Introduction
As a traditional Chinese medicine (TCM), the dried
secretion from skin glands of Bufo gargarizans (toad venom)
has been used in the treatment of various types of inflammation in
China for thousands of years (1).
Previous studies reported that toad venom has an activity in tumor
inhibition. For instance, extraction of toad venom inhibited the
growth of non-small cell lung cancer (2,3). Active
components of toad venom, including telocinobufagin, marinobufagin,
bufalin, bufotalin and resibufogenin, were reported to exhibit a
tumor-inhibiting activity (4–6). Bufalin and cardiotonic steroid, isolated
from toad venom, were identified to cause apoptosis in human
prostate and breast cancer cells by upregulating the expression of
caspase family genes in tumor cells (7,8). Bufalin
can also suppress cell proliferation and inhibit the migration and
invasion of tumor cells, although the molecular mechanisms remain
unknown (6,9,10). By
contrast, arenobufagin, another major component of toad venom, was
reported as a specific inhibitor of VEGF-mediated angiogenesis
(6).
Compared with the anti-tumor activity of the
aforementioned small molecules from toad venom of B.
gargarizans, studies on the anti-tumor activity of skin
secretion from other amphibians are focused on peptides that are
considered to be more sensitive and/or specific than small
molecules (11–14). In previous studies, a large number of
anti-tumor peptides were reported from different toads and frogs
(15–17). Peptides from the magainin family were
isolated from African clawed frog (Xenopus laevis) skin
(18,19). Maganin 1 and maganin 2, two peptides
with 23 amino acid residues, were reported to inhibit cell
proliferation in NCI-H82 and NCI-H526 cells at a low dose (19,20). Since
maganin 2 has a high specific cytotoxicity, its derivative via
amino acid modification has been approved as a novel medicine in
cancer treatment (21,22). In addition, maximin and bombinin
families, identified in Bombina maxima and Bombina
orientalis, were reported to inhibit the proliferation of tumor
cells (23).
However, the active peptides of toad venom from
B. gargarizans are unknown since toad venom is always
prepared into the dried form from secretion of toad, a procedure
that possibly denatures or damages active proteins and peptides.
The identification and detection of possible anti-tumor peptides
from skin gland secretions of B. gargarizans remains a
challenge, and has not been studied. In the present study, protein
components and their anti-tumor activity of fresh toad venom (FTV)
were compared with those of dried toad venom (DTV). Furthermore,
basic fibroblast growth factor (bFGF), one of the growth factors
involved in angiogenesis in cancer metastasis (24–26), was
selected as a biomarker for cancers to investigate protein
components from toad venom. A bFGF-immobilized affinity column was
used to capture active components that can interact with bFGF. The
bFGF can inhibit the apoptosis induced by oridonin in L929 tumor
cells (26), and cause tumor
migration by regulating several pathways (27,28). The
mechanism of anti-tumor activity of active components was
preliminarily studied.
Materials and methods
Collection and treatment of toad
venom
FTV was collected from the Laboratory Animal Center
at Zhejiang University (Hangzhou, China) according to the method
recommended in Pharmacopoeia of People's Republic of China
(1). To prepare soluble fraction of
toad venom, 0.6 g of FTV was extracted with 10 ml PBS (0.2 M, pH
7.2) in a 15 ml tube. The tube was incubated at 16°C for 4 h on an
agitator at 210 rpm and followed by centrifugation of 12,000 × g at
4°C for 15 min. Supernatant was collected as soluble fraction of
FTV. Another 0.6 g of FTV was baked at 37°C for 24 h to prepare
DTV. The soluble fraction of DTV was prepared using the method
described for the preparation of the soluble fraction of FTV.
Cell culture
Human SH-SY5Y-EGFP cells were purchased from
Hangzhou Neuropeptide Biological Science and Technology, Inc., Ltd.
(NUPTEC; Hangzhou, China) and maintained as described previously
(29) with a little modification.
Human SH-SY5Y, Hep G2 and HUVEC cells were purchased from Type Cell
Culture Collection of the Chinese Academy of Sciences (Beijing,
China). HUVEC cells were cultured in endothelial cell medium (ECM)
medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) while
other cells were cultured in DMEM (HyClone; GE Healthcare Life
Sciences). The two mediums were supplemented with 10% fetal bovine
serum (FBS) (FBS; HyClone; GE Healthcare Life Sciences) and 0.3%
penicillin-streptomycin solution (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany), at 37°C under a 5% CO2 and 95% air
humidified atmosphere in a CO2 cell incubator (Sanyo,
Moniguchi, Osaka, Japan). The medium was replaced every other
day.
High-pressure liquid chromatography
(HPLC) analysis
HPLC analysis of samples was performed on an Agilent
1260 Liquid Chromatographer (Agilent, Santa Clara, CA, USA).
Soluble fractions from FTV and DTV were filtrated by a 0.2 µm
filter (Axygen; Corning, Inc., Corning, NY, USA) and 30 µl of each
filtrated sample was loaded onto an Agilent reverse phase (RP) C8
column (Agilent) and eluted at a flow rate of 1 ml/min in a
gradient of 5% buffer B (100% acetonitrile) (Scharlab, Barcelona,
Spain) vs. 90% buffer A (0.1% H3PO4 in water)
for 10 min. Components in eluates were detected at 214 nm.
Isolation of total proteins from
soluble fraction of toad venom
Soluble fractions of FTV and DTV stored at −80°C
freezer were incubated on ice until they were completely thawed. In
total, 24 ml of pre-cold acetone at −80°C was mixed with 6 ml of
thawed soluble fractions of FTV or DTV. The mixtures were
centrifuged at 4°C, 13,400 × g for 10 min. The supernatant was
discarded, and subsequently, pelleted proteins were re-dissolved in
6 ml of double distilled water.
Determination of cytotoxicity
Human SH-SY5Y-EGFP cells were seeded in 96-well
plates (Corning, Inc.) at an initial density of 1.0×104
cells per well and incubated at 37°C for 24 h. Cells were treated
with soluble fraction of FTV and DTV, and their isolated proteins
were prepared from 0, 0.1625, 0.325, 0.75, 1.5, 3 and 6 mg toad
venom wt/ml at 37°C for 24 h. The MTT Cell Proliferation and
Cytotoxicity Assay kit (Sangon; Biotech, Co., Ltd., Shanghai,
China) was used to determine cytotoxicity. MTT, whose reducing
capacity indicates cellular activity, was added to each well and
incubated at 37°C for 4 h, according to the manufacturer's
protocol. Formazan solution was added to each well to resolve MTT
formazan crystals. Absorbance at 490 nm, indicating cellular
activity, was determined on a Mustiskan FC scanning multi-well
spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Protein analysis
For SDS-PAGE analysis, protein samples were prepared
as aforementioned. The soluble fraction of FTV or DTV (10 µl) were
mixed with 5 µl 5X SDS PAGE sample loading buffers (Sangon,
Biotech, Co., Ltd.) and mixtures were boiled for 5 min. The samples
were then loaded onto a 12% SDS-PAGE gel and electrophoresed for 40
min at 110V. Proteins on the gel were detected by Coomassie
brilliant blue R250 staining (Ourchem, Shanghai, China).
Identification of active
components
A bFGF-immobilized affinity column (NUPTEC) was used
to identify active peptides from the soluble fraction of toad
venom. In total, 20 ml soluble fraction of toad venom was loaded to
a bFGF affinity column and the same volume of sample was loaded to
a human serum albumin (HSA) affinity column (NUPTEC) as a control.
Subsequent to loading, the column was eluted by 5 column volumes of
50 mM Tris-HCl buffer (pH 7.2) and 50 mM PBS (pH 6.0) in order,
followed by elution of 3 column volumes of 100 mM glycine buffer
(pH 3.0) for active components. To identify active components, an
Agilent 1260 HPLC system was used to detect absorbance at 214 nm.
Collected eluates from affinity columns were loaded onto an Agilent
C-8 column and eluted at the flow rate of 1 ml/min using a 3%
buffer B (100% acetonitrile) against a 97% buffer A (0.1%
H3PO4 in water) for 10 min.
bFGF-inhibiting assay of bFGF-binding
components
SH-SY5Y cells (1×104) were plated into
96-well plates in triplicate and incubated at 37°C in a 5%
CO2 and 95% air humidified atmosphere in a
CO2 cell incubator for 24 h, followed by treatment with
a final concentration of 1 µM bFGF (NUPTEC). The bFGF-binding
component was added to medium at various doses of 0, 5, 25, 50 and
100 µg/ml (final concentration) along with bFGF. Morphology of the
cells was visualized using a Leica DM3000B microscope (Leica
Microsystems GmbH, Wetzlar, Germany; magnification, ×200) and
images were captured using LAS software (version 4.2; Leica
Microsystems GmbH) and the length of 150 neurites was measured
randomly for each sample at day 1, 2, 3, 4 and 5, respectively.
Cytotoxicity assay of bFGF-binding
components
SH-SY5Y, Hep G2 and HUVEC cells (1×104)
were plated into 96-well plates in triplicate and incubated at 37°C
for 24 h as aforementioned. SH-SY5Y and Hep G2 cells were cultured
in DMEM while HUVEC cells were cultured in ECM. Cells were then
treated with bFGF-binding component at various doses of 0, 5, 25,
50 and 100 µg/ml (final concentration), respectively. Cell
viability was determined using the MTT Cell Proliferation and
Cytotoxicity Assay kit after 24 h treatment.
Reverse transcription-polymerase chain
reaction (RT-PCR) assay
Gene expression of caspase-3 and caspase-8 was
detected by RT-PCR analysis. SH-SY5Y cells were plated into a
6-well plate (Corning, Inc.) at an initial density of
1.0×105 cells/well. After 24 h, cells were treated with
bFGF-binding component at various doses of 0, 5, 25 and 50 µg/ml
(final concentration). Total RNAs were extracted by TRIzol reagent
(Thermo Fisher Scientific, Inc.) and reverse-transcribed into cDNAs
by FastQuant RT kit (Tiangen Biotech, Beijing, China), according to
the manufacturer's protocol. Primers for caspase-3 (accession no.
BC016926; www.ncbi.nlm.nih.gov), caspase-8
(accession no. AH007578; www.ncbi.nlm.nih.gov) and β-actin (accession no.
DQ407611.1; www.ncbi.nlm.nih.gov) were designed and synthesized by
Sangon (Biotech, Co., Ltd.) for RT PCR analysis (Table I). The PCRs were performed at 95°C for
2 min, followed by 25 cycles of denaturing at 95°C for 15 sec,
annealing at 61°C for 15 sec and extending at 72°C for 45 sec. The
PCR products were analyzed on 1% agarose gels and signal
intensities were quantified by Quantity One (version 4.5; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
 | Table I.Primers used in reverse
transcription-polymerase chain reaction. |
Table I.
Primers used in reverse
transcription-polymerase chain reaction.
| Primers | Sequences |
|---|
| Caspase-3 |
|
|
Forward |
CTGGACTGCGGTATTGAGAC |
|
Reverse |
CCGGGTGCGGTAGAGTAAGC |
| Caspase-8 |
|
|
Forward |
CTGCAGAGGGAACCTGGTACATCC |
|
Reverse |
CATCAATCAGAAGGGAAGA |
| β-actin |
|
|
Forward |
GATCATTGCTCCTCCTGAG |
|
Reverse |
ACTCCTGCTTGCTGATCCA |
Western blot analysis
SH-SY5Y cells were cultured and treated as
aforementioned. After 24 h, cells were collected with ice-cold PBS
to 1.5 ml centrifuge tubes, and centrifuged at 4°C and 200 × g for
5 min. The supernatant was discarded and collected cells were
immediately lysed with lysis buffer (20 mM sucrose, 1 mM EDTA, 20
µM Tris-HCl, pH 7.2, 1 mM DTT, 10 mM KCl, 1.5 mM MgCl2,
containing 1 mM phenylmethanesulfonyl fluoride, PMSF). Lysed cells
were then centrifuged at 4°C, 13,400 × g for 10 min and total
protein was collected. Protein concentrations were determined to
normalize different samples using Pierce BCA Protein kit (Thermo
Fisher Scientific, Inc.), following the instructions. Total
proteins were then loaded to 12% SDS-PAGE gel and electrophoresed
for 40 min at 110V and then transferred to a polyvinylidene
fluoride (PVDF) membrane (Bio-Rad Laboratories, Inc.). The membrane
was then blocked with 5% skim milk, and incubated with the
anti-caspase-3 (cat. no. ER30804; 1:2,000) or anti-caspase-8 (cat.
no. ET1603-16; 1:2,000) primary antibodies (Hua'an Biotech,
Hangzhou, China), and goat anti-rabbit IgG secondary antibodies
(HRP-conjugated; cat. no. HA1001-100; 1:2,000; Hua'an Biotech),
respectively. The target proteins were visualized using an enhanced
chemiluminescence detection system (Tiangen Biotech).
Statistical analysis
Significance of differences between treatments and
control were analyzed by analysis of variance using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA) and considered significant at
P<0.05.
Results
Different pattern of components from
FTV and DTV
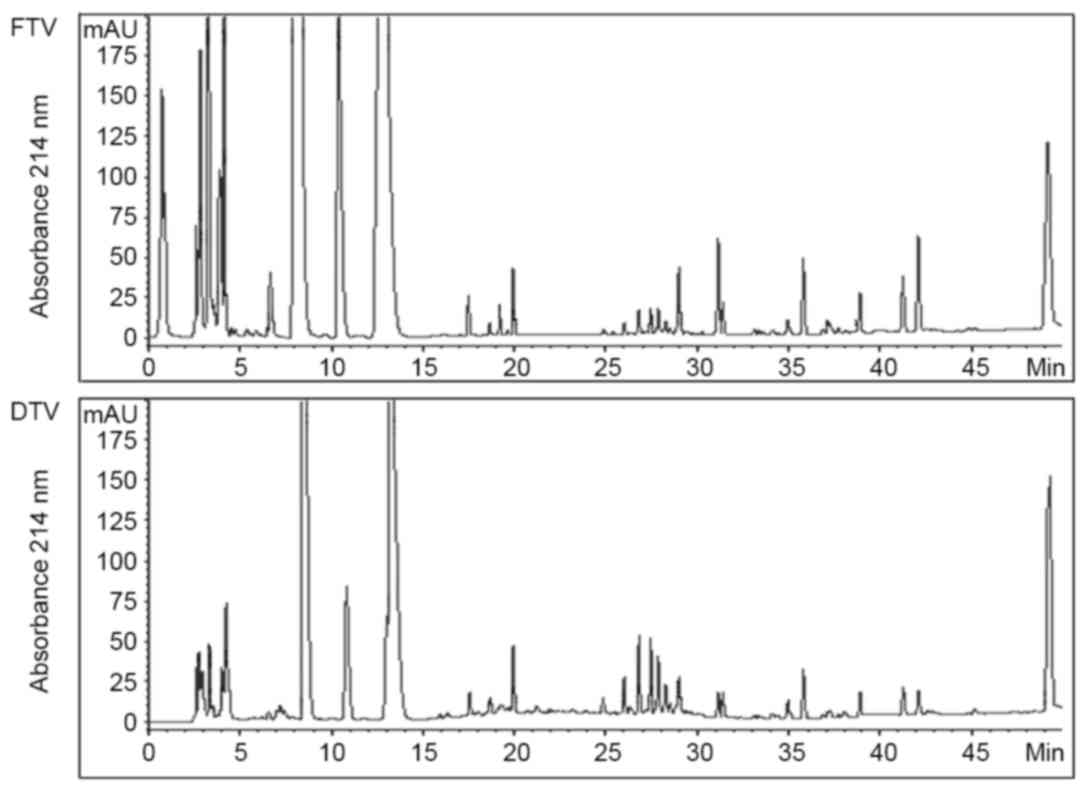
By HPLC analysis, no significant differences in
soluble fractions were identified between FTV and DTV on
chromatography beyond 15 min retention time, although there were a
number of chromatographic peaks showing difference in peak area
(Fig. 1). However, there was an
apparent difference between FTV and DTV within the first 15 min
retention time. Particularly, FTV had three much larger peaks than
DTV, and one large peak that was not observed in DTV within the
first 5 min retention time (Fig. 1),
indicating that FTV had more polar molecules than DTV.
Different cytotoxicity of components
from FTV and DTV
Based on different patterns of components from FTV
and DTV (Fig. 1), cytotoxicity of
soluble fractions from FTV and DTV was compared in human SH-SY5Y
cells to establish a link between HPLC chromatography and
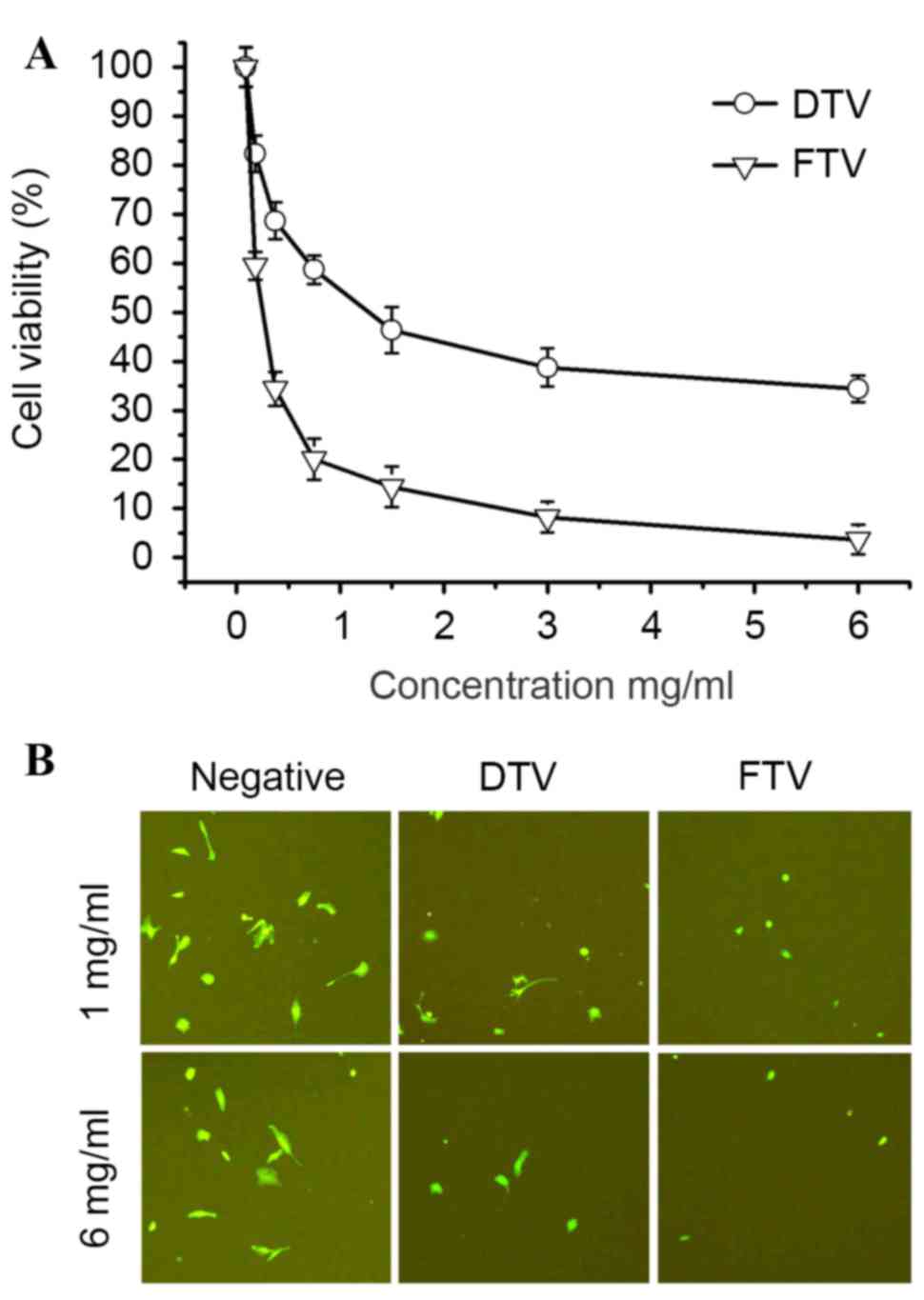
cytotoxicity. MTT assay demonstrated that cell survival decreased
in SH-SY5Y cells treated by soluble fractions from FTV and DTV in a
dose-dependent manner, indicating the cytotoxicity of FTV and DTV.
However, FTV had a more significant cytotoxicity compared with DTV
(P=0.15, 0.0089, 0.0024, 0.0019, 0.0056, 0.0027 and 0.0014 at
concentrations 0, 0.1625, 0.325, 0.75, 1.5, 3 and 6 mg/ml,
respectively; Fig. 2A).
Alternatively, the cytotoxicity was also compared in
EGFP-expressing SH-SY5Y cells. As fluorescence-imaged in Fig. 2B, FTV and DTV demonstrated
cytotoxicity as compared with controls, however, FTV was more
cytotoxic compared with DTV.
Protein components of toad venom and
their cytotoxicity
According to HPLC analysis of soluble fractions of
toad venom, the main difference between FTV and DTV was polar
molecular components (Fig. 1).
Water-soluble protein/peptide is one type of highly polar
biomolecules. Thus, water-soluble protein patterns were compared
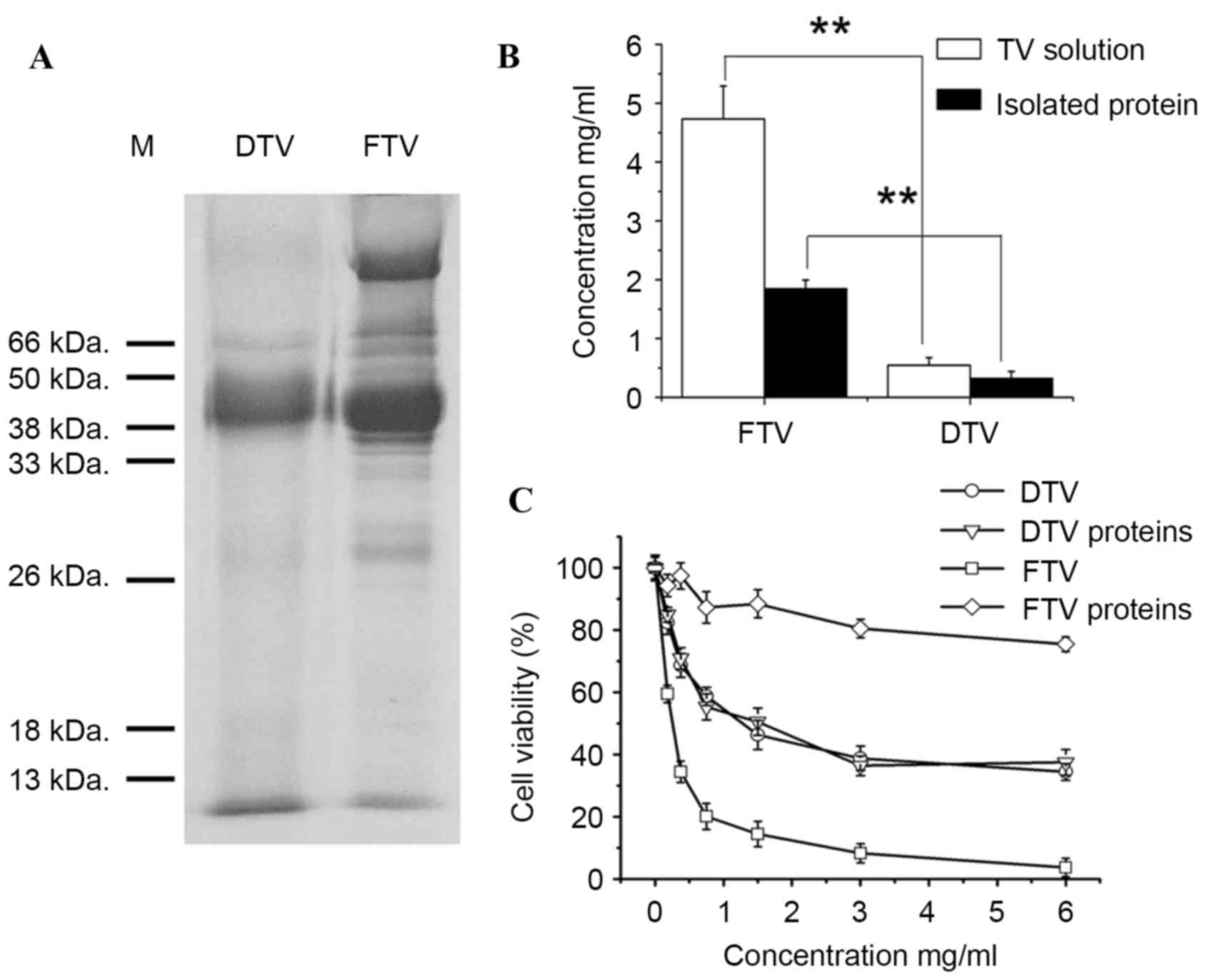
between FTV and DTV by SDS-PAGE analysis. As shown in Fig. 3A, FTV had more water-soluble protein
bands than DTV, consistent with the evidence that FTV had more and
larger peaks on HPLC chromatography compared with DTV within the
first 5 min of retention time (Fig.
1). Subsequent protein analysis demonstrated that soluble
fraction of FTV has a significantly higher protein level than DTV
(P=0.0025 and P=0.0064, respectively; Fig. 3B).
Furthermore, to determine whether difference in
water-soluble protein component of toad venom contributed to the
cytotoxicity, protein fractions were isolated from soluble
fractions of FTV and DTV, and the cytotoxicity was investigated in
human SH-SY5Y cells. Consistent with the aforementioned results
that FTV demonstrated a higher cytotoxicity than DTV (Fig. 2), FTV was more toxic to SH-SY5Y cells
than DTV (P=0.21, 0.0072, 0.0055, 0.0031, 0.0021, 0.0051 and 0.0060
and concentrations 0, 0.1625, 0.325, 0.75, 1.5, 3 and 6 mg/ml,
respectively; Fig. 3C). In parallel
to this observation, the protein fraction of FTV (FTV proteins) was
also more toxic to cells than the protein fraction of DTV (DTV
proteins) (P=0.21, 0.0072, 0.0055, 0.0031, 0.0021, 0.0051 and
0.0060 and 0, 0.1625, 0.325, 0.75, 1.5, 3 and 6 mg/ml,
respectively), and notably, the latter revealed only slight
toxicity (Fig. 3C), suggesting that
protein fraction is involved in the cytotoxicity of toad venom and
toxic proteins only retained in FTV.
Identification of bFGF-binding
components from FTV
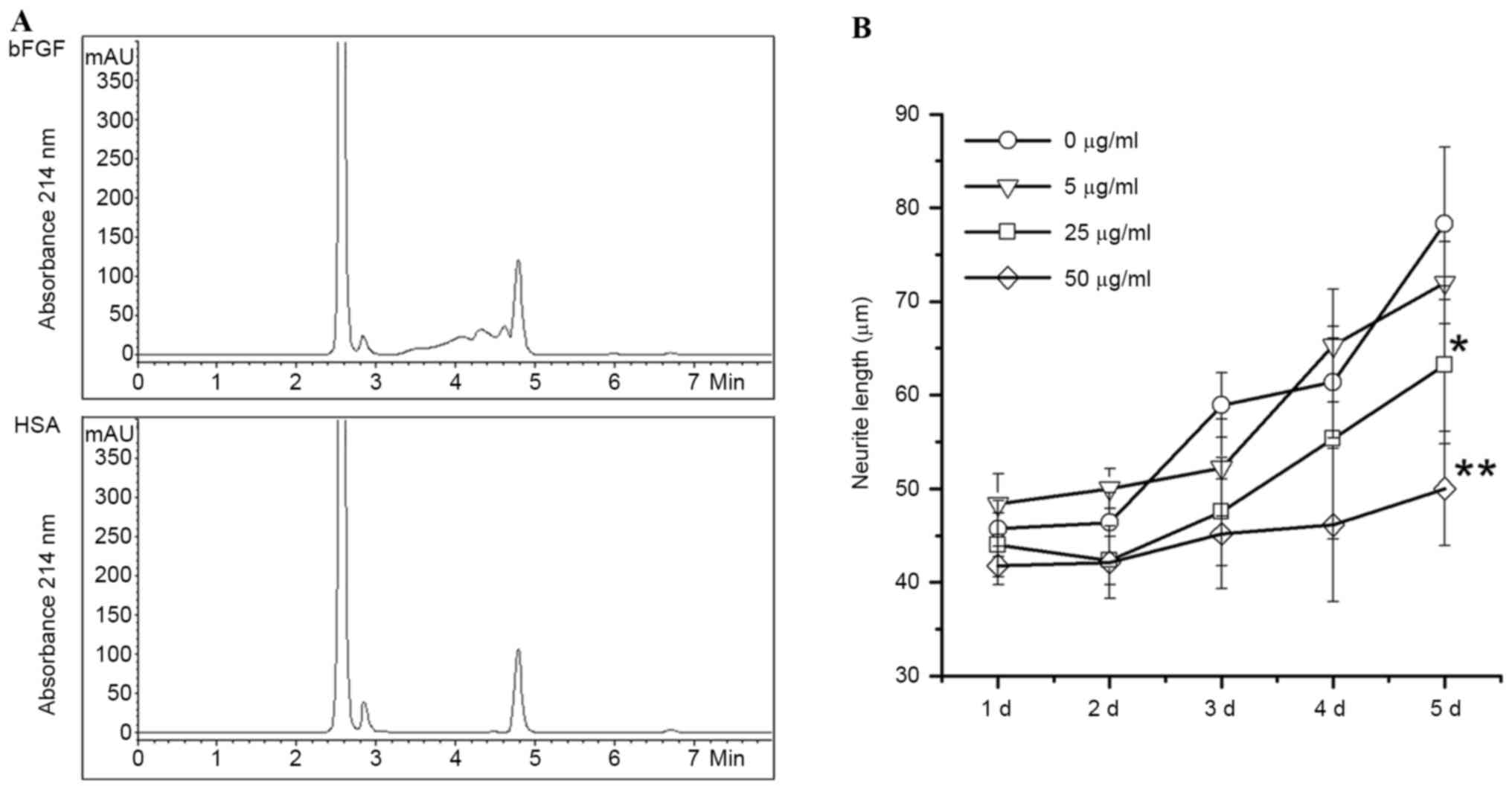
bFGF is one of the biomarkers in angiogenesis for
cancer metastasis. In order to identify anti-tumor components from
FTV, a bFGF-immobilized affinity column was used to capture
bFGF-binding components from FTV proteins, followed by
identification of HPLC analysis. As shown in Fig. 4A, the bFGF-affinity column captured
three specific peaks identified by HPLC chromatography, but the
HSA-affinity control column did not.
To verify the activity of bFGF-binding components of
FTV, eluates from the bFGF-affinity column were used to prohibit
bFGF-induced neurite outgrowth of SH-SY5Y cells. Data revealed that
the neurite outgrowth of human SH-SY5Y cells induced by bFGF is
significantly prohibited by bFGF-binding components from FTV in a
dose-dependent manner compared with the control (P=0.028 and 0.0081
at concentrations 25 and 50 µg/ml, respectively; Fig. 4B).
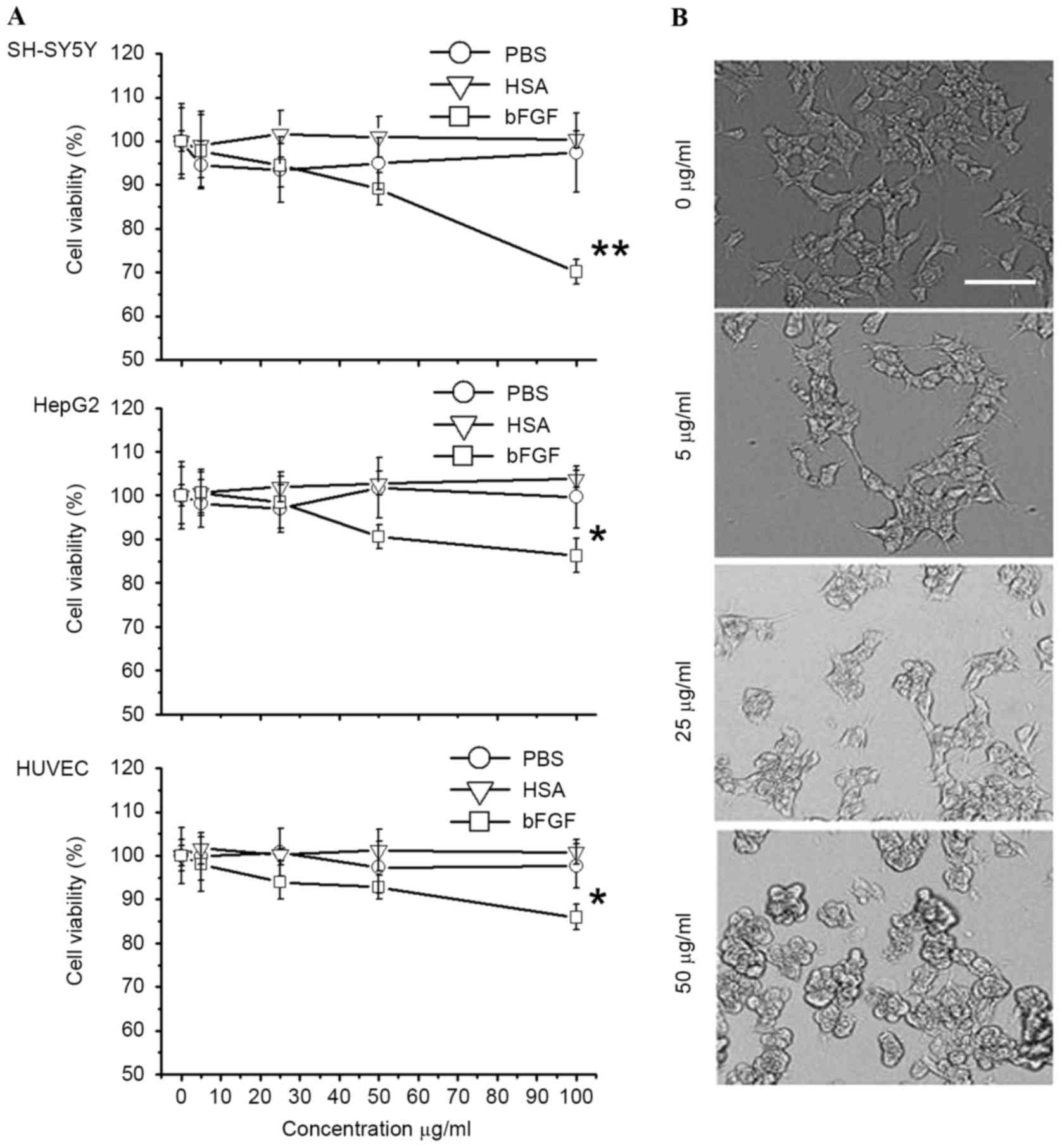
Apoptosis induced by bFGF-binding
components
In the present study, the cytotoxicity of
bFGF-binding components to human SH-SY5Y, Hep G2 and HUVEC cells
was shown to be specific (P=0.0014, 0.031 and 0.035 for SH-SY5Y,
HepG2 and HUVEC cells, respectively), since PBS and HSA-binding
components did not show cytotoxicity (Fig. 5A). Particularly in the SH-SY5Y cells,
the morphology of cells was greatly affected following treatment
with bFGF-binding components (Fig.
5B). To further investigate the mechanism of cytotoxicity
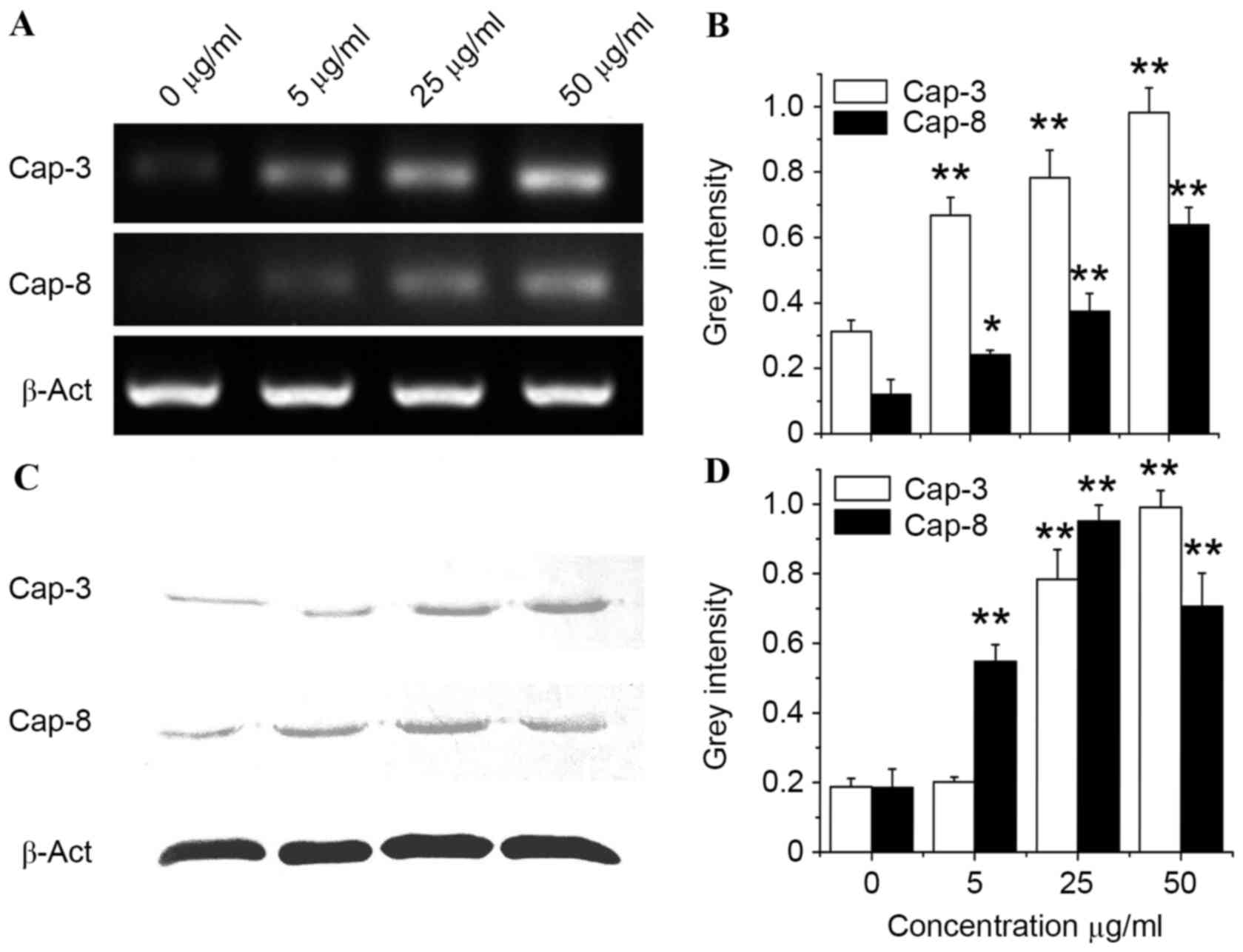
induced by bFGF-binding components, gene and protein expression of
caspase-3 and caspase-8 (two biomarkers for apoptosis) was studied
by analysis of mRNA and protein level in SH-SY5Y cells treated with
bFGF-binding components. Data from RT-PCR, as well as western blot
analysis, revealed that gene (P=0.0075, 0.0043 and 0.0031 at
concentrations 5, 25 and 50 mg/ml, respectively, vs. the Cap-3
control; P=0.022, 0.0077 and 0.0059 at concentrations 5, 25 and 50
mg/ml, respectively, vs. the Cap-8 control) and protein (P=0.13,
0.0037 and 0.0023 at concentrations 5, 25 and 50 mg/ml,
respectively, vs. the Cap-3 control; P=0.0090, 0.0041 and 0.0055 at
concentrations 5, 25 and 50 mg/ml, respectively, vs. the Cap-8
control) expression of caspase-3 and caspase-8 were significantly
upregulated by bFGF-binding components in a dose-dependent manner
(Fig. 6), suggesting that
bFGF-binding components can induce cytotoxicity, possibly via
activation of apoptosis in human SH-SY5Y cells.
Discussion
Anti-tumor peptides secreted from the skin of frogs
and toads have been extensively reported and they give a promising
progress in cancer research and clinical practice (14,15,19,30).
However, these peptides exhibit a high diversity in different
species, including Pipidae and Discoglossidae. B.
gargarizans is one of the most important species of
Bufonidae in China, and the majority of studies are focused
on the anti-tumor activity of small molecular components (4,6,7,13). It has
not been reported that this species has anti-tumor peptides
secreted from skin glands. In the present study, components from
B. gargarizans that contributed to the inhibition of
proliferation of SH-SY5Y neuroblastoma cells were identified.
DTV has been used as a traditional medicine in China
for thousands of years (1). In the
present study, DTV was identified to possess fewer peptides than
FTV, since treatment may be harmful for peptides when FTV is
prepared for DTV. This is supported by the observation in the
present study that FTV had more protein bands and components than
DTV, as evidenced by HPLC and SDS-PAGE analysis. Along with the
difference in protein components, FTV had more significant
cytotoxicity in SH-SY5Y neuroblastoma cells than DTV and isolated
proteins/peptides from FTV were more cytotoxic to tumor cells than
those from DTV.
To identify active peptides from FTV, a
bFGF-immobilized affinity column was used to capture bFGF-binding
components from FTV protein fractions, and three potential peptides
that could inhibit the proliferation of bFGF-dependent cells
including HUVEC and SH-SY5Y, were identified (31,32). In
parallel to the inhibition of cell proliferation, these
bFGF-binding components could also upregulate the gene and protein
expression of caspase-3 and caspase-8 in tumor cells, suggesting
that the cytotoxicity induced by bFGF-binding components possibly
came from the activation of apoptosis, and that the anti-tumor
activity of bFGF-binding components isolated from FTV was
contributed by inhibition of bFGF activity and down-regulation of
bFGF gene expression. This is consistent with bFGF being markedly
involved in tumor growth (31–33).
Acknowledgements
The present study was supported by the startup fund
(grant no. 2012FR017) from Zhejiang A&F University in China,
Zhejiang Provincial Natural Science Foundation (grant no.
LQ14H280007) in China, and Prior project (grant no. T12B13292014)
from Hangzhou Hygiene Bureau Zhejiang, China.
References
|
1
|
The State Pharmacopoeia Commission of PR
China, . Pharmacopoeia of the People's Republic of China. 2010. 1.
Chemical Industry Publishing House; Beijing: pp. 360. 2010
|
|
2
|
Meng Z, Yang P, Shen Y, Bei W, Zhang Y, Ge
Y, Newman RA, Cohen L, Liu L, Thornton B, et al: Pilot study of
huachansu in patients with hepatocellular carcinoma, nonsmall-cell
lung cancer, or pancreatic cancer. Cancer. 115:5309–5318. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang SJ, Zhang YT, Zhao JH, Shen LN, Shi
F and Feng NP: Preparation and in vitro anti-tumor properties of
toad venom extract-loaded solid lipid nanoparticles. Pharmazie.
68:653–660. 2013.PubMed/NCBI
|
|
4
|
Qiao L, Huang YF, Cao JQ, Zhou YZ, Qi XL
and Pei YH: One new bufadienolide from Chinese drug ‘Chan'Su’. J
Asian Nat Prod Res. 10:233–237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Ma X, Li F, Wang J, Chen H, Wang G,
Lv X, Sun C and Jia J: Preparative separation and purification of
bufadienolides from Chinese traditional medicine of ChanSu using
high-speed counter-current chromatography. J Sep Sci. 33:1325–1330.
2010.PubMed/NCBI
|
|
6
|
Zhang DM, Liu JS, Deng LJ, Chen MF, Yiu A,
Cao HH, Tian HY, Fung KP, Kurihara H, Pan JX and Ye WC:
Arenobufagin, a natural bufadienolide from toad venom, induces
apoptosis and autophagy in human hepatocellular carcinoma cells
through inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis.
34:1331–1342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu Z, Li E and Liu Y, Gao Y, Sun H, Wang
Y, Wang Z, Liu X, Wang Q and Liu Y: Bufalin induces the apoptosis
of acute promyelocytic leukemia cells via the downregulation of
survivin expression. Acta Haematol. 128:144–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong SH, Kim GY, Chang YC, Moon SK, Kim WJ
and Choi YH: Bufalin prevents the migration and invasion of T24
bladder carcinoma cells through the inactivation of matrix
metalloproteinases and modulation of tight junctions. Int J Oncol.
42:277–286. 2013.PubMed/NCBI
|
|
9
|
Chen YY, Lu HF, Hsu SC, Kuo CL, Chang SJ,
Lin JJ, Wu PP, Liu JY, Lee CH, Chung JG and Chang JB: Bufalin
inhibits migration and invasion in human hepatocellular carcinoma
SK-Hep1 cells through the inhibitions of NF-κB and matrix
metalloproteinase-2/−9-signaling pathways. Environ Toxicol.
30:74–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhai XF, Fang FF, Liu Q, Meng YB, Guo YY
and Chen Z: MiR-181a contributes to bufalin-induced apoptosis in
PC-3 prostate cancer cells. BMC Complement Altern Med. 13:3252013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miller YE: Bombesin-like peptides: From
frog skin to human lung. Am J Respir Cell Mol Biol. 3:189–190.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simmaco M, Severini C, De Biase D, Barra
D, Bossa F, Roberts JD, Melchiorri P and Erspamer V: Six novel
tachykinin- and bombesin-related peptides from the skin of the
Australian frog Pseudophryne guntheri. Peptides. 11:299–304. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee WH, Li Y, Lai R, Li S, Zhang Y and
Wang W: Variety of antimicrobial peptides in the Bombina maxima
toad and evidence of their rapid diversification. Eur J Immunol.
35:1220–1229. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
King JD, Leprince J, Vaudry H, Coquet L,
Jouenne T and Conlon JM: Purification and characterization of
antimicrobial peptides from the Caribbean frog, Leptodactylus
validus (Anura: Leptodactylidae). Peptides. 29:1287–1292. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bevins CL and Zasloff M: Peptides from
frog skin. Annu Rev Biochem. 59:395–414. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bechinger B, Zasloff M and Opella SJ:
Structure and orientation of the antibiotic peptide magainin in
membranes by solid-state nuclear magnetic resonance spectroscopy.
Protein Sci. 2:2077–2084. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Westerhoff HV, Zasloff M, Rosner JL,
Hendler RW, De Waal A, Vaz Gomes A, Jongsma PM, Riethorst A and
Juretić D: Functional synergism of the magainins PGLa and
magainin-2 in Escherichia coli, tumor cells and liposomes. Eur J
Biochem. 228:257–264. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohsaki Y, Gazdar AF, Chen HC and Johnson
BE: Antitumor activity of magainin analogues against human lung
cancer cell lines. Cancer Res. 52:3534–3538. 1992.PubMed/NCBI
|
|
19
|
Baker MA, Maloy WL, Zasloff M and Jacob
LS: Anticancer efficacy of Magainin2 and analogue peptides. Cancer
Res. 53:3052–3057. 1993.PubMed/NCBI
|
|
20
|
Giacometti A, Ghiselli R, Cirioni O,
Mocchegiani F, D'Amato G, Orlando F, Sisti V, Kamysz W, Silvestri
C, Naldoski P, et al: Therapeutic efficacy of the magainin analogue
MSI-78 in different intra-abdominal sepsis rat models. J Antimicrob
Chemother. 54:654–660. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shin SY, Kang JH, Jang SY, Kim Y, Kim KL
and Hahm KS: Effects of the hinge region of cecropin
A(1–8)-magainin 2(1–12), a synthetic antimicrobial peptide, on
liposomes, bacterial and tumor cells. Biochim Biophys Acta.
1463:209–218. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takeshima K, Chikushi A, Lee KK, Yonehara
S and Matsuzaki K: Translocation of analogues of the antimicrobial
peptides magainin and buforin across human cell membranes. J Biol
Chem. 278:1310–1315. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gibson BW, Tang DZ, Mandrell R, Kelly M
and Spindel ER: Bombinin-like peptides with antimicrobial activity
from skin secretions of the Asian toad, Bombina orientalis. J Biol
Chem. 266:23103–23111. 1991.PubMed/NCBI
|
|
24
|
Kubo H, Cao R, Brakenhielm E, Makinen T,
Cao Y and Alitalo K: Blockade of vascular endothelial growth factor
receptor-3 signaling inhibits fibroblast growth factor-2-induced
lymphangiogenesis in mouse cornea. Proc Natl Acad Sci USA. 99:pp.
8868–8873. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Demirkesen C, Büyükpinarbasili N,
Ramazanoğlu R, Oğuz O, Mandel NM and Kaner G: The correlation of
angiogenesis with metastasis in primary cutaneous melanoma: A
comparative analysis of microvessel density, expression of vascular
endothelial growth factor and basic fibroblastic growth factor.
Pathology. 38:132–137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang J, Wu L, Tashiro S, Onodera S and
Ikejima T: Fibroblast growth factor-2 suppresses oridonin-induced
L929 apoptosis through extracellular signal-regulated
kinase-dependent and phosphatidylinositol 3-kinase-independent
pathway. J Pharmacol Sci. 102:305–313. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu JF, Crepin M, Liu JM, Barritault D and
Ledoux D: FGF-2 and TPA induce matrix metalloproteinase-9 secretion
in MCF-7 cells through PKC activation of the Ras/ERK pathway.
Biochem Biophys Res Commun. 293:1174–1182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suyama K, Shapiro I, Guttman M and Hazan
RB: A signaling pathway leading to metastasis is controlled by
N-cadherin and the FGF receptor. Cancer Cell. 2:301–314. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qian Y, Zheng Y and Tiffany-Castiglioni E:
Valproate reversibly reduces neurite outgrowth by human SY5Y
neuroblastoma cells. Brain Res. 1302:21–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ferreira PM, Lima DJ, Debiasi BW, Soares
BM, Kda C Machado, Jda C Noronha, Dde J Rodrigues, Sinhorin AP,
Pessoa C and Vieira GM Jr: Antiproliferative activity of Rhinella
marina and Rhaebo guttatus venom extracts from Southern Amazon.
Toxicon. 72:43–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li D, Wang H, Xiang JJ, Deng N, Wang PP,
Kang YL, Tao J and Xu M: Monoclonal antibodies targeting basic
fibroblast growth factor inhibit the growth of B16 melanoma in vivo
and in vitro. Oncol Rep. 24:457–463. 2010.PubMed/NCBI
|
|
32
|
Polec A, Fedorcsak P, Eskild A and Tanbo
TG: The interplay of human chorionic gonadotropin (hCG) with basic
fibroblast growth factor and adipokines on angiogenesis in vitro.
Placenta. 35:249–253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu Z, Shi H, Mei Q, Shen Y and Xu J:
Effects of macrophage metalloelastase on the basic fibroblast
growth factor expression and tumor angiogenesis in murine colon
cancer. Dig Dis Sci. 57:85–91. 2012. View Article : Google Scholar : PubMed/NCBI
|