Introduction
Breast cancer is the leading cause of
cancer-associated mortality for the female population worldwide;
there were an estimated 234,190 novel cases and 40,730 mortalities
in 2015 in the USA (1,2). Treatment of breast cancer typically
includes surgery followed by adjuvant chemotherapy, radiotherapy or
hormone therapy (3). There has been a
significant effort to improve the prognosis of patients with breast
cancer; however, breast cancer remains a highly prevalent and
lethal malignancy (4,5). Local recurrence and distant metastasis
are the main reasons for the poor prognosis associated with breast
cancer (6). It was previously
reported that between 30 and 75% of patients with breast cancer
developed disease recurrence or metastasis following surgery and
adjuvant treatment (7). The local
recurrence or metastasis of breast cancer is the leading cause for
the deterioration of breast cancer, and is responsible for >90%
of breast cancer-associated mortalities (8). Therefore, improving the understanding of
the molecular mechanisms of breast cancer recurrence and metastasis
is particularly important.
MicroRNAs (miRNAs), small non-coding RNAs of 19–24
nucleotides, may be critical regulators of cancer pathogenesis and
progression (9). miRNAs negatively
regulate the expression of specific mRNA targets by directly
binding miRNA recognition elements, typically in the 3′
untranslated region (3′UTR) of target mRNAs, resulting in the
inhibition of translation and/or degradation of the target mRNA,
thus downregulating the expression of the associated protein
products (10,11). Each type of miRNA may modulate the
expression of numerous mRNAs simultaneously; therefore, miRNAs
serve critical roles in biological functions, including cell
proliferation, cell cycle progression, migration, invasion,
metastasis, apoptosis, metabolism and the immune response (12–18).
Accumulating studies have indicated that there are associations
between miRNA expression and the clinical features of patients with
cancer, including tumor size, tumor-node-metastasis (TNM) stage,
development, survival time, disease recurrence and metastasis
(19–22). miRNAs have been reported to be
frequently dysregulated in various types of human cancer, and may
function as tumor suppressors or oncogenes in tumorigenesis and
tumor development (23). miRNAs are
therefore regarded as attractive targets for the development of
improved cancer therapies.
In the present study, it was demonstrated that the
expression levels of miR-215 were lower in breast cancer tissues
and cell lines. In addition, enforced miR-215 expression inhibited
the proliferation and invasion of breast cancer cells. AKT
threonine serine/kinase 1 (AKT1) was identified as a novel direct
target of miR-215 in breast cancer. On the basis of these results,
miR-215 may be suitable for development as a therapeutic target in
the treatment of breast cancer.
Materials and methods
Clinical samples and cell lines
Breast cancer and matched adjacent non-tumor breast
tissues were obtained from 56 patients who underwent surgery at the
First Affiliated Hospital of Harbin Medical University (Harbin,
China) between September 2013 and February 2014. None of the
patients had received chemotherapy, radiotherapy or adjuvant
hormonal therapy prior to surgery. All samples were snap frozen in
liquid nitrogen and stored at −80°C. The present study was approved
by the Ethics Committee of the First Affiliated Hospital of Harbin
Medical University. Written informed consent for research purposes
was obtained from each patient.
Human breast cancer cells (MCF-7A, MDA-MB-231,
MDA-MB-453, BT-474 and SK-BR-3) and HEK293T cells were obtained
from the American Type Culture Collection (Manassas, VA, USA).
MCF-10A, normal mammary epithelial cells, were purchased from the
Cell Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). Cells were maintained in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS), 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a
humidified atmosphere containing 5% CO2.
miRNA and small interfering RNA
(siRNA) transfection
An miR-215 mimic and an miRNA negative control (NC)
were synthesized by Shanghai GenePharma Co., Ltd. (Shanghai,
China). AKT1 siRNA and scrambled siRNA were purchased from
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The breast cancer
cells were seeded into 6-well plates and grown to 60% confluence.
They were transfected with miRNA or siRNA using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cells with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. For detecting miR-215
expression, total RNA was reverse-transcribed into complementary
DNA with a PrimeScript reverse transcription reagent kit (Takara
Bio, Inc., Otsu, Japan). qPCR was performed with a SYBR Premix Ex
Taq™ II kit (Takara Bio, Inc.) on an Applied Biosystems
7500 Sequence Detection system (Thermo Fisher Scientific,
Inc.).
The reaction system contained 10 µl SYBR Premix Ex
Taq II, 2 µl cDNA (200 ng), 0.8 µl forward primer, 0.8 µl reverse
primer, 0.4 µl ROX Reference Dye and 6 µl ddH2O. The amplification
was performed with cycling conditions as follows: 5 min at 95°C,
followed by 40 cycles of 95°C for 30 sec and 65°C for 45 sec.
Relative expression levels of miR-215 were evaluated using the
2−ΔΔCq method (24). The
primer sequences used for qPCR were: miR-215 forward,
5′-ACACTCCAGCTGGGATGACCTATGAATTG-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′; U6 snRNA forward,
5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′. All experiments were run in
triplicate.
MTT assay
An MTT assay was performed to detect the rate of
cell proliferation. Breast cancer cells were seeded in a 96-well
plate, at a density of 3×103 cells per well, following
transfection with miR-215 mimic or siRNA. The extent of
proliferation was then assessed at 24, 48, 72 and 96 h
post-transfection. A 20 µl volume of MTT solution (5 mg/ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to each
well prior to incubation at 37°C for 4 h. The supernatant was
removed and cells were resuspended in 150 µl dimethylsulfoxide. The
absorbance of the cells at 490 nm was detected using a microplate
reader. All experiments were performed in triplicate.
Cell invasion assay
A Transwell chamber with 8 µm pores (BD Biosciences,
San Jose, CA, USA) was used to evaluate cell invasive capacity. The
filter of the top chamber was pre-coated with Matrigel (BD
Biosciences). Briefly, 4×104 miR-215 mimic- or
siRNA-transfected cells were suspended in 200 µl FBS-free DMEM, and
added to the upper chamber. The lower chambers were filled with 500
µl DMEM supplemented with 20% FBS as a chemoattractant. At 48 h
after incubation, the noninvading cells that remained on the upper
surface of the Transwell chamber were removed with a cotton swab.
The invading cells on the lower surface were fixed with 100%
methanol and stained with 0.1% crystal violet. Cells that had
invaded to the lower surface were counted using a light microscope
in five randomly selected fields.
Bioinformatics analysis
TargetScan online software (www.targetscan.org/) and miRanda (www.microrna.org) were used for miR-215 target gene
prediction. From the potential targets, AKT1 was selected as it has
previously been demonstrated to contribute to breast cancer
progression (25).
Western blot analysis
At 72 h after transduction with the miR-215 mimic or
siRNA, total protein was extracted from cells using a Total Protein
Extraction kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China)
and its concentration was determined using a Pierce bicinchoninic
acid protein quantitation kit (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Equal amounts of protein
(30 µg) were separated using SDS-PAGE (10% gel) and gels were
electroblotted onto polyvinylidene fluoride membranes (Merck KGaA).
The membranes were blocked with 5% skimmed milk in TBS containing
0.1% Tween-20, and then incubated with the following primary
antibodies: Mouse anti-human AKT1 monoclonal antibody (ab54752,
1:1,000; Abcam, Cambridge, UK) and mouse anti-human β-actin
monoclonal antibody (ab8226, 1:1,000; Abcam). Following overnight
incubation at 4°C, the membranes were probed with a goat anti-mouse
secondary antibody (ab6789, 1:5,000; Abcam) for 1 h at room
temperature. The bands were visualized using Electrogenerated
chemiluminescence (GE Healthcare Life Sciences, Chalfont, UK).
β-Actin was used as a loading control for protein level
normalization.
Luciferase reporter assay
The luciferase reporter vectors, pGL3-AKT1-3′UTR
wild-type 1 (Wt1), 2 (Wt2), mutant 1 (Mut1) and mutant 2 (Mut2),
were synthesized by Shanghai GenePharma Co., Ltd. For the
luciferase reporter assay, breast cancer cells were cultured in
24-well plates and transfected with luciferase reporter vectors,
along with the miR-215 mimic or NC, using Lipofectamine 2000. At 48
h after transfection, luciferase activities were measured with the
Dual-Luciferase Reporter Assay system (Promega Corporation,
Madison, WI, USA) according to the manufacturer's protocol. Firefly
luciferase activity was normalized to Renilla luciferase
activity.
Statistical analysis
Data were presented as mean ± standard deviation.
Statistical analysis was performed using Student's t-tests or
one-way analysis of variance plus multiple comparisons using SPSS
16.0 software (SPSS, Inc., Chicago, IL, USA). SNK was used to
compare between two groups in multiple groups. For all analyses,
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-215 is downregulated in breast
cancer tissues and cell lines
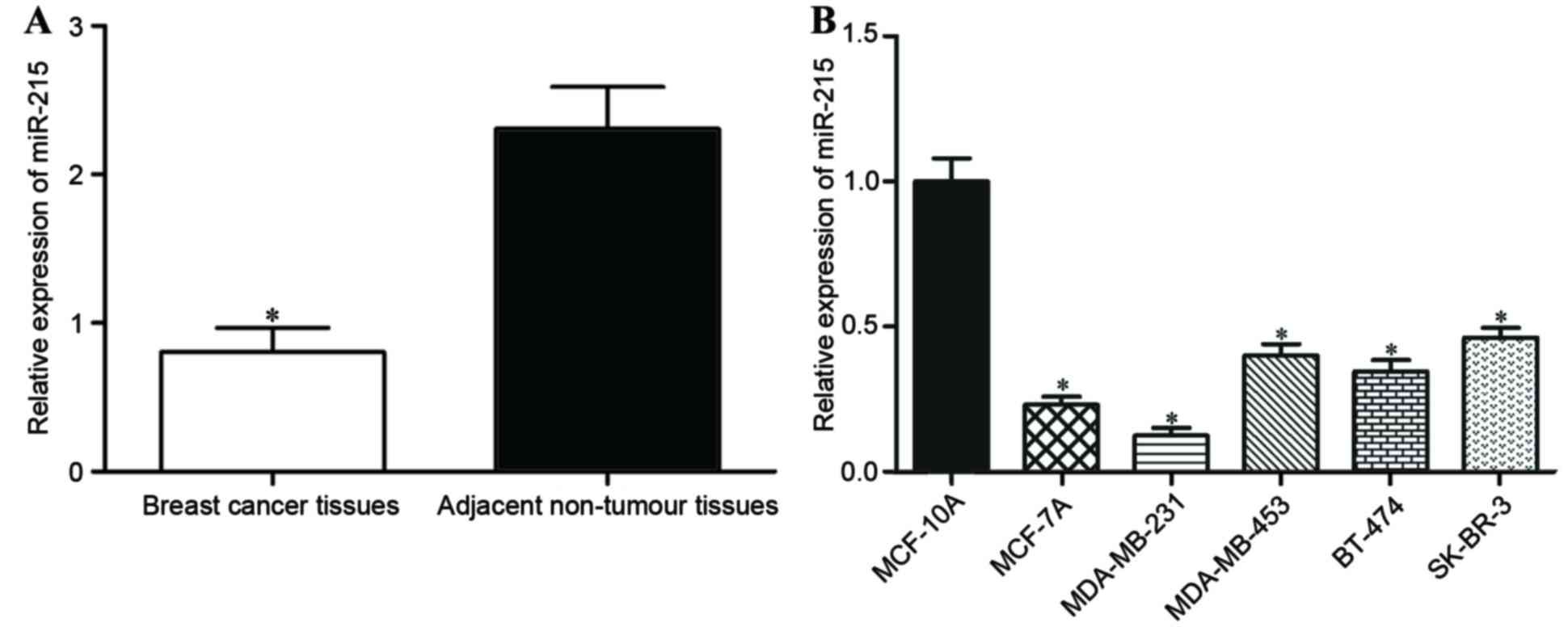
To evaluate the role of miR-215 in breast cancer,
RT-qPCR was performed to measure the expression levels of miR-215
in breast cancer tissues and matched adjacent non-tumor breast
tissues. The expression of miR-215 in breast cancer tissues was
significantly decreased compared with in matched adjacent non-tumor
breast tissues (P<0.05; Fig.
1A).
For further characterization of miR-215 expression
in breast cancer, its expression in a normal mammary epithelial
cell line (MCF-10A) and breast cancer cell lines (MCF-7A,
MDA-MB-231, MDA-MB-453, BT-474 and SK-BR-3) was also quantified.
RT-qPCR analysis demonstrated that miR-215 expression was
significantly decreased in all the breast cancer cell lines
compared with MCF-10A (P<0.05; Fig.
1B). The lowest level of miR-215 expression was in MCF-7A and
MDA-MB-231 cells, which were selected for use in further
experiments.
Effects of miR-215 on the
proliferation and invasion of breast cancer cells
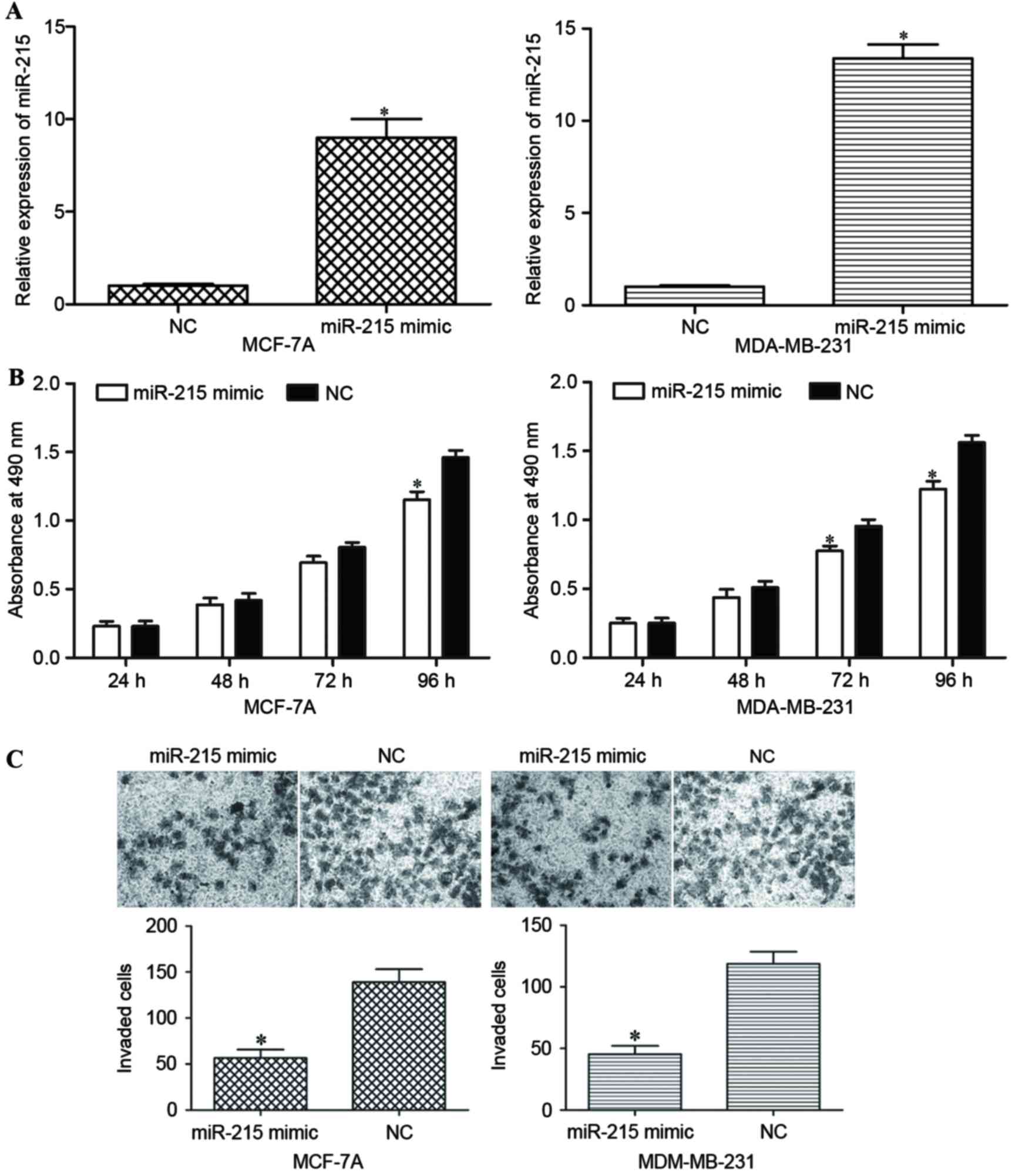
Subsequent to transfecting MCF-7A and MDA-MB-231
cells with the miR-215 mimic or NC, the expression level of miR-215
was measured using RT-qPCR. Transfection with the miR-215 mimic led
to a significant increase in its expression in MCF-7A and
MDA-MB-231 cells (P<0.05; Fig.
2A).
An MTT assay was performed to evaluate the effect of
miR-215 on the proliferation of MCF-7A and MDA-MB-231 cells. MCF-7A
and MDA-MB-231 cells transfected with the miR-215 mimic
proliferated at a decreased rate compared with the cells
transfected with NC (P<0.05; Fig.
2B).
An invasion assay was performed to investigate the
effect of miR-215 on the invasive capacity of breast cancer cells.
Following transfection with the miR-215 mimic, the invasive
capacity of MCF-7A and MDA-MB-231 cells was significantly decreased
compared with NC-transfected cells (Fig.
2C). Taken together, the data indicated that miR-215 inhibited
the proliferation and invasion of breast cancer cells.
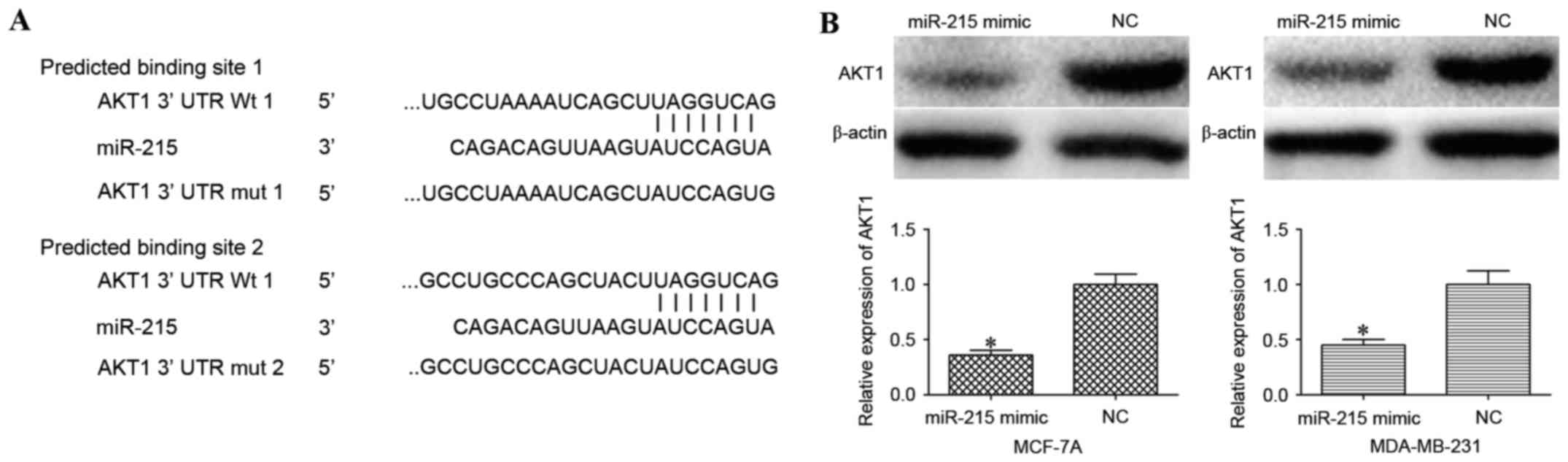
Overexpression of miR-215 decreases
the level of AKT1 protein
In order to investigate the underlying molecular
mechanism of miR-215 in the progression of breast cancer,
TargetScan and miRanda were used to screen for target genes of
miR-215. From the potential targets, AKT1 was selected as it had
previously been demonstrated to contribute to breast cancer
progression. As illustrated in Fig.
3A, the 3′UTR of AKT1 contains two predicted binding sites for
miR-215.
The effect of miR-215 overexpression on AKT1 protein
expression in MCF-7A and MDA-MB-231 cells was evaluated using
western blot analysis. AKT1 was significantly downregulated in
miR-215 mimic-transfected MCF-7A and MDA-MB-231 cells compared with
cells transfected with NC (P<0.05; Fig. 3B).
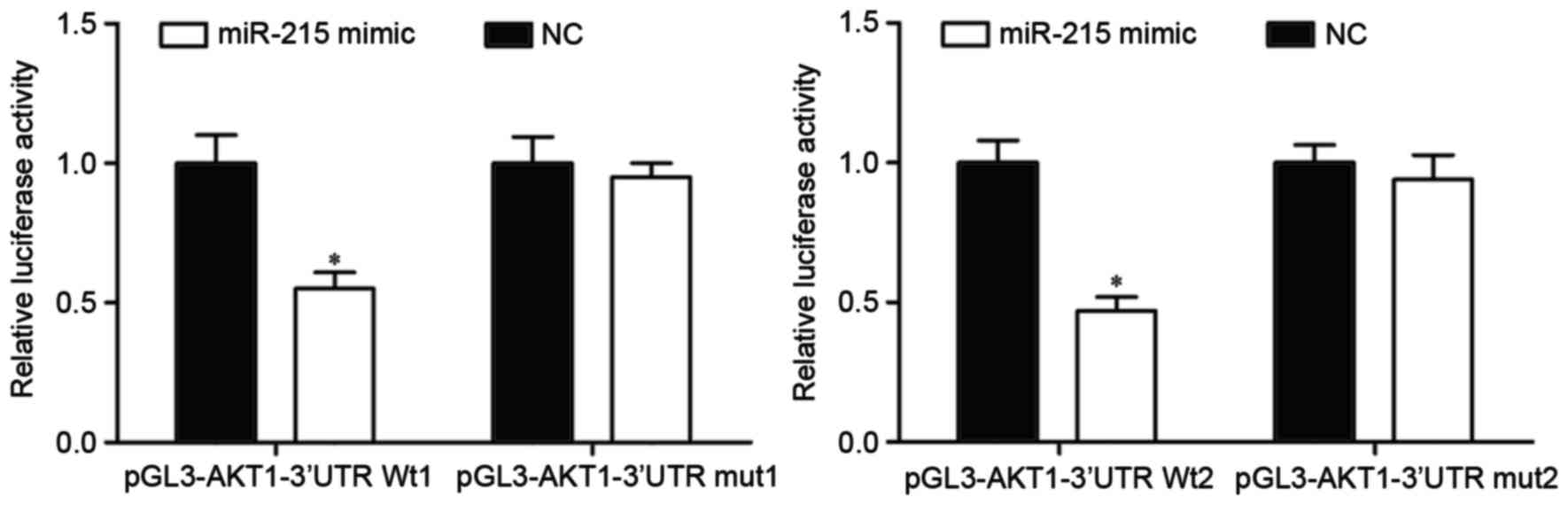
AKT1 is a direct target of
miR-215
To determine whether miR-215 could directly target
AKT1, a luciferase reporter assay was performed. Luciferase
reporter vectors with the miR-215 mimic or NC were introduced into
HEK293T cells. miR-215-transfected HEK293T cells further
transfected with the pGL3-AKT1-3′UTR Wt1 and Wt2 luciferase report
vectors exhibited a significant decrease in luciferase activity
compared with NC-transfected cells with the reporter vectors
(P<0.05; Fig. 4). By contrast,
pGL3-AKT1-3′UTR Mut1 and Mut2 luciferase reporter vectors abrogated
the repression of luciferase activity associated with miR-215
overexpression in HEK293T cells. These results suggested that AKT1
was a direct target of miR-215.
AKT1 promotes the proliferation and
invasion of breast cancer cells
The results of the present study had demonstrated
that miR-215 overexpression inhibited the proliferative and
invasive capacity of breast cancer cells, and AKT1 had been
implicated as a direct target of miR-215. Therefore the association
of AKT1 with the proliferation and invasion of breast cancer cells
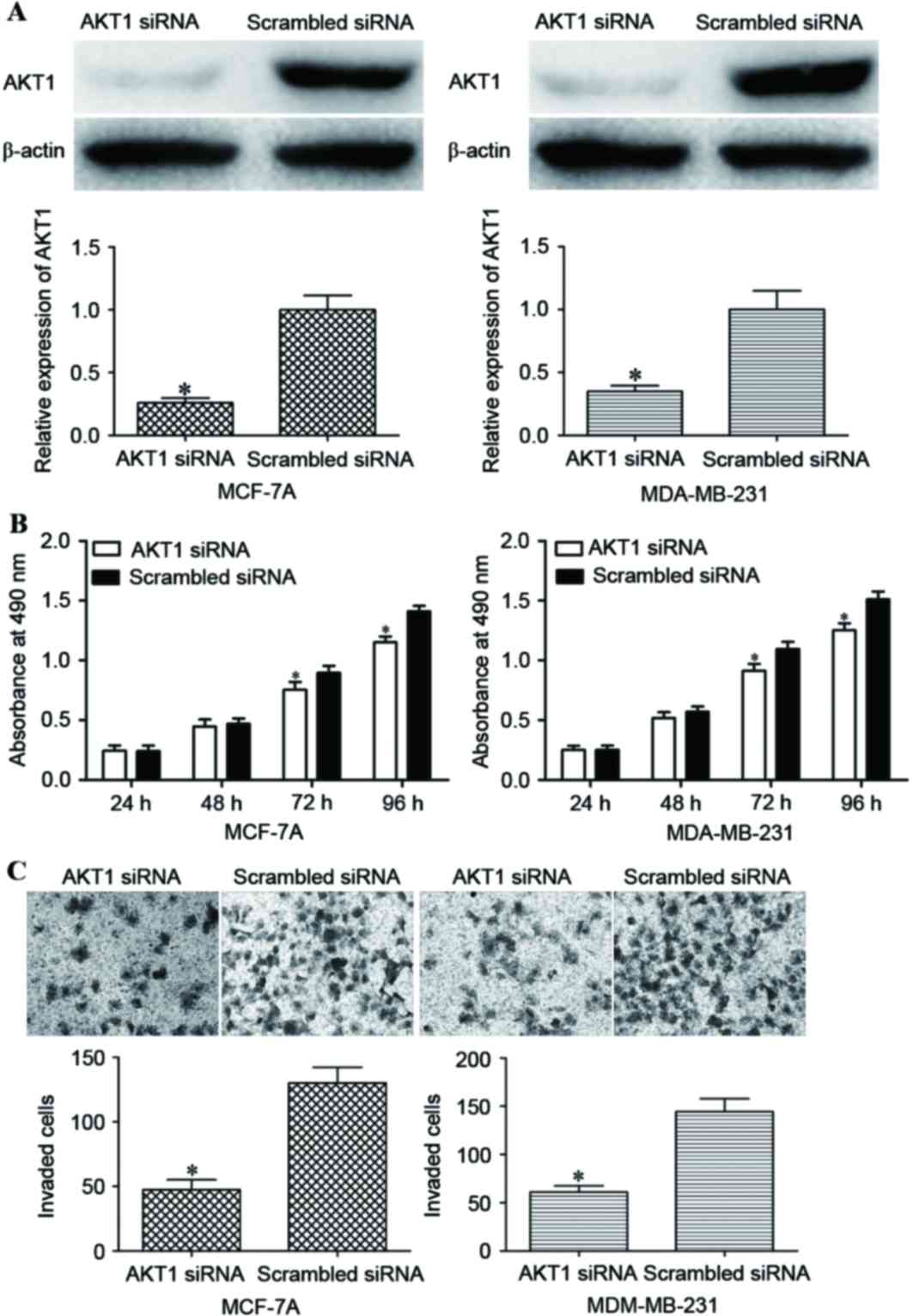
was investigated. AKT1 siRNA was used to decrease AKT1 expression.
Following transfection, western blot analysis demonstrated that
AKT1 protein expression was decreased in the AKT1 siRNA groups
compared with in the scrambled siRNA groups (P<0.05; Fig. 5A).
An MTT assay revealed that AKT1 siRNA transfection
inhibited the proliferation of MCF-7A and MDA-MB-231 cells compared
with transfection with scrambled siRNA (P<0.05; Fig. 5B). In addition, AKT1 siRNA decreased
the invasive capacity of MCF-7A and MDA-MB-231 cells compared with
the scrambled siRNA groups (P<0.05; Fig. 5C). These data indicated that the
reduced expression of AKT1 inhibited cell proliferation and
invasion in a similar manner to miR-215 overexpression in MCF-7A
and MDA-MB-231 cells, which suggested that AKT1 was the functional
target of miR-215 in breast cancer.
Discussion
In the present study, it was identified that miR-215
was significantly downregulated in breast cancer tissues and cell
lines compared with adjacent non-tumor breast tissues and a normal
human breast epithelial cell line, respectively. miR-215
overexpression inhibited the proliferation and invasion of breast
cancer cells. In addition, AKT1 was identified as a novel target of
miR-215; therefore, it was hypothesized that miR-215 acted as a
tumor suppressor through the downregulation of AKT1 in breast
cancer cells. The results suggested that miR-215 should be
investigated as a potential therapeutic agent in the treatment of
breast cancer.
Previous studies identified that miR-215 was
upregulated in gastric (26–28) and cervical (29) cancer. The expression level of miR-215
was demonstrated to be associated with the International Federation
of Gynecology and Obstetrics stage, the histological grade, and the
extents of vascular invasion and lymph node metastasis of cervical
cancer; the 5-year survival rate was lower in patients with stage
II cervical cancer who exhibited miR-215 overexpression (29). However, previous studies have also
demonstrated that miR-215 may be downregulated in epithelial
ovarian (30), pancreatic (31), non-small cell lung (32), breast (33) and colon (34) cancer. In the study on pancreatic
cancer, a low expression level of miR-215 was significantly
associated with a large tumor size, an advanced TNM stage, a high
extent of lymph node metastasis and vessel invasion, and lower
overall survival time; multivariate regression analysis
demonstrated that miR-215 underexpression was an independent
unfavorable prognostic factor for patients (31). miR-215 expression was also reported to
be associated with a higher tumor grade, human epidermal growth
factor receptor 2 (HER2)-positivity, HER2-positive breast cancer
subtype and lymph node metastasis in breast cancer (33). These studies have indicated that
miR-215 may be a diagnostic and prognostic biomarker for a number
of types of cancer.
In certain types of cancer, miR-215 has been
identified as a tumor suppressor. For example, in epithelial
ovarian cancer, ectopic miR-215 expression suppressed cell
proliferation, promoted apoptosis and increased sensitivity to
chemotherapy drugs (30). Ge et
al (35) reported that miR-215
overexpression inhibited cell proliferation, promoted apoptosis and
increased sensitivity to chemotherapy drugs in ovarian cancer
cells. Hou et al (32) also
demonstrated that the restoration of the expression of miR-215
inhibited proliferation and invasion and promoted apoptosis in
vitro, and suppressed tumorigenicity and metastasis in
vivo, in non-small cell lung cancer cells.
However, in gastric cancer and cervical cancer,
miR-215 has previously been demonstrated to act as an oncogene.
Previous studies demonstrated that miR-215 enhanced the
proliferation, migration, invasion and metastasis of gastric cancer
cells (26–28). These previous studies may appear
contradictory, in that miR-215 acted as an oncogene in certain
types of cancer, and a tumor suppressor in others. This
contradiction may be explained by the ‘imperfect complementarity’
of the interactions between miRNAs and target genes (36).
miRNAs perform critical functions in a variety of
cellular processes by binding to the 3′UTR of target mRNAs
(10,11). Several targets of miR-215 have been
identified, including runt-related transcription factor 1 (28) and RB transcriptional corepressor 1
(26) in gastric cancer, X-linked
inhibitor of apoptosis in epithelial ovarian cancer (30), and zinc finger E-box binding homeobox
2 in pancreatic cancer (31) and
non-small cell lung cancer (32). In
our study, AKT1 was validated as a novel target of miR-215 through
four experiments. Firstly, TargetScan online software and miRanda
predicted that the AKT1 3′UTR contained two miR-215 seed matches.
Secondly, western blot analysis results demonstrated that miR-215
mimic decreased the AKT1 expression level of breast cancer cells.
Thirdly, a luciferase report assay revealed that miR-215 targeted
the AKT1 3′UTR. Finally, the consequences of reducing AKT1
expression were similar to miR-215 overexpression in breast cancer
cells. These results have prompted us to hypothesize that AKT1 is a
direct target of miR-215, and that miR-215 produces its anti-tumor
effects in breast cancer cells through the downregulation of
AKT1.
AKT1 is a highly conserved serine/threonine kinase
and a crucial factor of the PI3K/AKT pathway, which regulates
various cellular processes, including proliferation, apoptosis,
migration, invasion and metabolism (37,38). AKT1
is one of the most frequently activated kinases in human cancer
(39,40). It has been demonstrated that AKT1 is
upregulated in a number of types of cancer, including prostate and
breast cancer, and ovarian carcinomas (41). Therefore, AKT1 is considered a
valuable therapeutic target for several types of cancer. As it has
been identified that miR-215 targeted AKT1 to inhibit breast cancer
cell proliferation and invasion, miR-215/AKT1-based therapy may be
a promising treatment for breast cancer.
In conclusion, the present study has demonstrated
that the level of miR-215 expression was decreased in breast
cancer, and that miR-215 overexpression inhibited the proliferation
and invasion of breast cancer cells by targeting AKT1. The present
study has provided novel insight into the molecular mechanisms
underlying the proliferation and metastasis of breast cancer.
However, as the regulatory role of miR-215 in the rapid
proliferation and metastasis of breast cancer may be complex, the
topic requires further investigation in further studies.
References
|
1
|
Singh R, Yadav V, Kumar S and Saini N:
MicroRNA-195 inhibits proliferation, invasion and metastasis in
breast cancer cells by targeting FASN HMGCR, ACACA and CYP27B1. Sci
Rep. 5:174542015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eroles P, Tormo E, Pineda B, Espin E and
Lluch A: MicroRNAs in breast cancer: One more turn in regulation.
Curr Drug Targets. 17:1083–1100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakamura S, Yagata H, Ohno S, Yamaguchi H,
Iwata H, Tsunoda N, Ito Y, Tokudome N, Toi M, Kuroi K and Suzuki E:
Multi-center study evaluating circulating tumor cells as a
surrogate for response to treatment and overall survival in
metastatic breast cancer. Breast Cancer. 17:199–204. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gong Y, He T, Yang L, Yang G, Chen Y and
Zhang X: The role of miR-100 in regulating apoptosis of breast
cancer cells. Sci Rep. 5:116502015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Muenst S, Däster S, Obermann EC, Droeser
RA, Weber WP, von Holzen U, Gao F, Viehl C, Oertli D and Soysal SD:
Nuclear expression of snail is an independent negative prognostic
factor in human breast cancer. Dis Markers. 35:337–344. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu L, Ju F, Zhao H and Ma X: MicroRNA-134
modulates resistance to doxorubicin in human breast cancer cells by
downregulating ABCC1. Biotechnol Lett. 37:2387–2394. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zielinska HA, Bahl A, Holly JM and Perks
CM: Epithelial-to-mesenchymal transition in breast cancer: A role
for insulin-like growth factor I and insulin-like growth
factor-binding protein 3? Breast Cancer (Dove Med Press). 7:9–19.
2015.PubMed/NCBI
|
|
9
|
Pdel C Monroig, Chen L, Zhang S and Calin
GA: Small molecule compounds targeting miRNAs for cancer therapy.
Adv Drug Deliv Rev. 81:104–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith L, Baxter EW, Chambers PA, Green CA,
Hanby AM, Hughes TA, Nash CE, Millican-Slater RA, Stead LF,
Verghese ET and Speirs V: Down-regulation of miR-92 in breast
epithelial cells and in normal but not tumour fibroblasts
contributes to breast carcinogenesis. PLoS One. 10:e01396982015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fei B and Wu H: MiR-378 inhibits
progression of human gastric cancer MGC-803 cells by targeting
MAPK1 in vitro. Oncol Res. 20:557–564. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Z, Yin B, Wang B, Ma Z, Liu W and Lv
G: MicroRNA-210 promotes proliferation and invasion of peripheral
nerve sheath tumor cells targeting EFNA3. Oncol Res. 21:145–154.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Misawa A, Katayama R, Koike S, Tomida A,
Watanabe T and Fujita N: AP-1-Dependent miR-21 expression
contributes to chemoresistance in cancer stem cell-like SP cells.
Oncol Res. 19:23–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohdaira H, Sekiguchi M, Miyata K and
Yoshida K: MicroRNA-494 suppresses cell proliferation and induces
senescence in A549 lung cancer cells. Cell Prolif. 45:32–38. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao Q, Liu F, Ji K, Liu N, He Y, Zhang W
and Wang L: MicroRNA-381 inhibits the metastasis of gastric cancer
by targeting TMEM16A expression. J Exp Clin Cancer Res. 36:292017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tomasetti M, Santarelli L, Neuzil J and
Dong L: MicroRNA regulation of cancer metabolism: Role in tumour
suppression. Mitochondrion. 19:29–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rusek AM, Abba M, Eljaszewicz A, Moniuszko
M, Niklinski J and Allgayer H: MicroRNA modulators of epigenetic
regulation, the tumor microenvironment and the immune system in
lung cancer. Mol Cancer. 14:342015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hurst DR, Edmonds MD and Welch DR:
Metastamir: The field of metastasis-regulatory microRNA is
spreading. Cancer Res. 69:7495–7498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang H, Liu P, Yang L and Xie X, Ye F, Wu
M, Liu X, Chen B, Zhang L and Xie X: miR-185 suppresses tumor
proliferation by directly targeting E2F6 and DNMT1 and indirectly
upregulating BRCA1 in triple-negative breast cancer. Mol Cancer
Ther. 13:3185–3197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pu Q, Huang Y, Lu Y, Peng Y, Zhang J, Feng
G, Wang C, Liu L and Dai Y: Tissue-specific and plasma microRNA
profiles could be promising biomarkers of histological
classification and TNM stage in non-small cell lung cancer. Thorac
Cancer. 7:348–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song J, Bai Z, Zhang J, Meng H, Cai J,
Deng W, Bi J, Ma X and Zhang Z: Serum microRNA-21 levels are
related to tumor size in gastric cancer patients but cannot predict
prognosis. Oncol Lett. 6:1733–1737. 2013.PubMed/NCBI
|
|
23
|
Bartels CL and Tsongalis GJ: MicroRNAs:
Novel biomarkers for human cancer. Ann Biol Clin (Paris).
68:263–272. 2010.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ju X, Katiyar S, Wang C, Liu M, Jiao X, Li
S, Zhou J, Turner J, Lisanti MP, Russell RG, et al: Akt1 governs
breast cancer progression in vivo. Proc Natl Acad Sci USA. 104:pp.
7438–7443. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deng Y, Huang Z, Xu Y, Jin J, Zhuo W,
Zhang C, Zhang X, Shen M, Yan X, Wang L, et al: MiR-215 modulates
gastric cancer cell proliferation by targeting RB1. Cancer Lett.
342:27–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu YJ and Fan Y: MiR-215/192 participates
in gastric cancer progression. Clin Transl Oncol. 17:34–40. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li N, Zhang QY, Zou JL, Li ZW, Tian TT,
Dong B, Liu XJ, Ge S, Zhu Y, Gao J and Shen L: miR-215 promotes
malignant progression of gastric cancer by targeting RUNX1.
Oncotarget. 26:4817–4828. 2016.
|
|
29
|
Liang H, Li Y, Luo RY and Shen FJ:
MicroRNA-215 is a potential prognostic marker for cervical cancer.
J Huazhong Univ Sci Technolog Med Sci. 34:207–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ge G, Zhang W, Niu L, Yan Y, Ren Y and Zou
Y: miR-215 functions as a tumor suppressor in epithelial ovarian
cancer through regulation of the X-chromosome-linked inhibitor of
apoptosis. Oncol Rep. 35:1816–1822. 2016.PubMed/NCBI
|
|
31
|
Li QW, Zhou T, Wang F, Jiang M, Liu CB,
Zhang KR, Zhou Q, Tian Z and Hu KW: MicroRNA-215 functions as a
tumor suppressor and directly targets ZEB2 in human pancreatic
cancer. Genet Mol Res. 14:16133–16145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hou Y, Zhen J, Xu X, Zhen K, Zhu B, Pan R
and Zhao C: miR-215 functions as a tumor suppressor and directly
targets ZEB2 in human non-small cell lung cancer. Oncol Lett.
10:1985–1992. 2015.PubMed/NCBI
|
|
33
|
Zhou SW, Su BB, Zhou Y, Feng YQ, Guo Y,
Wang YX, Qi P and Xu S: Aberrant miR-215 expression is associated
with clinical outcome in breast cancer patients. Med Oncol.
31:2592014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Karaayvaz M, Pal T, Song B, Zhang C,
Georgakopoulos P, Mehmood S, Burke S, Shroyer K and Ju J:
Prognostic significance of miR-215 in colon cancer. Clin Colorectal
Cancer. 10:340–347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ge G, Zhang W, Niu L, Yan Y, Ren Y and Zou
Y: miR-215 functions as a tumor suppressor in epithelial ovarian
cancer through regulation of the X-chromosome-linked inhibitor of
apoptosis. Oncol Rep. 35:1816–1822. 2016.PubMed/NCBI
|
|
36
|
Yu Z, Ni L, Chen D, Zhang Q, Su Z, Wang Y,
Yu W, Wu X, Ye J, Yang S, et al: Identification of miR-7 as an
oncogene in renal cell carcinoma. J Mol Histol. 44:669–677. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Agarwal E, Brattain MG and Chowdhury S:
Cell survival and metastasis regulation by Akt signaling in
colorectal cancer. Cell Signal. 25:1711–1719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kang B, Hao C, Wang H, Zhang J, Xing R,
Shao J, Li W, Xu N, Lu Y and Liu S: Evaluation of
hepatic-metastasis risk of colorectal cancer upon the protein
signature of PI3K/AKT pathway. J Proteome Res. 7:3507–3515. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang G, Liu Z, Xu H and Yang Q:
miR-409-3p suppresses breast cancer cell growth and invasion by
targeting Akt1. Biochem Biophys Res Commun. 469:189–195. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun M, Wang G, Paciga JE, Feldman RI, Yuan
ZQ, Ma XL, Shelley SA, Jove R, Tsichlis PN, Nicosia SV and Cheng
JQ: AKT1/PKBalpha kinase is frequently elevated in human cancers
and its constitutive activation is required for oncogenic
transformation in NIH3T3 cells. Am J Pathol. 159:431–437. 2001.
View Article : Google Scholar : PubMed/NCBI
|