Introduction
Breast cancer is the most frequently occurring
cancer type in women and the second most common cause of brain
metastases (1). The occurrence of
brain metastases is typically associated with a limited survival
time as well as reduced quality of life. Brain metastases usually
occur late in the disease course of breast cancer, and are uncommon
at the time of initial diagnosis of breast cancer (2,3). The risk
factors for brain metastases include young age, tumor stage, human
epidermal growth factor receptor (HER2)-positivity,
triple-negativity, number of metastatic sites (n>2) and large
tumor size (4–9). It has been estimated that 10–15% of
patients with breast cancer will develop brain metastases (10), while postmortem studies have detected
brain metastases in up to 30% of patients (11). The median time of brain metastases
occurrence is 2–3 years after the initial diagnosis of breast
cancer (12). Life expectancy is
largely reduced following the diagnosis of brain metastases, with
survival time ranging from 2 to 16 months (4). Although screening for brain metastases
is not recommended as part of routine clinical care in asymptomatic
patients, Miller et al (6)
detected brain metastases in 15% of patients presenting with
disseminated breast cancer at the initial screening. Treatment of
brain metastases is challenging due to a number of factors; the
number and location of brain metastases, performance status of the
patient and biological subtype must be taken into consideration
(13).
There is growing evidence that the risk of distant
metastases differs according to the biological subtype of breast
cancer (3,14). Compared with luminal subtypes,
HER2-positive (15) and
triple-negative (TN) breast cancer tend to spread significantly
more often to the brain. However little is known about the
subtype-specific outcomes with regard to brain metastases-free
survival (BMFS) and survival following brain metastases (SFBM). The
present study aimed to determine subtype-specific survival rates
among breast cancer patients with brain metastases. The results
suggest that in-depth knowledge of the natural history of brain
metastases and their clinical outcome may aid in individualizing
treatment strategies.
Materials and methods
Study patients
The present study retrospectively analyzed a cohort
of patients with breast cancer who were treated at the Hannover
Medical School (Hannover, Germany) between January 1st, 2004 and
December 31st, 2010. A total of 1,147 patients who met all
inclusion criteria were identified from the Hannover Clinical
Cancer Register database. The inclusion criteria were primary
invasive breast cancer, no previous cancer and no simultaneous
cancer of other origin. The exclusion criteria were benign diseases
of the breast, ductal carcinoma in situ, microinvasive
carcinoma, missing hormone receptor (HR) and/or HER2 receptor
status, and rare histology (atypical carcinoid tumor, sclerosing
sweat duct carcinoma, signet ring cell carcinoma, sarcoma,
myoepithelioma, carcinosarcoma and phyllodes tumor). All patients
provided written informed consent and the study was approved by the
local ethics committee.
Intrinsic breast cancer subtype
Each primary breast cancer tumor was assessed for HR
and HER2 expression by immunohistochemistry (IHC).
Immunohistochemical staining was part of routine diagnostics and
performed according to the American Society of Clinical Oncology
(ASCO)/College of American pathologists (CAP) Clinical Practice
Guidelines (16,17). The results of this staining was taken
from patients' records. HER2-negativity by clinical assay was
defined as IHC 0/1+ or 2+, confirmed by a fluorescence in
situ hybridization (FISH)/chromogenic in situ
hybridization amplification ratio of <2.0. Estrogen receptor
(ER) and/or progesterone receptor (PR) IHC expression of ≥10% was
considered positive. Hormone receptor positivity was defined as ER
or PR were positive. Intrinsic subtypes were assigned as follows:
Luminal subtype, HR+/HER2-; HER2-enriched subtypes, HR±/HER2+; and
TN subtype, HR-/HER2-. The results were assigned according to the
ASCO/CAP guidelines (16,17).
Outcomes
Overall survival (OS) was defined as the time from
the initial breast cancer diagnosis to the final follow-up or
mortality. BMFS was defined as the time from breast cancer
diagnosis to the diagnosis of brain metastases. SFBM was defined as
the time from diagnosis of brain metastases to the date of
mortality or last follow-up.
Statistical analysis
Statistical analysis was performed using IBM SPSS
Statistics version 22 (IBM SPSS, Armonk, NY, USA) and GraphPad
Prism 5 (GraphPad Inc., La Jolla, CA, USA) to create figures.
Categorical data were compared with χ2 test or Fisher's
exact test, as appropriate. Group differences were calculated using
Kruskal-Wallis test for nonparametric data. Survival was estimated
by the Kaplan-Meier method and compared with the log-rank
(Mantel-Cox) test between breast cancer subtypes. Cox regression
analysis was performed to evaluate the hazard ratio and
corresponding 95% confidence interval (95% CI). P≤0.05 was
considered to indicate a statistically significant difference. All
survival times were calculated in days for the purpose of precise
results.
Results
Patient and tumor characteristics
Patient and tumor characteristics are specified in
Table I. Among the 1,147 total
patients, 770 patients (67.13%) had luminal-type, 202 (17.61%) had
HER2-enriched and 175 (15.26%) had TN breast cancer. Among the
group of HER2-enriched tumors, 113 (9.85%) were HR-positive and 89
(7.76%) were HR-negative.
 | Table I.Baseline characteristics of 1,147
breast cancer patients. |
Table I.
Baseline characteristics of 1,147
breast cancer patients.
|
|
| HER2-enriched |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | Luminal | HR+/HER2+ | HR-/HER2+ | TN | P-value |
|---|
| Number of patients (%
of total) | 770 (67.13) | 113 (9.85) | 89 (7.80) | 175 (15.26) | – |
| Age at diagnosis,
years |
|
|
|
| <0.001 |
|
Median | 57 | 53 | 50 | 49 |
|
|
Interquartile range | 47.0–67.0 | 44.5–64.0 | 42.0–61.0 | 38.0–60.0 |
|
| Grade [n (%)] |
|
|
|
| <0.001 |
| G1 | 78 (10.13) | 1 (0.88) | 1 (1.12) | 3 (1.71) |
|
| G2 | 469 (60.91) | 48 (42.48) | 25 (28.09) | 28 (16.00) |
|
| G3 | 199 (25.84) | 59 (52.21) | 58 (65.17) | 132 (75.43) |
|
| G4 | 0 (0.00) | 2 (1.77) | 0 (0.00) | 5 (2.86) |
|
| GX | 24 (3.12) | 3 (2.65) | 5 (5.62) | 7 (4.00) |
|
| Histology [n
(%)] |
|
|
|
| <0.001 |
| Invasive
ductal carcinoma | 596 (77.40) | 104 (92.04) | 81 (91.01) | 145 (82.86) |
|
| Invasive
lobular carcinoma | 118 (15.32) | 6 (5.31) | 2 (2.25) | 3 (1.71) |
|
|
Other | 56 (7.27) | 3 (2.65) | 6 (6.74) | 27 (15.43) |
|
| pT stagea [n (%)] |
|
|
|
| <0.001 |
| pT1 | 397 (51.56) | 49 (43.36) | 27 (30.34) | 63 (36.00) |
|
| pT2 | 208 (27.01) | 29 (25.66) | 28 (31.46) | 52 (29.71) |
|
|
pT3 | 34 (4.42) | 2 (1.77) | 4 (4.49) | 5 (2.86) |
|
|
pT4 | 13 (1.69) | 2 (1.77) | 2 (2.25) | 3 (1.71) |
|
|
Missing/unknown | 118 (15.32) | 31 (27.43) | 28 (31.46) | 52 (29.71) |
|
| pN
stagea [n (%)] |
|
|
|
| <0.001 |
|
pN0 | 402 (52.21) | 39 (34.51) | 25 (28.09) | 72 (41.14) |
|
|
pN1 | 162 (21.04) | 25 (22.12) | 13 (14.61) | 28 (16.00) |
|
|
pN2 | 41 (5.32) | 8 (7.08) | 10 (11.24) | 13 (7.43) |
|
|
pN3 | 38 (4.94) | 8 (7.08) | 11 (12.36) | 7 (4.00) |
|
|
Missing/unknown | 127 (16.49) | 33 (29.20) | 30 (33.71) | 55 (31.43) |
|
| ypT
stagea (n=160) [n
(%)] |
|
|
|
| <0.001 |
|
ypT0 | 6 (3.75) | 3 (1.88) | 6 (3.75) | 17 (10.63) |
|
|
ypTis | 3 (1.88) | 2 (1.25) | 9 (5.63) | 2 (1.25) |
|
|
ypT1 | 28 (17.50) | 10 (6.25) | 7 (4.38) | 12 (7.50) |
|
|
ypT2 | 19 (11.88) | 5 (3.13) | 0 (0.00) | 9 (5.63) |
|
|
ypT3 | 7 (4.38) | 1 0.63) | 0 (0.00) | 1 (0.63) |
|
|
ypT4 | 4 (2.50) | 1 (0.63) | 2 (1.25) | 3 (1.88) |
|
|
Missing/unknown | 1 (0.63) | 0 (0.00) | 1 (0.63) | 1 (0.63) |
|
| ypN
stagea (n=147) [n
(%)] |
|
|
|
| 0.065 |
|
ypN0 | 26 (17.69) | 8 (5.44) | 16 (10.88) | 31 (21.09) |
|
|
ypN1 | 16 (10.88) | 9 (6.12) | 6 (4.08) | 5 (3.40) |
|
|
ypN2 | 13 (8.84) | 3 (2.04) | 1 (0.68) | 3 (2.04) |
|
|
ypN3 | 3 (2.04) | 0 (0.00) | 1 (0.68) | 1 (0.68) |
|
|
Missing/unknown | 3 (2.04) | 0 (0.00) | 1 (0.68) | 1 (0.68) |
|
| Metastases
stagea [n (%)] |
|
|
|
| 0.032 |
| M0 | 716 (92.99) | 98 (86.73) | 77 (86.52) | 160 (91.43) |
|
| M1 | 46 (5.97) | 13 (11.50) | 8 (8.99) | 10 (5.71) |
|
| MX | 8 (1.04) | 2 (1.77) | 4 (4.49) | 5 (2.86) |
|
| Surgery |
|
|
|
| 0.018 |
| No | 29 (3.77) | 5 (4.42) | 1 (1.12) | 3 (1.71) |
|
|
Yes | 738 (95.84) | 105 (92.92) | 88 (98.88) | 172 (98.29) |
|
|
Unknown | 3 (0.39) | 3 (2.65) | 0 (0.00) | 0 (0.00) |
|
| Chemotherapy
(adjuvant/neoadjuvant) [n (%)] |
|
|
|
| <0.001 |
| No | 419 (54.42) | 21 (18.58) | 9 (10.11) | 25 (14.29) |
|
|
Yes | 296 (38.44) | 82 (72.57) | 72 (80.90) | 134 (76.57) |
|
|
Missing/unknown | 55 (7.14) | 10 (8.85) | 8 (8.99) | 16 (9.14) |
|
| Antihormone therapy
[n (%)] |
|
|
|
| <0.001 |
| No | 49 (6.36) | 11 (9.73) | 82 (92.13) | 167 (95.43) |
|
|
Yes | 535 (69.48) | 80 (70.80) | 6 (6.74) | 6 (3.43) |
|
|
Unknown | 186 (24.16) | 22 (19.47) | 1 (1.12) | 2 (1.14) |
|
| Anti-HER2 therapy
[n (%)] |
|
|
|
| <0.001 |
| No | 752 (97.66) | 20 (17.70) | 13 (14.61) | 172 (98.29) |
|
|
Yes | 3 (0.39) | 83 (73.45) | 70 (78.65) | 1 (0.57) |
|
|
Unknown | 15 (1.95) | 10 (8.85) | 6 (6.74) | 2 (1.14) |
|
| Distant metastases
(overall) [n (%)] |
|
|
|
| <0.001 |
| No | 652 (84.68) | 91 (80.53) | 62 (69.66) | 125 (71.43) |
|
|
Yes | 118 (15.32) | 22 (19.47) | 27 (30.34) | 50 (28.57) |
|
| Brain metastases at
breast cancer diagnosis [n (%)] |
|
|
|
| 0.020 |
| No | 768 (99.74) | 110 (97.35) | 88 (98.88) | 172 (98.29) |
|
|
Yes | 2 (0.26) | 3 (2.65) | 1 (1.2) | 3 (1.71) |
|
| Brain metastases
(overall) [n (%)] |
|
|
|
| <0.001 |
| No | 758 (98.44) | 106 (93.81) | 76 (85.39) | 153 (87.43) |
|
|
Yes | 12 (1.56) | 7 (6.19) | 13 (14.61) | 22 (12.57) |
|
Distant metastases were found in 77 of the 1,147
patients (6.71%) at the time of diagnosis of breast cancer; in
total, 217 patients (18.92%) developed distant metastases during
the course of the disease. There were 54 patients (4.71%) who
developed brain metastases, including 9 (11.69%) who already had
brain metastases at the time of initial breast cancer diagnosis.
Among those with brain metastases, 12 patients (1.56%) had luminal,
20 (9.90%) had HER2-enriched and 22 (12.57%) had TN primary breast
cancer (P<0.001). The number and the treatment of brain
metastases among these patients are shown in Table II. Between the various intrinsic
subtypes of breast cancer, there were no significant differences in
the number of brain metastases or the type of treatment, with the
exception of antihormone therapy.
 | Table II.Number of brain metastases and
treatment within the subgroup of 54 breast cancer patients who
developed brain metastases. |
Table II.
Number of brain metastases and
treatment within the subgroup of 54 breast cancer patients who
developed brain metastases.
|
|
| HER2-enriched |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | Luminal | HR+/HER2+ | HR-/HER2+ | TN | P-value |
|---|
| Number of patients
(% of total) | 12 (22.22) | 7 (12.96) | 13 (24.07) | 22 (40.74) | – |
| Number of brain
metastases [n (%)] |
|
|
|
| 0.395 |
| 1 | 1 (8.33) | 3 (42.86) | 3 (23.08) | 4 (18.18) |
|
| ≥2 | 5 (41.67) | 3 (42.86) | 8 (61.54) | 17 (77.27) |
|
|
Missing/unknown | 3 (25.00) | 1 (14.29) | 2 (15.38) | 1 (4.55) |
|
| Treatment of brain
metastases [n (%)] |
|
|
|
| 0.664 |
|
Surgery |
|
|
|
|
|
|
No | 7 (58.33) | 2 (28.57) | 6 (46.15) | 12 (54.55) |
|
|
Yes | 5 (41.67) | 5 (71.43) | 6 (46.15) | 8 (36.36) |
|
|
Unknown | 0 (0.00) | 0 (0.00) | 1 (7.69) | 2 (9.09) |
|
| Radiotherapy |
|
|
|
| 0.350 |
| No | 5 (41.67) | 2 (28.57) | 2 (15.38) | 2 (9.09) |
|
|
Yes | 7 (58.33) | 5 (71.43) | 10 (76.92) | 18 (81.82) |
|
|
Unknown | 0 (0.00) | 0 (0.00) | 1 (7.69) | 2 (9.09) |
|
| Chemotherapy |
|
|
|
| 0.346 |
| No | 11 (91.67) | 4 57.14) | 7 (53.85) | 13 (59.09) |
|
|
Yes | 1 (8.33) | 3 (42.86) | 4 (30.77) | 7 (31.82) |
|
|
Unknown | 0 (0.00) | 0 (0.00) | 2 (15.38) | 2 (9.09) |
|
| Antihormone
therapy |
|
|
|
| 0.021 |
| No | 9 (75.00) | 4 (57.14) | 12 (92.31) | 20 (90.91) |
|
|
Yes | 3 (25.00) | 3 (42.86) | 0 (0.00) | 0 (0.00) |
|
|
Unknown | 0 (0.00) | 0 (0.00) | 1 (7.69) | 2 (9.09) |
|
| Immunotherapy |
|
|
|
| 0.217 |
| No | 12 (100.0) | 5 (71.43) | 9 (69.23) | 19 (86.36) |
|
|
Yes | 0 (0.00) | 2 (28.57) | 3 (23.08) | 1 (4.55) |
|
|
Unknown | 0 (0.00) | 0 (0.00) | 1 (7.69) | 2 (9.09) |
|
Overall survival in the entire study
population
The OS time in the entire study cohort [n=1,147;
median, 1,376 days (46 months)] differed significantly according to
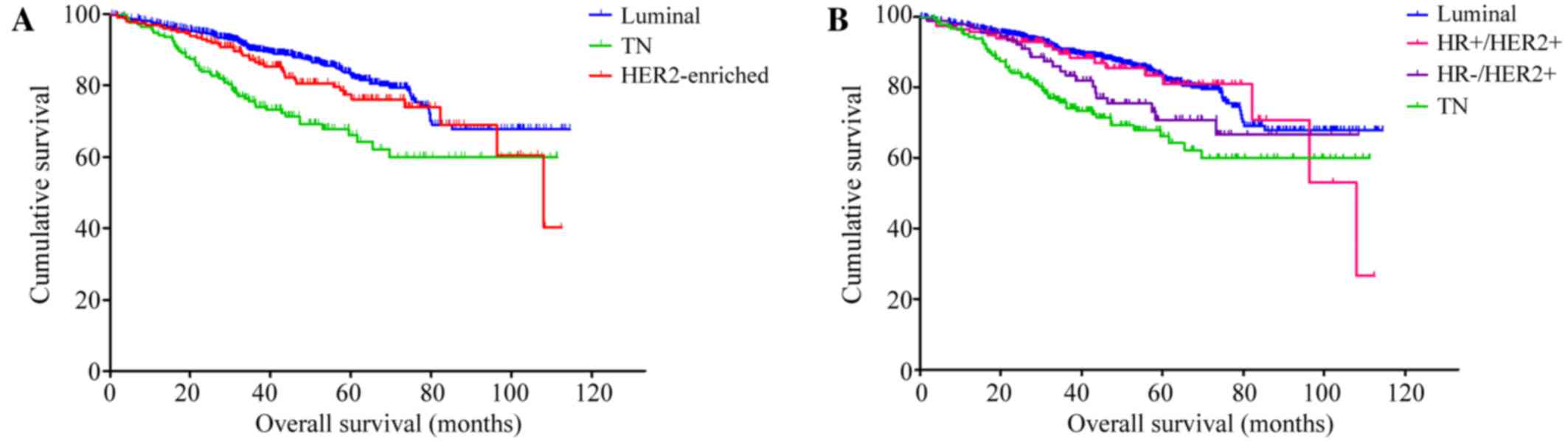
breast cancer subtype (P<0.001; Fig.
1A). Patients with TN breast cancer had a significantly shorter
OS than patients with luminal breast cancer (hazard ratio, 2.20;
95% CI, 1.59–3.04; P<0.001) and patients with HER2-enriched
tumors (hazard ratio, 1.66; 95% CI, 1.11–2.50; P=0.015). There was
no significant difference between the luminal and HER2-positive
breast cancer subtypes (hazard ratio, 1.32; 95% CI, 0.93–1.89;
P=0.123). Of the 202 patients with HER2-positive breast cancer, 153
(75.74%) received anti-HER2 therapy whereas 33 patients (16.34%)
did not; in the remaining 16 cases (7.92%), this information was
not available.
It is well-recognized that HER2-positive tumors are
of a heterogeneous nature (18).
Therefore, a comparison was also performed after dividing the
patients into four distinct subgroups: Luminal, HR+/HER2+,
HR-/HER2+ and TN subtypes. From this analysis, significant
differences in OS were detected (P<0.001; Fig. 1B): Patients with HR-/HER2+ cancer had
a significantly reduced OS compared with those with luminal breast
cancer (P=0.049; hazard ratio, 1.58; 95% CI, 1.00–2.49); and
patients with TN cancer had a significantly poorer OS compared with
those with luminal (P<0.001; hazard ratio, 2.20; 95% CI,
1.59–3.04) and those with HR+/HER2+ cancer (P=0.011; hazard ratio,
1.97; 95% CI, 1.17–3.33).
Survival outcomes in patients with
brain metastases
Among the 54 patients who developed brain
metastases, the median BMFS was 600 days (20 months) (95% CI,
379.15–820.85 days) and differed significantly by breast cancer
subtype. The median BMFS was 1,003 days (33 months) (95% CI,
840.05–1,165.95 days) in luminal, 514 days (17 months) (95% CI,
283.91–744.09 days) in HER2-enriched and 460 days (15 months) (95%
CI, 154.33–765.67 days) in TN breast cancer patients (P=0.045;
Fig. 2). In addition, slight
differences in BMFS were observed when comparing the four distinct
breast cancer subtypes (P=0.069). Patients with HER2-positive
breast cancer demonstrated a significantly shorter BMFS compared
with patients with the luminal subtype (hazard ratio, 2.62; 95% CI,
1.19–5.77; P=0.017), irrespectively of whether anti-HER2 therapy
was received.
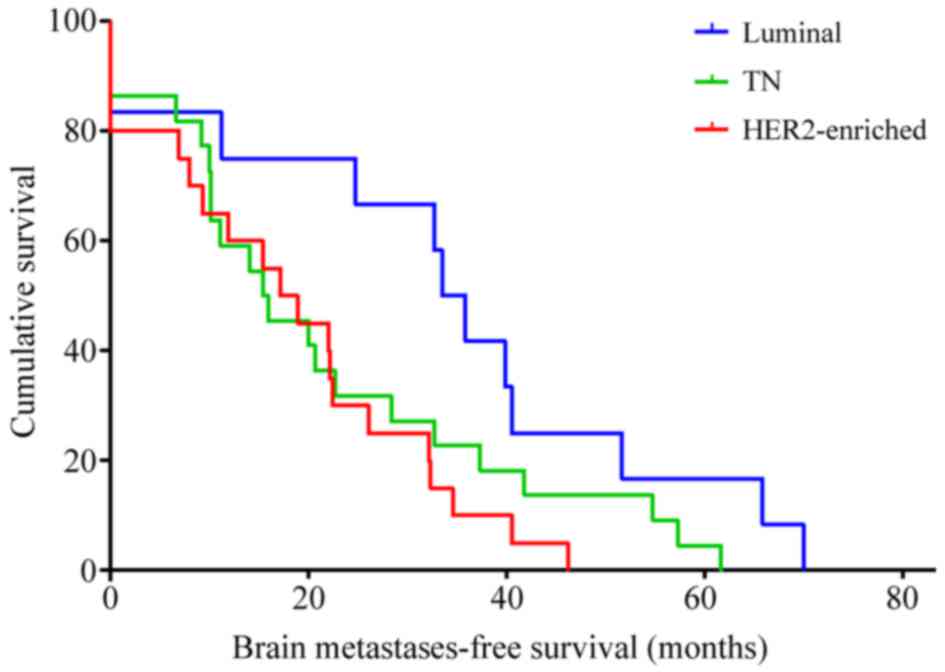 | Figure 2.Brain metastases-free survival of
breast cancer patients by subtype estimated by Kaplan-Meier method
(n=54): Luminal [median, 1,003 days (33 months); 95% CI,
840.05–1,165.95 days], HER2-enriched [median, 514 days (17 months);
95% CI, 283.91–744.09 days], TN [median, 460 days (15 months); 95%
CI, 154.33–765.67 days]. CI, confidence interval; HER2, human
epidermal growth factor receptor 2; TN, triple-negative. |
The median duration of SFBM was 246 days (8 months)
(95% CI, 128.65–363.35 days) and this differed significantly among
the subtypes (P=0.029; Fig. 3A): The
median duration of SFBM was 386 days (13 months) (95% CI,
0.00–914.26 days) in luminal, 310 days (10 months) (95% CI,
0.00–658.19 days) in HER2-enriched and 147 days (5 months) (95% CI,
109.64–184.36 days) in TN breast cancer patients.
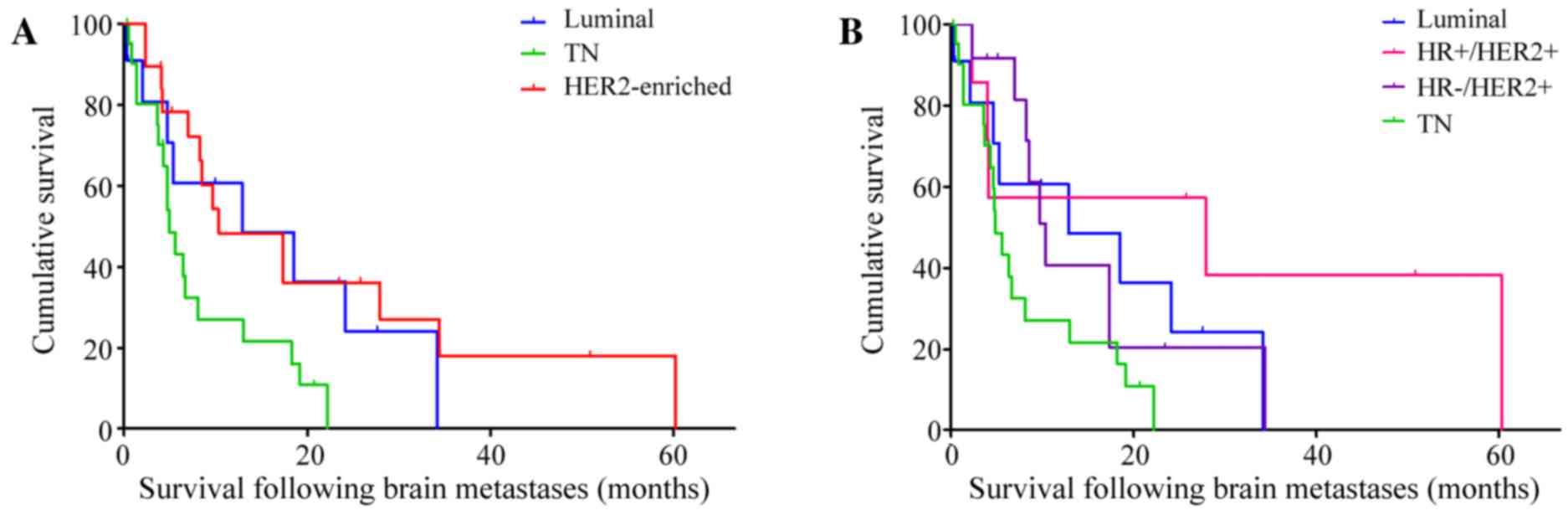 | Figure 3.Survival following brain metastases of
breast cancer patients (n=54) by subtype, estimated by Kaplan-Meier
method. (A) Survival according to luminal (median, 13 months; 95%
CI, 0.00–914.26 days), HER2-enriched (median, 10 months; 95% CI,
0.00–658.19 days) and TN (median, 5 months; 95% CI, 109.64–184.36
days) subtypes. (B) Survival according to luminal (median, 13
months; 95% CI, 0.00–914.26 days), HR+/HER2+ (median, 28 months;
95% CI, 0.00–2,301.57 days), HR-/HER2+ (median, 10 months; 95% CI,
227.49–392.51 days), TN (median, 5 months; 95% CI, 109.64–184.36
days). CI, confidence interval; HER2, human epidermal growth factor
receptor 2; TN, triple-negative; HR, hormone receptor. |
With regard to luminal, HR+/HER2+, HR-/HER2+ and TN
breast cancer subtypes, the median durations of SFBM were 386 days
(13 months) (95% CI, 0.00–914.26 days), 837 days (28 months) (95%
CI, 0.00–2,301.57 days), 310 days (10 months) (95% CI,
227.49–392.51 days) and 147 days (5 months) (95% CI, 109.64–184.36
days), respectively (P=0.042; Fig.
3B). Patients with TN cancer had a significantly shorter SFBM
compared with that of HER2-positive patients (P=0.013; hazard
ratio, 2.66; 95% CI, 1.23–5.73), particularly those with the
HR+/HER2+ subtype (P=0.013; hazard ratio, 4.44; 95% CI,
1.36–14.49).
OS in the 54 patients with brain metastases did not
differ significantly between breast cancer subtypes (P=0.180). The
median OS times were 1,282 days (43 months) (95% CI,
817.03–1,746.97 days) in patients with HER2-enriched breast cancer,
664 days (22 months) (95% CI, 338.79–989.21 days) in patients with
TN breast cancer and 1,690 days (56 months) (95% CI,
1,038.21–2,341.79 days) in patients with luminal breast cancer.
Discussion
The incidence of brain metastasis detection in
breast cancer is increasing due to advances in imaging technologies
and the introduction of novel therapies resulting in longer
survival times (9,19). In-depth understanding of the natural
history of brain metastases can aid in the optimization of
treatment and follow-up strategies.
It is accepted that the risk of metastasis and the
survival times vary significantly among breast cancer subtypes,
which was confirmed in the current single-institution cohort study.
Patients with TN breast cancer had a significantly decreased OS
compared with those with luminal or HER2-positive breast cancer
subtypes. However, no significant difference in OS was identified
between luminal and HER2-positive breast cancer, which is most
likely attributable to the fact that the majority of HER2-positive
patients that were included in this study received HER2-targeted
treatment.
Due to the limited number of patients in the current
study, OS did not significantly differ among patients with brain
metastases with different intrinsic subtypes. However numerically,
OS was longest in patients with luminal breast cancer [1,690 days
(56 months)] compared with patients with HER2-enriched [1,282 days
(43 months)] and TN cancers [664 days (22 months)]. These
differences may be explained, in part, by the differences in BMFS;
metastases of luminal breast cancer occur rather late in the course
of the disease (4,20). In fact, it was demonstrated that BMFS
varies significantly between breast cancer subtypes, with luminal
breast cancer patients showing the most favorable outcome. The
median BMFS was 33 months in luminal compared to 17 months in
HER2-enriched and 15 months in TN breast cancer patients
(P=0.045).
SFBM significantly differed in the current study
cohort (P=0.042). TN patients had the poorest survival time (5
months) compared with luminal (13 months), HR+/HER2+ (28 months)
and HR-/HER2+ (10 months) tumors, respectively. These findings are
consistent with previous reports demonstrating that the median
length of SFBM is <6 months in patients with TN breast cancers
(21–23). This indicates that treatment
strategies for TN patients with brain metastases should be
carefully selected and should acknowledge the limited prognosis. By
contrast, SFBM was doubled in HER2-enriched cancer cases (10
months) compared with TN breast cancers (5 months), despite similar
BMFS times; this may reflect the high efficiency of HER2-targeted
treatment strategies (21,24).
With regard to brain metastases in cases of the
luminal subtype, data varies among studies; certain authors have
reported a median SFBM similar to that of TN patients (22), speculating that the lack of further
treatment options later in the course of the disease could explain
the poor prognosis. By contrast, the present data and that of
Niwińska et al (23)
demonstrated median SFBMs in luminal tumors of 13 months and 15
months, respectively. In the present study, the survival time of
this subgroup was longer than that of patients with TN or HR-/HER2+
breast cancer.
In the past, HER2-positive breast cancer has been
considered as a single disease entity. However, there is mounting
evidence to suggest that HER2-positive breast cancers are
clinically and biologically heterogeneous (18). This is recognized by the St. Gallen's
criteria, which divide HER2-positive disease into two groups:
ER+/HER2+ and ER-/HER2+ (25). In the
present study, ~75% of the patients with HER2-positive breast
cancer received HER2-targeted treatment. The OS in HR-/HER2+
patients was significantly shorter compared with that of patients
with luminal breast cancer (P=0.049; hazard ratio, 1.58; 95% CI,
1.00–2.49). By contrast, the OS of HR+/HER2+ patients was
comparable to that of luminal breast cancer. These findings are in
line with previous studies, which have shown that adjuvant
treatment with trastuzumab is associated with a 40% increase in
disease-free survival and OS times in HR+/HER2+ cancers as compared
with HR-/HER2+ cancers (26,27).
There are several limitations of the present study.
All patients included in this retrospective analysis were treated
at a single institution between 2004–2010, and only 54 patients
with brain metastases met all inclusion and exclusion criteria of
the study. Therefore, subgroup analysis must be interpreted with
caution. Due to the small and varying subgroup sizes of the
patients with brain metastases, a distinct multivariate analysis
was not appropriate. In addition, immunohistochemical staining and
FISH analysis were used to define subtypes of breast cancer, rather
than gene expression analysis. However, considerable efforts have
been made to ensure the high-quality of immunohistochemical
analysis of steroid hormone receptors and HER2 status (28). Despite these efforts, immunostaining
remains only a surrogate marker of intrinsic molecular breast
cancer subtypes. Furthermore, since brain metastases tissue was not
available for all cases, biological discordance between the primary
breast cancer and the brain metastases cannot be excluded.
In conclusion, the prognosis of breast cancer
subtypes varies significantly in patients with brain metastases.
This could have important implications for treatment and follow-up
strategies. Patients with luminal breast cancer have a low risk of
developing brain metastases per se, and symptom-based
clinical follow-up seems appropriate. Patients with HER2-positive
or TN breast cancer have a significantly higher risk of developing
brain metastases. Compared to TN breast cancer, the survival times
of metastatic HER2-positive breast cancer have improved
significantly over the past years due to the availability of novel
powerful HER2-directed drugs (19,29).
Therefore this subgroup of patients may benefit from closer
clinical and imaging follow-up examinations.
References
|
1
|
Lassman AB and DeAngelis LM: Brain
metastases. Neurol Clin. 21:1–23, vii. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stark AM: Neurosurgical treatment of
breast cancer metastases to the neurocranium. Patholog Res Int.
2011:5498472010.PubMed/NCBI
|
|
3
|
Sihto H, Lundin J, Lundin M, Lehtimäki T,
Ristimäki A, Holli K, Sailas L, Kataja V, Turpeenniemi-Hujanen T,
Isola J, et al: Breast cancer biological subtypes and protein
expression predict for the preferential distant metastasis sites: A
nationwide cohort study. Breast Cancer Res. 13:R872011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weil RJ, Palmieri DC, Bronder JL, Stark AM
and Steeg PS: Breast cancer metastasis to the central nervous
system. Am J Pathol. 167:913–920. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heitz F, Rochon J, Harter P, Lueck HJ,
Fisseler-Eckhoff A, Barinoff J, Traut A, Lorenz-Salehi F and du
Bois A: Cerebral metastases in metastatic breast cancer:
Disease-specific risk factors and survival. Ann Oncol.
22:1571–1581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miller KD, Weathers T, Haney LG, Timmerman
R, Dickler M, Shen J and Sledge GW Jr: Occult central nervous
system involvement in patients with metastatic breast cancer:
Prevalence, predictive factors and impact on overall survival. Ann
Oncol. 14:1072–1077. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heitz F, Harter P, Lueck HJ,
Fissler-Eckhoff A, Lorenz-Salehi F, Scheil-Bertram S, Traut A and
du Bois A: Triple-negative and HER2-overexpressing breast cancers
exhibit an elevated risk and an earlier occurrence of cerebral
metastases. Eur J Cancer. 45:2792–2798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pestalozzi BC, Zahrieh D, Price KN,
Holmberg SB, Lindtner J, Collins J, Crivellari D, Fey MF, Murray E,
Pagani O, et al: Identifying breast cancer patients at risk for
central nervous system (CNS) metastases in trials of the
international breast cancer study group (IBCSG). Ann Oncol.
17:935–944. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niikura N, Saji S, Tokuda Y and Iwata H:
Brain metastases in breast cancer. Jpn J Clin Oncol. 44:1133–1140.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
DiStefano A, Yap Y Yong, Hortobagyi GN and
Blumenschein GR: The natural history of breast cancer patients with
brain metastases. Cancer. 44:1913–1918. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsukada Y, Fouad A, Pickren JW and Lane
WW: Central nervous system metastasis from breast carcinoma.
Autopsy study. Cancer. 52:2349–2354. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng X and Hung MC: Breast cancer brain
metastases. Cancer Metastasis Rec. 26:635–643. 2007. View Article : Google Scholar
|
|
13
|
Leone JP and Leone BA: Breast cancer brain
metastases: The last frontier. Exp Hematol Oncol. 4:332015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kennecke H, Yerushalmi R, Woods R, Cheang
MC, Voduc D, Speers CH, Nielsen TO and Gelmon K: Metastatic
behavior of breast cancer subtypes. J Clin Oncol. 28:3271–3277.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palmieri D, Bronder JL, Herring JM, Yoneda
T, Weil RJ, Stark AM, Kurek R, Vega-Valle E, Feigenbaum L,
Halverson D, et al: Her-2 overexpression increases the metastatic
outgrowth of breast cancer cells in the brain. Cancer Res.
67:4190–4198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American society of
clinical oncology/college of American pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hammond ME, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American society of clinical oncology/college of
American pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. J Clin Oncol. 28:2784–2795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Blows FM, Driver KE, Schmidt MK, Broeks A,
van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO,
Blomqvist C, et al: Subtyping of breast cancer by
immunohistochemistry to investigate a relationship between subtype
and short and long term survival: A collaborative analysis of data
for 10,159 cases from 12 studies. PLoS Med. 7:e10002792010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rostami R, Mittal S, Rostami P, Tavassoli
F and Jabbari B: Brain metastasis in breast cancer: A comprehensive
literature review. J Neurooncol. 127:407–414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Berghoff A, Bago-Horvath Z, De Vries C,
Dubsky P, Pluschnig U, Rudas M, Rottenfusser A, Knauer M, Eiter H,
Fitzal F, et al: Brain metastases free survival differs between
breast cancer subtypes. Br J Cancer. 106:440–446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anders CK, Deal AM, Miller CR, Khorram C,
Meng H, Burrows E, Livasy C, Fritchie K, Ewend MG, Perou CM and
Carey LA: The prognostic contribution of clinical breast cancer
subtype, age and race among patients with breast cancer brain
metastases. Cancer. 117:1602–1611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nam BH, Kim SY, Han HS, Kwon Y, Lee KS,
Kim TH and Ro J: Breast cancer subtypes and survival in patients
with brain metastases. Breast Cancer Res. 10:R202008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niwińska A, Murawska M and Pogoda K:
Breast cancer brain metastases: Differences in survival depending
on biological subtype, RPA RTOG prognostic class and systemic
treatment after whole-brain radiotherapy (WBRT). Ann Oncol.
21:942–948. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park YH, Park MJ, Ji SH, Yi SY, Lim DH,
Nam DH, Lee JI, Park W, Choi DH and Huh SJ: Trastuzumab treatment
improves brain metastasis outcomes through control and durable
prolongation of systemic extracranial disease in
HER2-overexpressing breast cancer patients. Br J Cancer.
100:894–900. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ: Panel members: Strategies for
subtypes-dealing with the diversity of breast cancer: Highlights of
the St Gallen international expert consensus on the primary therapy
of early breast cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Perez EA, Romond EH, Suman VJ, Jeong JH,
Davidson NE, Geyer CE Jr, Martino S, Mamounas EP, Kaufman PA and
Wolmark N: Four-year follow-up of trastuzumab plus adjuvant
chemotherapy for operable human epidermal growth factor receptor
2-positive breast cancer: Joint analysis of data from NCCTG N9831
and NSABP B-31. J Clin Oncol. 29:3366–3373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vaz-Luis I, Ottesen RA, Hughes ME, Marcom
PK, Moy B, Rugo HS, Theriault RL, Wilson J, Niland JC, Weeks JC and
Lin NU: Impact of hormone receptor status on patterns of recurrence
and clinical outcomes among patients with human epidermal growth
factor-2-positive breast cancer in the national comprehensive
cancer network: A prospective cohort study. Breast Cancer Res.
14:R1292012. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wasielewski Rv, Hasselmann S, Rüschoff J,
Fisseler-Eckhoff A and Kreipe H: Proficiency testing of
immunohistochemical biomarker assays in breast cancer. Virchows
Arch. 453:537–543. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yao H, He G, Yan S, Chen C, Song L, Rosol
TJ and Deng X: Triple-negative breast cancer: Is there a treatment
on the horizon? Oncotarget. Sep 27–2016.(Epub ahead of print). doi:
10.18632/oncotarget.1.2284.
|
|
30
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumours, UICC, International
Union against Cancer. 7th. Wiley-Blackwell; 2010
|