/ml leupeptin and 2
µg/ml aprotinin (Sigma-Aldrich; Merck KGaA). Samples were
centrifuged at 10,000 × g at 4°C for 10 min and the supernatants
were collected for immunoblotting according to a previously
described method (18). In brief,
cell lysates (50 µg protein/lane) were resolved in 8–10% SDS/PAGE
gels and transferred to polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). Membranes were then incubated in a
blocking buffer (5% nonfat milk in 20 mM Tris-HCl, 150 mM NaCl,
0.1% Tween-20) for 1 h at room temperature and subsequently
incubated with the aforementioned primary antibodies in the
blocking buffer overnight at 4°C. Following washing with TBS buffer
containing 0.1% Tween-20, the membranes were incubated with
corresponding horseradish-peroxidase conjugated secondary
antibodies (as aforementioned) at room temperature for 1 h. The
blots were visualized by the use of the aforementioned enhanced
chemiluminescent detection kit (Pierce; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The immunoblot
bands were quantified by densitometry analysis using Scion Image
4.0 software (Scion Corporation, Frederick, MD, USA) and the signal
intensity of each protein was normalized to the corresponding
signal intensity of β-actin.
Statistical analysis
The results are presented as the mean ± standard
deviation. One-way analysis of variance was used to evaluate
overall difference between the groups. The Student's t-test was
performed for pairwise comparison of normal distributed parameters.
P<0.05 was considered to indicate a statistically significant
difference.
Results
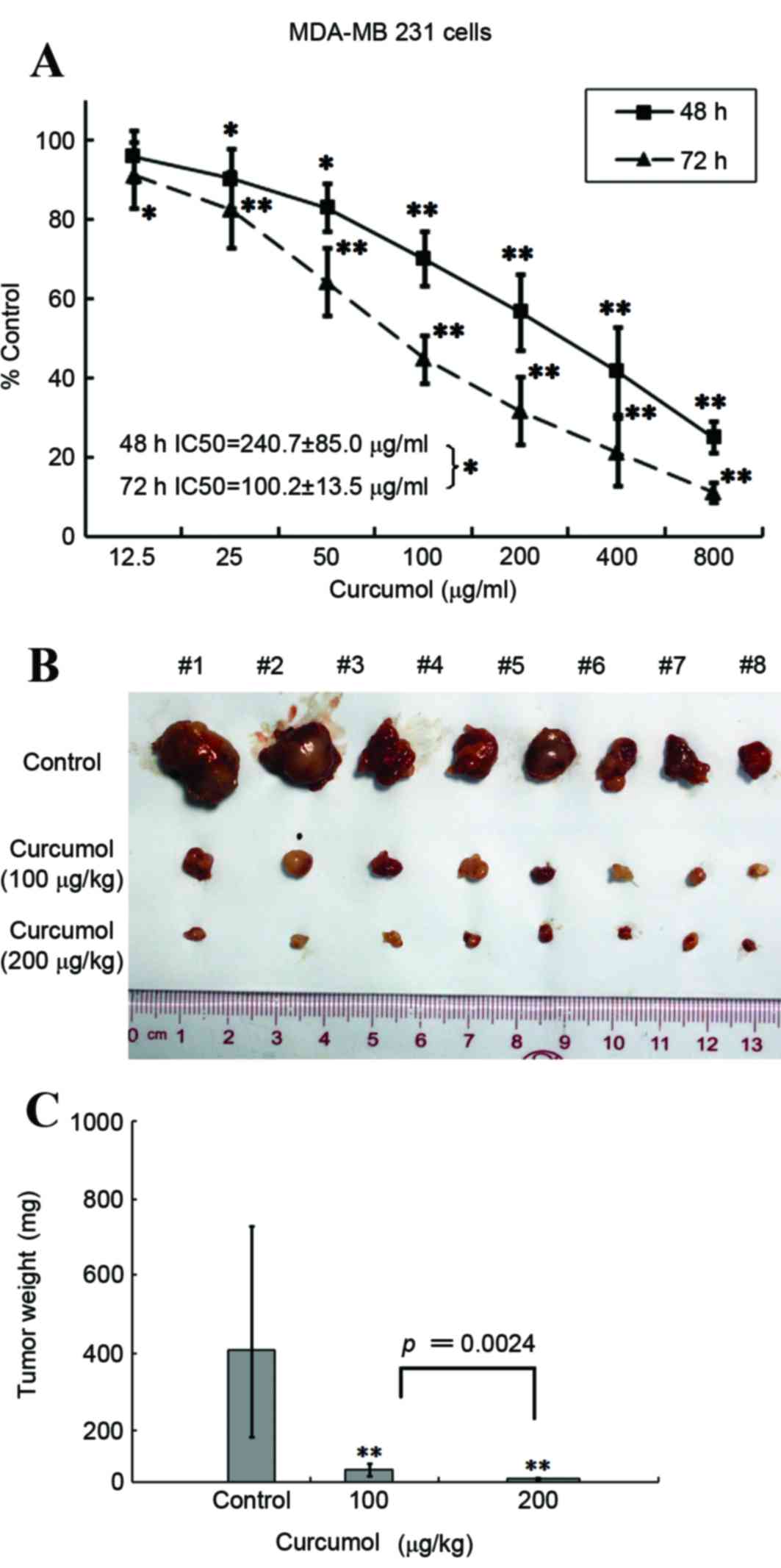
Curcumol suppresses the growth of TNBC
MDA-MB 231 cells in vitro and in vivo
To the best of our knowledge, this is the first time
that the effect of curcumol on TNBC MDA-MB-231 cell proliferation
has been determined. The cells were treated with 12.5–800 µg/ml
curcumol for 48 and 72 h, and cell viability was examined using an
MTT assay. As presented in Fig. 1A, a
significant dose- and time-dependent inhibition of TNBC MDA-MB-231
cell proliferation was observed when curcumol was administered at
concentrations >25 µg/ml (P<0.05; P<0.01). The
IC50 value was 240.7±85.0 µg/ml for 48 h and 100.2±13.5
µg/ml for 72 h.
Although recent studies have suggested that curcumol
may have anticancer properties, limited in vivo data has
previously been presented. Therefore, the present study aimed to
explore the effect of curcumol on TNBC MDA-MB-231 xenografts. Two
groups of female BALB/C nude mice bearing TNBC MDA-MB 231
xenografts were intraperitoneally injected with curcumol (100 or
200 µg/kg) every other day for ≤21 days. Compared with the vehicle
treatment group, the tumor volume was significantly reduced by
~90.4% in the 100 µg/kg (P=0.0024) of curcumol treatment group and
by ~97.5% in the 200 µg/kg group (P=0.0009; Fig. 1B and C). Combined, these results
suggest that curcumol is capable of blocking the growth of breast
cancer TNBC MDA-MB-231 cells in vitro and in
vivo.
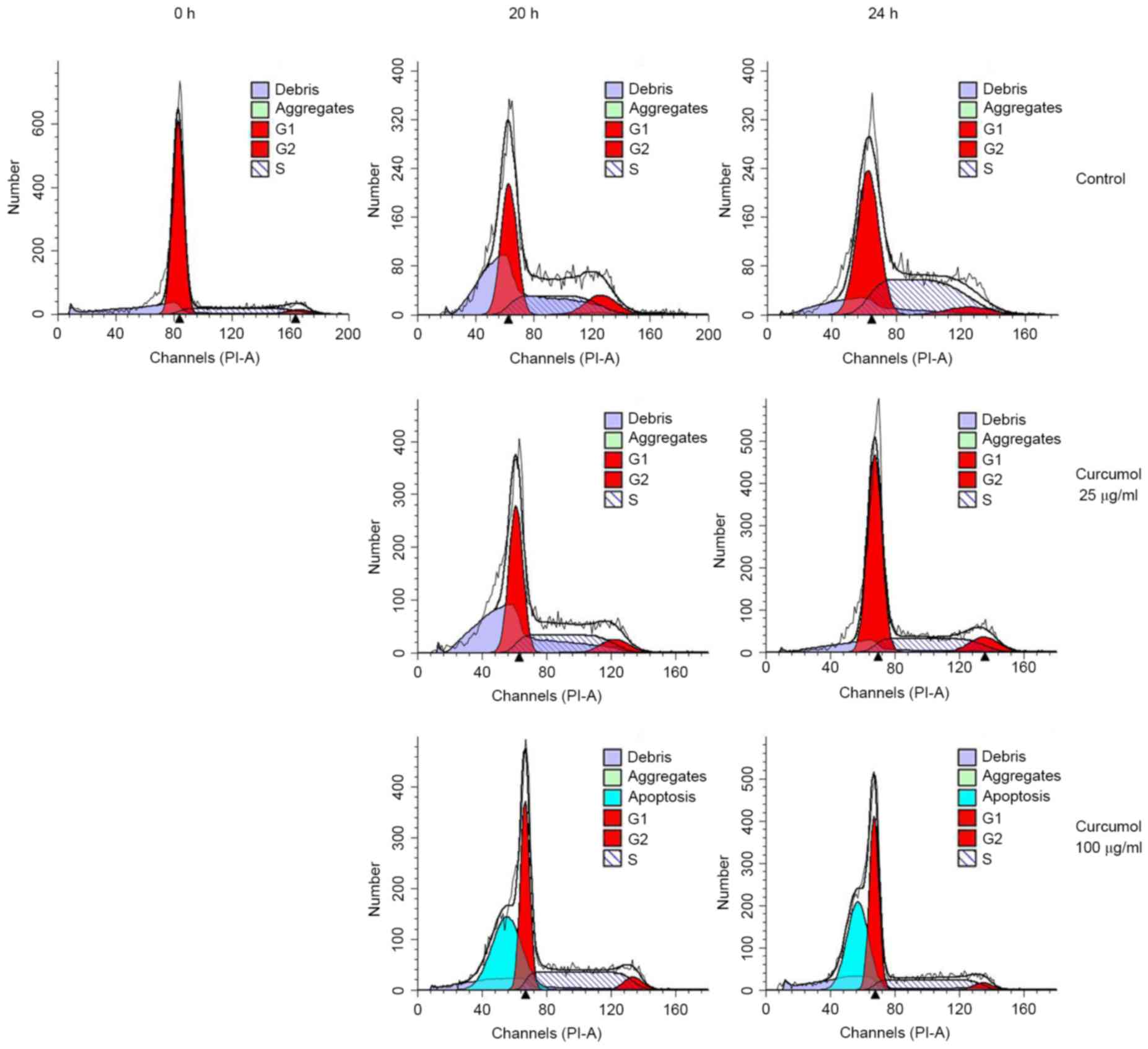
Curcumol induces G1 phase
arrest and sub-G1 phase cell accumulation
As the inhibition of cell proliferation may be due
to the arrest of cell growth, the present study investigated the
regulatory effects of curcumol on the cell-cycle distribution in
TNBC MDA-MB-231 cells. TNBC MDA-MB-231 cells were synchronized by
serum starvation as aforementioned. The synchronized cells were
stimulated with 25 or 100 µg/ml curcumol for 20 and 24 h at 37°C,
respectively. The cells were then collected, stained with PI and
subjected to flow cytometry analysis. The 0 h indicates the release
point of synchronized cells. The results demonstrated a significant
increase in the number of G1 phase cells, from
28.93–55.15% in the 100 µg/ml treatment group (P=0.0240), whereas
there was only a slight elevation in the group of cells treated
with 25 µg/ml curcumol (Fig. 2;
Table I). Notably, when the cells
were treated with a higher concentration of curcumol (100 µg/ml for
24 h), 41.44% of cells were observed to be in the hypodiploid
sub-G1 phase, which suggested that the inhibitory
effects of curcumol observed in TNBC MDA-MB-231 cells were
associated with the induction of apoptosis.
 | Table I.Induction of G1 arrest and
apoptosis in MDA-MB 231 cells by curcumol. |
Table I.
Induction of G1 arrest and
apoptosis in MDA-MB 231 cells by curcumol.
| Treatment | G1 | S |
G2/M | Apoptosis |
|---|
| 0 h | 86.95±3.39 | 8.51±2.08 | 4.53±1.78 | 0.00±0.00 |
| 20 h |
|
|
|
|
|
Control | 51.10±4.32 | 37.12±2.34 | 11.78±5.87 | 0.00±0.00 |
| 25.0
µg/ml | 56.18±6.52 | 32.48±4.94 | 11.41±1.63 | 0.00±0.00 |
| 100.0
µg/ml | 69.21±17.27 | 22.20±10.14 | 5.26±4.93 |
32.96±10.91b |
| 24 h |
|
|
|
|
|
Control | 28.93±12.34 | 50.01±9.54 | 1.01±11.25 | 0.00±0.00 |
| 25.0
µg/ml | 33.28±21.35 | 48.09±13.90 | 18.64±11.44 | 0.00±0.00 |
| 100.0
µg/ml |
55.15±10.32a | 38.36±5.61 | 6.49±5.5 |
41.44±15.37b |
Curcumol induces cell apoptosis
To confirm the aformentioned effect of curcumol on
the TNBC MDA-MB-231 cells, the cellular apoptotic response was
further investigated via two methodologies. Firstly, the apoptotic
morphological features were investigated using DAPI staining.
Following exposure to 50, 100 or 200 µg/ml curcumol for 48 h, TNBC
MDA-MB-231 cells exhibited the following apoptosis-associated
morphological changes: Nucleus shrinking, chromatin condensation
and the formation of dot-shaped nuclear fragments (Fig. 3A and B). By contrast, the control
group was observed to have fewer apoptotic cells.
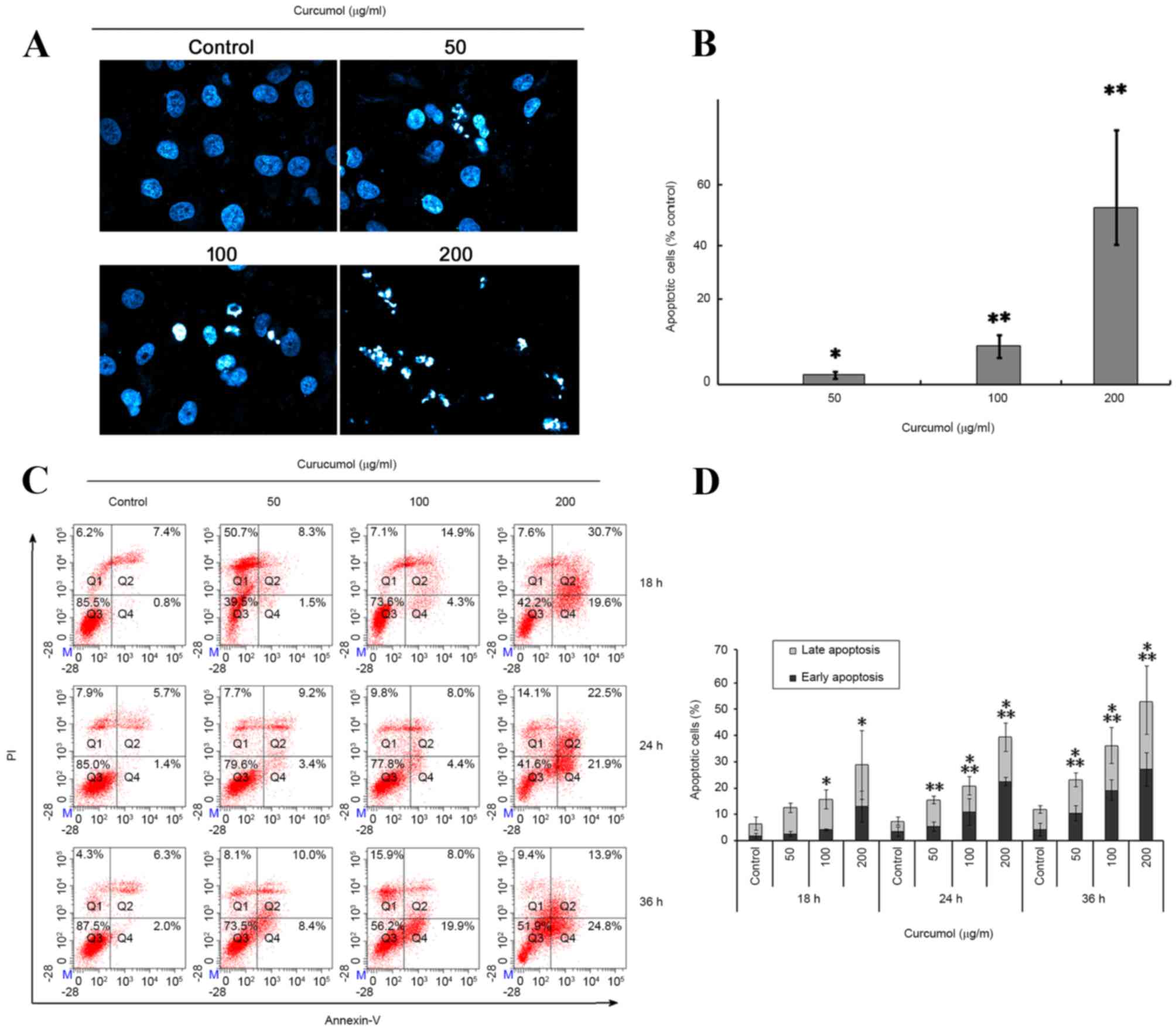 | Figure 3.Induction of apoptosis by curcumol in
TNBC MDA-MB-231 cells. (A) The percentage of apoptotic cells were
increased in MDA-MB-231 cells following exposure to curcumol (0,
50, 100 and 200 µg/ml) for 48 h by DAPI staining. The stained
nuclei were observed under a laser confocal fluorescence microscope
(original magnification, ×400). (B) Summary of apoptotic MDA-MB-231
cells staining with DAPI following treatment with curcumol. Data
was obtained by counting the apoptotic cells in ≥10 fields (×400).
Each bar represents the mean and the error bars represent the
standard deviation of three independent experiments (*P<0.05,
**P<0.01 vs. vehicle control). (C) Representative flow cytometry
data demonstrated increased apoptosis rates in cells treated with
the indicated concentrations of curcumol for 18, 24 and 48 h. Q1,
PI positive (necrosis); Q2, Annexin V and PI positive (late
apoptosis); Q3, negative immunofluorescence (living cells); Q4,
Annexin V positive (early apoptosis). (D) Summarized data of cell
apoptosis. Each bar represents the mean and the error bars
represent the standard deviation of three independent experiments.
*Early apoptosis, P<0.05 (vs. control); **Late apoptosis,
P<0.05 (vs. control). DAPI, 4,6-diamidino-2-phenylindole; FITC,
fluorescein isothiocyanate; PI, propidium iodide; TNBC,
triple-negative breast cancer. |
The present study also confirmed and quantified the
apoptotic cells using a flow cytometric Annexin V/PI double
staining assay. In the quadrant dot plot of double variable flow
cytometry, Q1 quadrant (Annexin V-/PI+) showed necrotic cells; Q2
quadrant (Annexin V+/PI+) stood for late apoptotic cells; Q3
quadrant (Annexin V-/PI-) showed living cells; and Q4 quadrant
(Annexin V+/PI-) represented early apoptotic cells. As presented in
Fig. 3C and D, treatment of TNBC
MDA-MB-231 cells with 50, 100 or 200 µg/ml curcumol increased the
early apoptotic cell population (Q4) from 1.8±0.95% (vehicle
control) to 2.53±1.05% (50 µg/ml), 4.1±0.26% (100 µg/ml) and
12.93±5.9% (200 µg/ml), at 18 h; 4.1±2.6% (vehicle control) to
5.33±1.77% (50 µg/ml), 10±5.04% (100 µg/ml) and 22.5±1.58% (200
µg/ml) at 24 h; 4.2±2.31% (vehicle control) to 9.7±3.43% (50
µg/ml), 19.1±3.96% (100 µg/ml) and 27.13±6.42% (200 µg/ml) at 36 h.
It also increased the late apoptotic cell population (Q2) from
5.96±3.2% (vehicle control) to 10.03±1.8% (50 µg/ml), 11.6±3.63%
(100 µg/ml) and 15.9±13.09% (200 µg/ml) at 18 h; 3.93±1.55%
(vehicle control) to 10.03±1.44% (50 µg/ml), 9.8±3.46% (100 µg/ml)
and 16.83±5.43% (200 µg/ml) at 24 h; 7.66±1.35% (vehicle control)
to 12.68±2.643% (50 µg/ml), 18.6±15.73% (100 µg/ml) and 24.4±12.34%
(200 µg/ml) at 36 h. These results indicated that curcumol induces
a marked level of apoptosis of TNBC MDA-MB-231 cells in a dose- and
time-dependent manner.
Curcumol upregulates the expression of
p73, PUMA and Bak
Previously published data have demonstrated that p73
may serve a critical role in the induction of cell death by
apoptosis in various p53-deficient cancer cell lines, including
TNBC 231 cells (7,19–21). In
order to understand the events involved in curcumol-mediated
apoptosis of TNBC MDA-MB 231-cells, the present study investigated
the expression patterns of p73 as well as of the p53-mediated
pro-apoptotic mediators PUMA and Bcl-2 associated X protein (Bax)
gene family, using immunoblotting. MCF7 cells, which express
wild-type p53 and exhibit overexpression of p53 and p21 following
exposure to adriamycin (1 µM, 24 h) (22), were used as a positive control for the
immunoblotting. As presented in Fig.
4, treatment with 25 µg/ml curcumol significantly increased p73
protein expression level (P=0.0016), and 100 µg/ml curcumol
increased the levels of p73, Bak and PUMA proteins (P=0.0016,
P=0.0263 and P=0.0093, respectively). No expression of p21 was
detected, which confirmed the dysfunction of p53 in the TNBC
MDA-MB-231 cells. The present study was unable to detect the
cleaved band of PARP-1 protein in the cells treated with curcumol
at the aforementioned dosages, which suggests a caspase-independent
apoptosis pathway. Notably, there was a 35.2% decrease of p53
expression detected in the cells treated with 100 µg/ml curcumol
(P=0.0268; Fig. 4). These data
suggest that curcumol-triggered apoptosis may occur via
upregulation of the apoptotic proteins p73, PUMA and Bak and the
downregulation of mutant p53 in TNBC MDA-MB 231 cells.
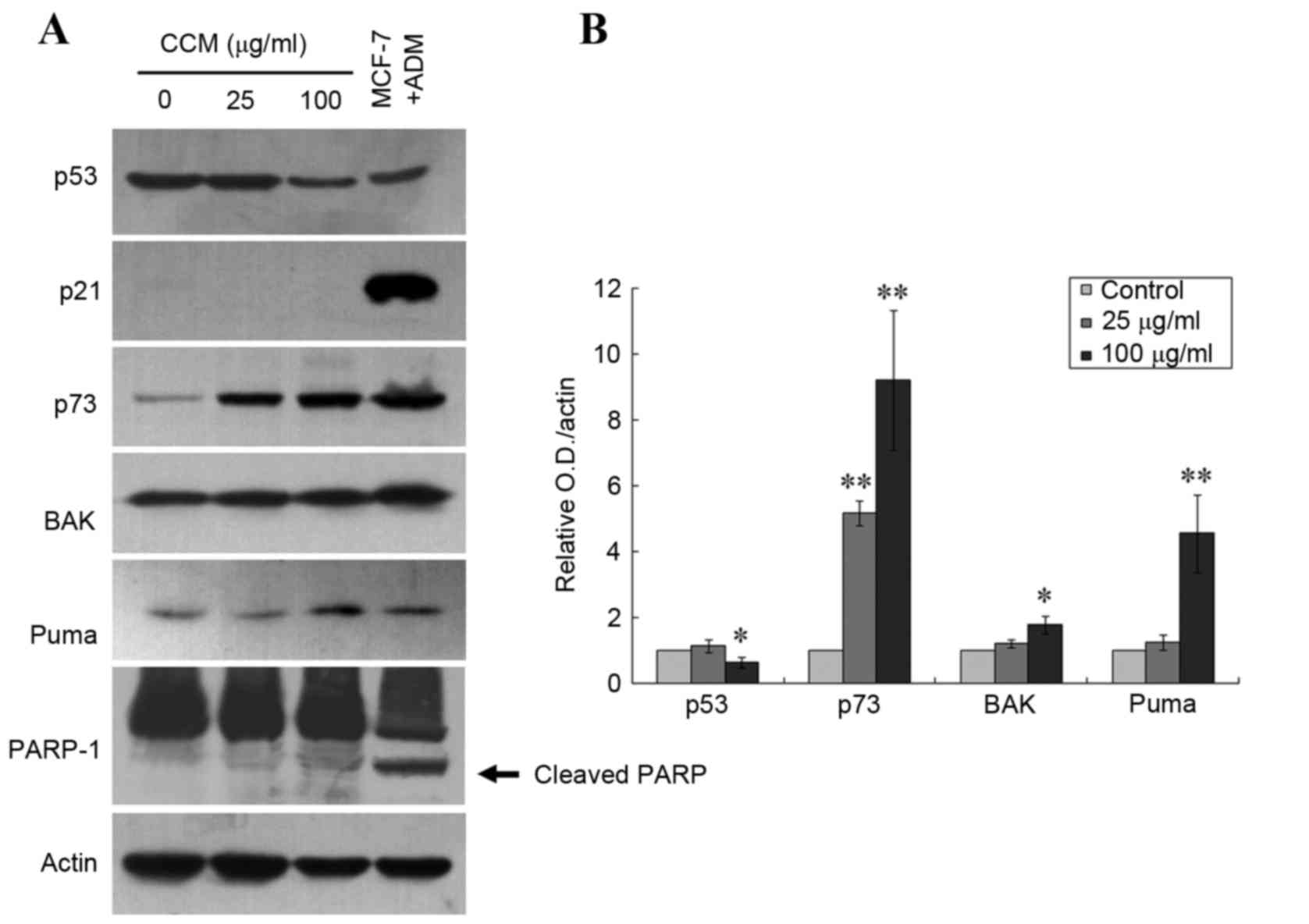 | Figure 4.Upregulation of p73, PUMA and Bak
expression by curcumol in TNBC MDA-MB-231 cells. (A) Immunoblotting
demonstrated increased protein levels of p73, PUMA, Bak and cleaved
PARP in MDA-MB-231 cells following treatment with 25 or 100 µg/ml
curcumol for 24 h. Actin was the loading control, and ADM-treated
MCF-7 cells served as the antibody controls. Cleavage of PARP is
indicated by the arrow. (B) Quantitative data for the relative
density of the proteins of interest. Each bar represents the mean ±
standard deviation of three independent experiments (*P<0.05,
**P<0.01 vs. the untreated control). PUMA, p53 upregulated
modulator of apoptosis; Bak, Bcl-2 antagonistic killer; PARP, poly
(ADP-ribose) polymerase; ADM, Adriamycin; MCF, macrophage
chemotactic factor; TNBC, triple-negative breast cancer. |
Discussion
Despite numerous advances in available cancer
therapies, TNBC, a subgroup of breast cancer that is often
accompanied with higher frequency of p53 gene mutations, remains a
challenge in clinical practice due to the lack of biological
targets and its resistance to chemo-radiotherapy (1–3). Natural
products, particularly those obtained from medicinal herbs,
including biochanin A, guercetin and curcumin, with apoptotic
activity have attracted great attention as potential sources for
the development of novel anticancer drugs (23–25). C.
wenyujin, a Chinese traditional herbal medicine, has
conventionally been used for the treatment of neoplasm and
inflammatory diseases (9). An
essential oil from C. wenyujin is listed as an anticancer
and antiviral drug in the Pharmacopoeia of the P.R. China (11). Curcumol is one of the major
pharmacological components of the aforementioned essential oil, and
has recently been reported to exhibit an anti-proliferative effect
on numerous human tumor cell lines, including lung, breast, ovary,
liver, gastric and intestinal cancer (14–17). To
the best of our knowledge, the present study is the first to
provide evidence of the anticancer effect of curcumol in a p53
mutant TNBC MDA-MB-231 cell line, via demonstrating a direct
inhibition of cellular viability and xenograft growth, and the
induction of apoptosis.
Apoptosis serves an essential role in cell
replacement, tissue remodeling and the removal of damaged cells
under normal conditions. The induction of apoptosis in malignant
cells is becoming a practical strategy for cancer treatment
(26,27). Thus, signaling pathways involved in
enhancing apoptosis are potential targets for identifying
innovative drug candidates. There are a number of previous reports
demonstrating that the oily extracts of C. wenyujin induce
cellular apoptosis and inhibit proliferation in certain cancer cell
lines (10,28–30). The
present study focused on curcumol, one of the active compounds
isolated from the curcuma oil (12).
Data obtained from flow cytometry and cellular morphology studies
demonstrate that curcumol is capable of inhibiting the growth of
TNBC MDA-MB 231 cells in a predominantly apoptotic manner. These
results are consistent with those observed in various other tumor
cell lines (14–17). Furthermore, the present study
supported the evidence indicating a marked effect of curcumol in a
BALB/c nude mice xenograft model. Therefore, it is postulated that
curcumol is capable of inducing apoptosis, which in turn causes
tumor cell death.
There are multiple forms of apoptosis; two
well-characterized apoptosis signaling pathways are the death
receptor pathway and the mitochondrial pathway (31,33). In
the death receptor pathway, the binding of death factors, including
the Fas ligand, tumor necrosis factor (TNF) or TNF-related
apoptosis-inducing ligand, to their corresponding receptors
triggers a signaling cascade that results in the activation of
initiatorcaspase-8, which in turn activates the downstream effector
caspase-3 leading to PARP cleavage (32). The mitochondrial pathway is initiated
by BH3-only proteins that induce the activation and oligomerization
of the Bcl-2 family members Bax and Bak (34). The oligomerized Bax/Bak then triggers
the release of cytochrome c from mitochondria into the
cytosol. Cytochrome c subsequently forms a complex with Apaf-1 and
activates caspase-9 and caspase-3 (33,35). As a
central integrator of apoptosis signaling pathways, mitochondria
also release other pro-apoptotic factors, including apoptosis
inducing factor (AIF), triggering a caspase-independent cell death
in a Bcl-2-dependent manner (36).
The tumor suppressor protein p53 mediates caspase-dependent and
caspase-independent cell death through numerous signaling pathways,
including the activation of Bcl-2 family member Bax, PUMA or AIF
(37,38). The loss of p53 function typically
results in the inhibition of apoptotic signaling pathways (39). p21 (CIP1/WAF1) is a regulator of cell
cycle progression that is known to be directly activated by
wild-type, but not mutant, p53. The lack of p21 expression may
serve as an indicator for p53 dysfunction (40,41).
As hypothesized, the expression of p21 in MDA-MB-231
cells following treatment of curcumol was not detected in the
present study, but a significant increase in p73 protein as well as
an upregulation of PUMA and Bax expression was observed. p73 is a
homolog of p53 and has a vital role in mediating apoptosis in a
variety of p53 deficient cancer cells (42–44). In
p73-induced apoptosis, p73 triggers the mitochondrial pathway by
directly transactivating the Bax and PUMA promoters (42). PUMA belongs to the BH3-only subgroup
of the Bcl-2 family. The expression of PUMA promotes the
mitochondrial translocation of Bax and formation of the Bax/Bak
complex, culminating in the induction of apoptosis (45). Notably, the cleaved band of the PARP-1
protein was not detected in the current study, suggesting that
curcumol-induced apoptosis in TNBC MDA-MB-231 cells may be a
caspase-independent method of cell death. Furthermore, Zhang et
al (17) recently reported that
curcumol induces caspase-independent apoptosis through the
mitochondrial pathway in human ASTC-a-1 lung adenocarcinoma
cells.
Notably, the present study observed a significant
decrease in the expression levels of mutant p53 following curcumol
treatment in TNBC MDA-MB-231 cells. Mutant p53 may not only lose
tumor suppressive activity, but may also gain functions that
promote malignant progression (46).
The effect of the downregulation of mutant p53 protein expression
may be associated with its anticancer properties.
The results of the present study have demonstrated
the effects of curcumol, including directly suppressing the growth
of human p53-mutant TNBC MDA-MB-231 cells via triggering apoptosis.
The underlying mechanisms may include an enhancement of p73, which
in turn transactivates the expression of PUMA and Bax, and inhibits
mutant p53 expression. These results indicate that curcumol may be
a promising anticancer compound for further investigation into
novel cancer therapeutics, particularly for TNBC.
Acknowledgements
The present study was supported by the Guangxi
Medical Science Research Center Fund (grant no. KFJJ2011-13) and
the Guangxi Liver Damage and Repair Key Laboratory Fund (grant no.
SYS201307).
Glossary
Abbreviations
Abbreviations:
|
IC50
|
half maximal inhibitory
concentration
|
|
PI
|
propidium iodide
|
|
PUMA
|
p53 upregulated modulator of
apoptosis
|
|
TNBC
|
triple-negative breast cancer
|
References
|
1
|
Turner NC and Reis-Filho JS: Tackling the
diversity of triple-negative breast Cancer. Clin Cancer Res.
19:6380–6388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oakman C, Viale G and Di Leo A: Management
of triple negative breast cancer. Breast. 19:312–321. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duffy MJ, Synnott NC, McGowan PM, Crown J,
O'Connor D and Gallagher WM: p53 as a target for the treatment of
cancer. Cancer Treat Rev. 40:1153–1160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Z and Sun Y: Targeting p53 for novel
anticancer therapy. Transl Oncol. 3:1–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lai D, Visser-Grieve S and Yang X: Tumour
suppressor genes in chemotherapeutic drug response. Biosci Rep.
32:361–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jost CA, Marin MC and Kaelin WG Jr: p73 is
a simian [correction of human] p53-related protein that can induce
apoptosis. Nature. 389:191–194. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tiwary R, Yu W, Sanders BG and Kline K:
α-TEA cooperates with chemotherapeutic agents to induce apoptosis
of p53 mutant, triple-negative human breast cancer cells via
activating p73. Breast Cancer Res. 13:R12011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoon MK, Ha JH, Lee MS and Chi SW:
Structure and apoptotic function of p73. BMB Rep. 48:81–90. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Membership of the 5th Pharmacopoeia
Commission, . Pharmacopoeia of the People's Republic of China
(English edition). Pharmacopeia Commission of PRC, . Chemical
Industry Press; Beijing: pp. 2302000
|
|
10
|
Liju VB, Jeena K and Kuttan R: An
evaluation of antioxidant, anti-inflammatory, and antinociceptive
activities of essential oil from Curcuma longa. L. Indian J
Pharmacol. 43:526–531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
State Pharmacopoeia Commission of P. R.
China, . Pharmacopoeia Commission of People's Republic of China.
China Medicinal Science and Technology Press; Beijing: pp. 2792005,
(In Chinese).
|
|
12
|
Dang YY, Li XC, Zhang QW, Li SP and Wang
YT: Preparative isolation and purification of six volatile
compounds from essential oil of Curcuma wenyujin using
high-performance centrifugal partition chromatography. J Sep Sci.
33:1658–1664. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hikino H, Meguro K, Sakurai Y and Takemoto
T: Structure of curcumol. Chem Pharm Bull (Tokyo). 14:1241–1249.
1966. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu JJ, Dang YY, Huang M, Xu WS, Chen XP
and Wang YT: Anti-cancer properties of terpenoids isolated from
Rhizoma Curcumae-a review. J Ethnopharmacol. 143:406–411. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo P, Wang YW, Weng BX, Li XK, Yang SL
and Ye FQ: Synthesis, anti-tumor activity, and structure-activity
relationships of curcumol derivatives. J Asian Nat Prod Res.
16:53–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang QL, Guo JQ, Wang QY, Lin HS, Yang ZP,
Peng T, Pan XD, Liu B, Wang SJ and Zang LQ: Curcumol induces
apoptosis in SPC-A-1 human lung adenocarcinoma cells and displays
anti-neoplastic effects in tumor bearing mice. Asian Pac J Cancer
Prev. 16:2307–2312. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang W, Wang Z and Chen T: Curcumol
induces apoptosis via caspases-independent mitochondrial pathway in
human lung adenocarcinoma ASTC-a-1 cells. Med Oncol. 28:307–314.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang X, Benovic JL and Wedegaertner PB:
Plasma membrane and nuclear localization of G protein coupled
receptor kinase 6A. Mol Biol Cell. 18:2960–2969. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan R, Meng Q, Hu H, Goldberg ID, Rosen
EM and Fan S: P53-independentdownregulation of p73 in human cancer
cells treated with Adriamycin. Cancer Chemother Pharmacol.
47:161–169. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rana S, Gupta K, Gomez J, Matsuyama S,
Chakrabarti A, Agarwal ML, Agarwal A, Agarwal MK and Wald DN:
Securinine induces p73-dependent apoptosis preferentially in
p53-deficient colon cancer cells. FASEB J. 24:2126–2134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong B, Prabhu VV, Zhang S, van den Heuvel
AP, Dicker DT, Kopelovich L and El-Deiry WS: Prodigiosin rescues
deficient p53 signaling and antitumor effects via upregulating p73
and disrupting its interaction with mutant p53. Cancer Res.
74:1153–1165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elmore LW, Rehder CW, Di X, McChesney PA,
Jackson-Cook CK, Gewirtz DA and Holt SE: Adriamycin-induced
senescence in breast tumor cells involves functional p53 and
telomere dysfunction. J Biol Chem. 277:35509–35515. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khan SI, Zhao J, Khan IA, Walker LA and
Dasmahapatra AK: Potential utility of natural products as
regulators of breast cancer-associated aromatase promoters. Reprod
Biol Endocrinol. 9:912011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Greenlee H: Natural products for cancer
prevention. Semin Oncol Nurs. 28:29–44. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
You L, An R, Liang K and Wang X:
Anti-breast cancer agents from Chinese herbal medicines. Mini Rev
Med Chem. 13:101–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meyn RE, Milas L and Stephens LC:
Apoptosis in tumor biology and therapy. Adv Exp Med Biol.
400B:657–667. 1997.PubMed/NCBI
|
|
27
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao Y, Yang FQ, Li SP, Hu G, Lee SM and
Wang YT: Essential oil of Curcuma wenyujin induces apoptosis in
human hepatoma cells. World J Gastroenterol. 14:4309–4318. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lim CB, Ky N, Ng HM, Hamza MS and Zhao Y:
Curcuma wenyujin extract induces apoptosis and inhibits
proliferation of human cervical cancer cells in vitro and in vivo.
Integr Cancer Ther. 9:36–49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Shi X, Zhang J, Zhang X and Martin
RC: Hepatic protection and anticancer activity of curcuma: A
potential chemopreventive strategy against hepatocellular
carcinoma. Int J Oncol. 44:505–513. 2014.PubMed/NCBI
|
|
31
|
Chowdhury I, Tharakan B and Bhat GK:
Current concepts in apoptosis: The physiological suicide program
revisited. Cell Mol Biol Lett. 11:506–525. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Strasser A, Jost PJ and Nagata S: The many
roles of FAS receptor signaling in the immune system. Immunity.
30:180–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Riedl SJ and Salvesen GS: The apoptosome:
Signalling platform of cell death. Nat Rev Mol Cell Biol.
8:405–413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Elkholi R, Floros KV and Chipuk JE: The
role of BH3-only proteins in tumor cell development, signaling, and
treatment. Genes Cancer. 2:523–537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ow YP, Green DR, Hao Z and Mak TW:
Cytochrome c: Functions beyond respiration. Nat Rev Mol Cell Biol.
9:532–542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pradelli LA, Bénéteau M and Ricci JE:
Mitochondrial control of caspase-dependent and -independent cell
death. Cell Mol Life Sci. 67:1589–1597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Estaquier J, Vallette F, Vayssiere JL and
Mignotte B: The mitochondrial pathways of apoptosis. Adv Exp Med
Biol. 942:157–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ren SX, Shen J, Cheng AS, Lu L, Chan RL,
Li ZJ, Wang XJ, Wong CC, Zhang L, Ng SS, et al: Correction: FK-16
derived from the anticancer peptide LL-37 induces
caspase-independent apoptosis and autophagic cell death in colon
cancer cells. PLoS One. 10:e01317502015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chipuk JE and Green DR: Dissecting
p53-dependent apoptosis. Cell Death Differ. 13:994–1002. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
el-Deiry WS, Tokino T, Velculescu VE, Levy
DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW and
Vogelstein B: WAF1, a potential mediator of p53 tumor suppression.
Cell. 75:817–825. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Z, Huang C, Li J and Shi X:
Vanadate-induced cell growth arrest is p53-dependent through
activation of p21 in C141 cells. J Inorg Biochem. 89:142–148. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Melino G, Bernassola F, Ranalli M, Yee K,
Zong WX, Corazzari M, Knight RA, Green DR, Thompson C and Vousden
KH: p73 Induces apoptosis via PUMA transactivation and Bax
mitochondrial translocation. J Biol Chem. 279:8076–8083. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ramadan S, Terrinoni A, Catani MV, Sayan
AE, Knight RA, Mueller M, Krammer PH, Melino G and Candi E: p73
inducesapoptosis by different mechanisms. Biochem Biophys Res
Commun. 331:713–717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ming L, Sakaida T, Yue W, Jha A, Zhang L
and Yu J: Sp1 and p73 activate PUMA following serum starvation.
Carcinogenesis. 29:1878–1884. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu J, Wang Z, Kinzler KW, Vogelstein B and
Zhang L: PUMA mediates the apoptotic response to p53 in colorectal
cancer cells. Proc Natl Acad Sci USA. 100:pp. 1931–1936. 2003;
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
van Oijen MG and Slootweg PJ:
Gain-of-function mutations in the tumor suppressor gene p53. Clin
Cancer Res. 6:2138–2145. 2000.PubMed/NCBI
|