Introduction
Prostate cancer (PCa) is the second most prevalent
cause of cancer-associated mortality globally (1). The incidence and mortality of PCa has
continually increased within the past decade in China, but remains
low compared with Western countries (2). The incidence of PCa in China is
predicted to increase further due to diet and lifestyle changes and
the aging population (3). Transrectal
systematic biopsy is the standard procedure for the detection of
PCa. The procedure is invasive and causes discomfort for patients;
however, 18 to 47% of cases of PCa may not be detected by this
method, whereas a number of clinically insignificant alterations to
the prostate may be misdiagnosed as PCa following detection by
systematic biopsy (4–7). Therefore, novel methods for the
effective and safe detection of clinically significant PCa are
required.
PCa tissues may exhibit increased stiffness due to
pathological alterations (8). Tissue
elasticity has potential as a novel diagnostic factor for PCa
(9). Real-time tissue elastography
(RTE) is a sonoelastography approach that uses colors to visualize
the variations in tissue elasticity or stiffness. In the diagnosis
of PCa, the sensitivity and specificity of RTE-targeted biopsy
varies from 51.1 to 91.7% and from 62.2 to 86.8%, respectively
(10–14). This is as the majority of previous
studies use the qualitative threshold ‘blue area’ for diagnosis,
which results in variability between the inter- and intra-observer.
However, RTE has not been quantitatively analyzed in targeted
biopsies for the detection of PCa.
Strain index is a quantitative parameter for
comparing the strain value of two tissues during histological
analysis. Zhang et al (15)
used the peak strain index for classifying benign and suspicious
malignant lesions in the peripheral zone of the prostate and
yielded higher sensitivity (74.5%) and specificity (83.3%).
However, whether peak strain index may aid the diagnosis of
clinically significant PCa has yet to be elucidated.
In the present study, the optimal peak strain index
in RTE-targeted biopsies was defined for the detection of PCa in
Chinese patients, and it was identified that RTE-targeted biopsy
coupled with the peak strain index may improve the detection rate
of clinically significant peripheral zone PCa.
Materials and methods
Patients with PCa
Between February 2011 and September 2013, patients
with lower urinary tract symptoms were examined for their serum
prostate specific antigen (PSA) prior to undergoing a digital
rectal examination (DRE) and a transrectal ultrasound (TRUS) at The
Second Affiliated Hospital of Soochow University (Jiangsu, China).
Patients presenting with an active urinary tract infection or acute
urinary retention were excluded from the present study. The
following criteria were used to determine the need for a prostate
biopsy: a) APSA value of ≥10 ng/ml; b) a PSA value of between 4 and
10 ng/ml, and a free-to-total PSA of <16%; c) DRE or TRUS
indicated a prostate nodule. The present study was approved by the
Institutional Ethics Committee of The Second Affiliated Hospital of
Soochow University and all patients provided written informed
consent prior to being enrolled onto the study.
RTE targeted biopsy
A Hitachi EUB-7500HV ultrasound system with a
EUP-V53W 7.5-MHz transrectal end-fire probe (Hitachi, Ltd., Tokyo,
Japan) was used in the RTE mode. The patient was in the left
decubitus position and elastograms were produced by manual
compression from the transverse plane and displayed with TRUS
images. The pressure and speed induced by manual compression was
adjusted by a visual indicator designed to decrease the
inter-observer variability. The strain of tissue was classified as
soft, moderate and hard according to the colors displayed, in which
red signifies high strain (soft), green indicates moderate strain
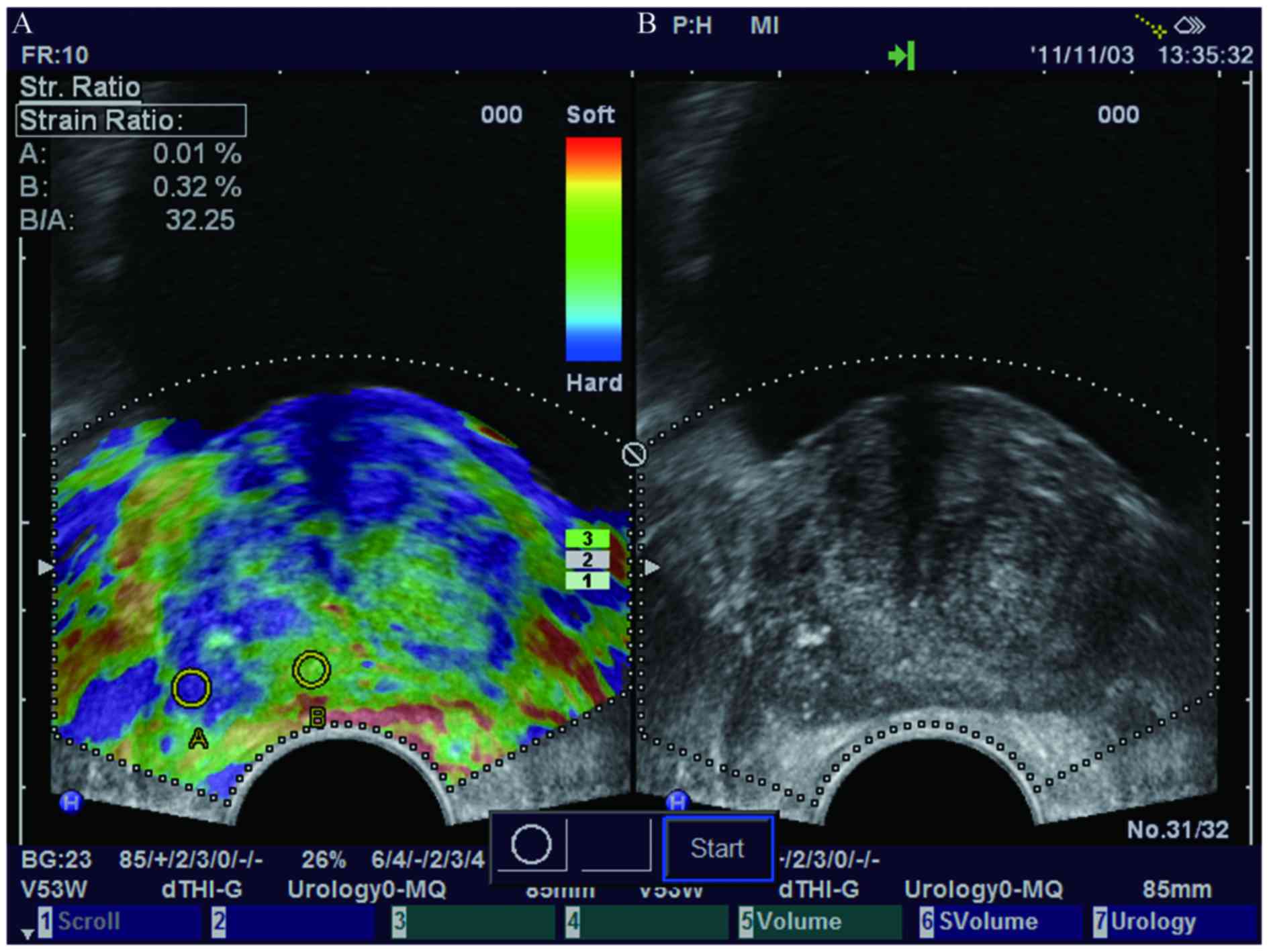
and blue indicates low strain (hard; Fig.
1). Hard lesions that present as blue areas in elastograms were
considered to be potential malignant lesions (16). Stable and reproducible elastograms
were recorded for further analysis. Regions with calcifications in
the prostate are stiff and may affect the elastogram results.
However, they are hyperechoic on the TRUS image and were able to be
identified and avoided during the biopsy (Fig. 1).
The quantitative parameter peak strain index was
calculated using the following formula: Strain ratio (SR) of the
surrounding reference tissue (B) that exhibited moderate elasticity
(green area) to SR of the peak elasticity (area with the highest
level of blue) region (A) (SRB/SRA). The
smallest size of the region of interest (ROI) in the RTE mode was
determined as the standard. A number of sections in the most
intense blue areas were measured to determine the highest
outstanding peak elasticity.
Two cores were obtained from the hardest area with
an 18-gauge biopsy needle. All examinations and targeted biopsies
were performed by a single examiner who was blind to the results of
the PSA test and other modalities.
Systematic biopsy
Following the RTE-targeted biopsy, a 10-core
systematic biopsy that was independent of the RTE and TRUS findings
was performed by a different examiner (The Second Affiliated
Hospital of Soochow University). A MyLab™90 ultrasound
system with an EC-123 7.5-MHz transrectal end-fire probe
(EsaoteSpA, Genova, Italy) was used in TRUS mode. The 10 cores
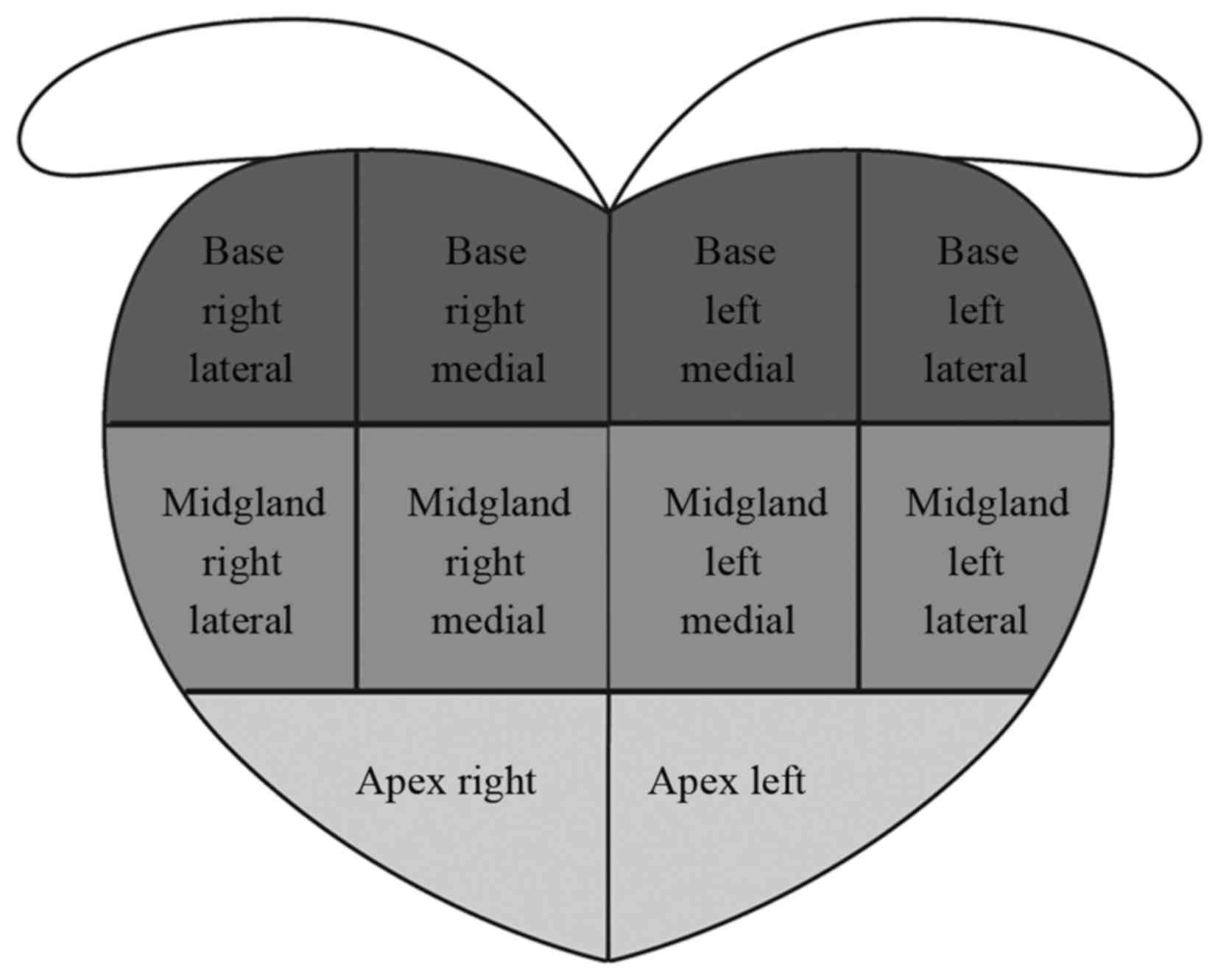
included 3 lateral and 2 medial cores in the left and right sides
(Fig. 2). All the cores were guided
by six dorsal gland sectors: Apex, middle and basement on the left
and right sides. The inner gland analysis was not included in the
results. The average time of RTE examination and targeted biopsy
for each patient was ~10 min.
Pathologic analysis
All cores were marked by identification numbers and
analyzed by a senior pathologist (Wuxi Affliated Hospital of
Nanjing University of Chinese Medicine) who was blind to the
results of the RTE and TRUS.
Statistical analysis
Peak strain index comparisons between malignant and
benign lesions were analyzed by the student's t-test or Wilcoxon
rank sum test. The diagnostic values of peak strain index and PSA
were assessed by receiver operating characteristic (ROC) curves.
Areas under the ROC curve (AUC) values between the peak strain
index and PSA were compared using a χ2 test. To evaluate
the significance of the differences between targeted biopsy and
systematic biopsy, McNemar's test was used. The sensitivities of
cancer detection for targeted biopsy, systematic biopsy and
targeted combined systematic biopsy were compared using a
χ2 test. The association between peak strain index and
Gleason scores was compared with Spearman correlation analysis. To
compare Gleason scores, the Wilcoxon rank sum test was performed.
Values are presented as the mean ± standard deviation. All
statistical calculations were performed with SAS software version
9.3 (SAS Institute Inc., Cary, NC, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
PSA and peak strain index value
A total of 141 patients were enrolled for
prospective analysis. The average age was 71.6 years (range,
49–90), the mean PSA value was 30 ng/ml (range, 0.5–190) and the
average prostate volume was 50.3 ml (range, 15.8–178.5). According
to the pathological results, PCa was detected in 51% (72/141)
patients. Patient characteristics, including age and PSA values,
are summarized in Table I. The age
and prostate volume in each group were similar, whereas the PSA
values in the malignant group were significantly higher compared
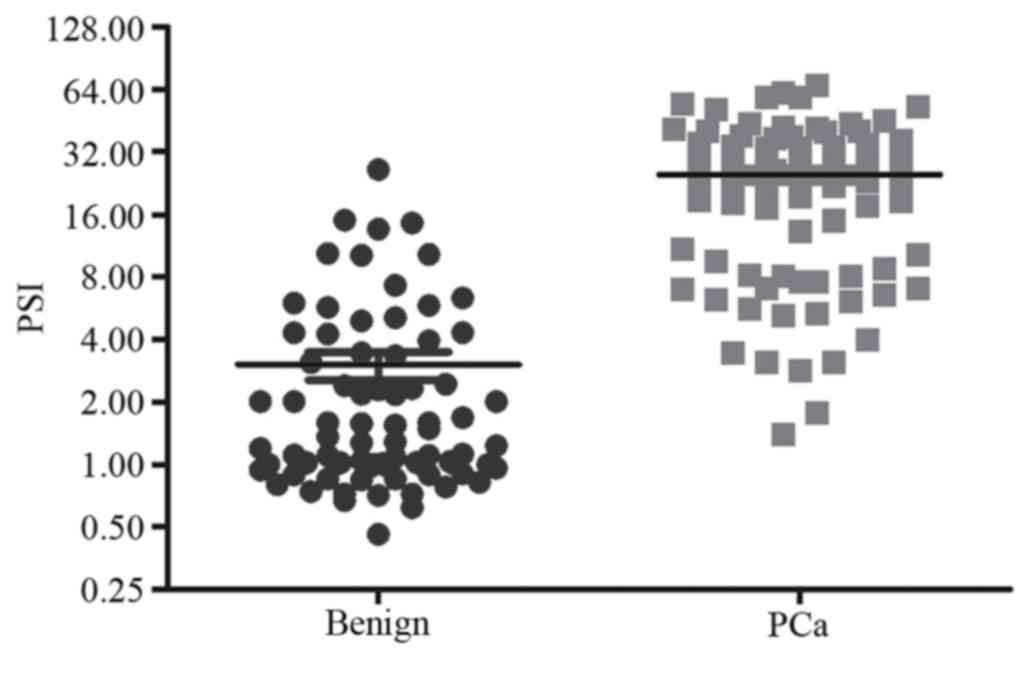
with the benign group (P<0.0001). The ranges of the peak strain
index value in malignant and benign lesions of the prostate were
1.39–66.86 and 0.46–26.31 (mean, 24.79 and 3.02, respectively;
P<0.0001; Fig. 3).
 | Table I.Characteristics of patients with
benign or malignant prostate lesions. |
Table I.
Characteristics of patients with
benign or malignant prostate lesions.
| Characteristic | Benign mean
(range) | Malignant mean
(range) | Overall mean
(range) | P-value |
|---|
| Number of
patients | 69 | 72 | 141 |
|
| Age, years | 70.50 (55–85) | 72.60 (49–90) | 71.60 (49–90) | 0.1422 |
| PSA, ng/ml | 10.40
(0.5–47.6) | 48.80
(1.1–190) | 30.00
(0.5–190) | <0.0001 |
| Prostate volume,
ml | 51.20
(24.7–178.5) | 49.40
(15.8–171) | 50.30
(15.8–178.5) | 0.1996 |
| Peak strain
index | 3.02
(0.5–26.3) | 24.79
(1.39–66.9) | 14.00
(0.5–66.9) | <0.0001 |
Characterization of biopsy cores
In 141 patients, 159 suspicious are as detected by
RTE were biopsied with 2 cores for each area. The positive
incidence of PCa in RTE-targeted biopsy cores was 44% (140/318
cores) and in systematic biopsy was 30.2% (426/1,410 cores). This
indicated that the RTE targeted biopsy core had a significantly
higher sensitively for detecting PCa (P<0.0001).
The majority of the positive cores in RTE-targeted
biopsy were identified in the apex and mid-gland (84% of positive
cores). Regarding the apex and mid-gland of the prostate, a higher
frequency of positive PCa cores were detected in the right side of
the prostate gland. However, using systematic biopsy, an increased
number of positive PCa cores were identified in the middle and base
of the gland. The distributions of PCa positive cores in the right
or left side were similar in these approaches (Table II).
 | Table II.Number of PCa cores detected by RTE
targeted biopsy and systematic biopsy. |
Table II.
Number of PCa cores detected by RTE
targeted biopsy and systematic biopsy.
|
| RTE targeted
biopsy | Systematic
biopsy |
|---|
|
|
|
|
|---|
| Core section | Right | Left | Overall (%) | Right | Left | Overall (%) |
|---|
| Apex | 38 | 20 | 58 (41) | 44 | 40 | 84 (20) |
| Midgland | 38 | 22 | 60 (43) | 92 | 87 | 179 (42) |
| Base | 10 | 12 | 22 (16) | 85 | 78 | 163 (38) |
| Total | 86 | 54 | 140 | 221 | 205 | 426 |
Detection of PCa inpatients using
RTE-targeted biopsy and systematic biopsy
Among the 72 patients diagnosed with PCa, 63 cases
(87.5%) were detected using RTE-targeted biopsy, 62 cases (86.1%)
using systematic biopsy and 53 cases (74%) of PCa were detected by
RTE-targeted and systematic biopsy. A total of 10 patients with PCa
were detected using RTE-targeted biopsy alone and 9 patients using
systematic biopsy alone. The sensitivity for cancer detection was
87.5% for RTE-targeted biopsy and 86.1% for systematic biopsy
(P=0.525).
Optimal peak strain index value for
the RTE-targeted biopsy
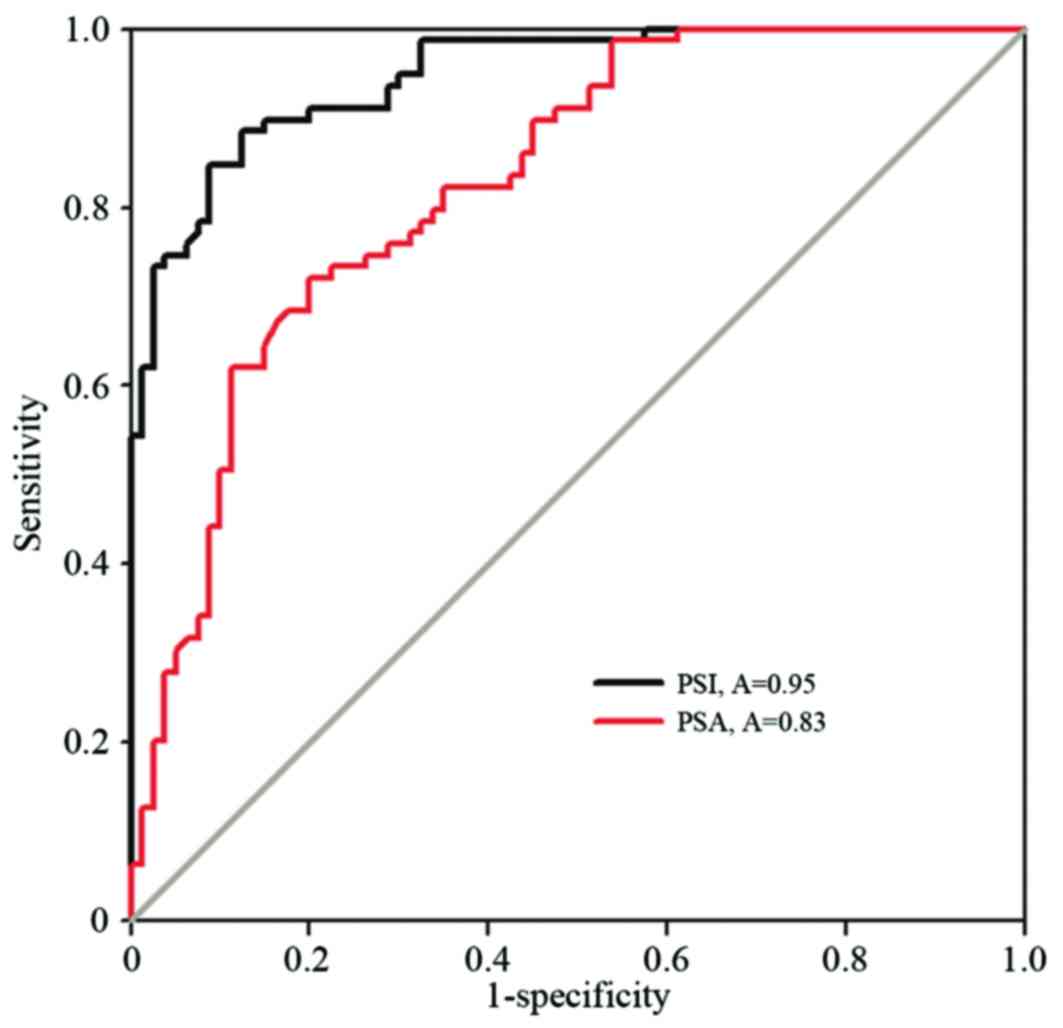
The higher peak strain index values demonstrated a
higher sensitivity and specificity for predicting PCa. When the
peak strain index was >5.97[AUC=0.95; 95% confidence interval
(CI), 0.92–0.98], PCa was predicted with the highest sensitivity
(87.5%; 63/72 cases) and specificity (85.5%; 53/69 cases). When the
PSA was >10.1 ng/ml (AUC=0.83; 95% CI, 0.76–0.89), the
sensitivity and the specificity for detecting PCa were 80% and
72.2%, respectively (Fig. 4).
RTE-targeted biopsy did not diagnose 9 patients with
PCa that had a lower peak strain index value (<5.97). The
majority of these 9 patients had multifocal and diffuse lesions in
the prostate with a lower PSA value, lower Gleason score and were
at an earlier clinical stage (Table
III).
 | Table III.Number of patients in each peak
strain group and PSA levels, Gleason score and clinical stage. |
Table III.
Number of patients in each peak
strain group and PSA levels, Gleason score and clinical stage.
|
|
| PSA, ng/ml | Gleason score | Clinical stage |
|---|
|
|
|
|
|
|
|---|
|
| Overall | <10 | 10–20 | >20 | ≤6 | 7 | ≥8 |
≤T2a | T2b |
≥T2c |
|---|
| Peak strain index
≥5.97 | 63 | 11 | 16 | 36 | 20 | 24 | 19 | 8 | 8 | 47 |
| Peak strain index
<5.97 | 9 | 3 | 3 | 3 | 7 | 1 | 1 | 1 | 0 | 8 |
| Total | 72 | 14 | 19 | 39 | 27 | 25 | 20 | 9 | 8 | 55 |
A total of 10 patients with a peak strain index of
≥5.97 were diagnosed as having a benign prostate lesion. Of these
10 cases, 2 cases were benign prostate hyperplasia (BPH), 4 were
BPH with chronic inflammation, 2 were granulomatous inflammation
and 2 were low-grade prostate intraepithelial neoplasia.
According to the guidelines of the American
Urological Association, the European Association of Urology and the
Chinese Urological Association, cases of PCa were classified as
low, moderate or high risk PCa (17–19)
Moderate and high risk PCa (considered to be clinically important)
must be treated as early as possible. Higher peak strain index
values were associated with clinically significant PCa (r=0.28;
P=0.017).
The Gleason scores of the 72 patients diagnosed with
PCa were between 5 and 9 and the number that scored 5–6, 7 or 8–9
were 27, 25 and 20, respectively (Table
IV). The overall positive incidence for RTE-targeted biopsy and
systematic biopsy were 87.5 and 86.1%, respectively (P=0.525).
There was no significant difference in the distribution of Gleason
scores between targeted biopsy and systematic biopsy (P=0.539).
When the Gleason score was ≥7, RTE targeted biopsy and systematic
biopsy detected 95.6 (43/45) and 84.4% (38/45) of PCa cases
(Table IV), respectively, and the
difference was statistically significant (P=0.0253). Therefore, an
RTE-targeted biopsy coupled with a peak strain index of ≥5.97 may
be able to detect a higher number of clinically significant cases
of PCa compared with systematic biopsy.
 | Table IV.Number of patients with PCa and
Gleason score distributions in the transrectal RTE targeted biopsy,
systematic biopsy and combination groups. |
Table IV.
Number of patients with PCa and
Gleason score distributions in the transrectal RTE targeted biopsy,
systematic biopsy and combination groups.
| Gleason score | TB | SB | TB+SB |
|---|
| 5–6 | 20 | 24 | 27 |
| 7 | 24 | 21 | 25 |
| 8–9 | 19 | 17 | 20 |
| Total | 63 | 62 | 72 |
Discussion
PCa tissue is stiffer compared with normal prostate
tissue (20). A number of previous
studies, which applied the qualitative stiffness threshold ‘blue
area’, indicated that RTE-guided biopsy was effective for detecting
PCa (4,10,21,22).
Nygård et al (23) established
that the frequency of positive cores was significantly higher in
RTE-targeted biopsies compared with standard systematic biopsies.
Another previous study indicated that additional patients that were
not detected using 10-core biopsies were detected using
RTE-targeted four-core biopsy (24).
These previous studies suggest that the application of RTE-guided
biopsy may be effective in prostate cancer detection and this is
concordant with the current study that uses the objective
quantitative parameter of stiffness in its approach.
Peak strain index is an objective quantitative
parameter that reflects the stiffest region of the PCa tissue and
has been established to be effective in distinguishing benign from
malignant areas in the breast and thyroid gland (25,26). A
previous study demonstrated that the peak strain index in PCa
lesions was higher compared with benign lesions with a threshold
value of 17.4 (15). In the present
study, the threshold value of the peak strain index was lower (5.97
vs. 17.4). The reasons for this variation may be that the methods
used for calculating the peak strain index were varied. The size of
the ROI was standardized as the smallest area compared with the SR
results in other patients in the current study. It was important to
select the appropriate site for the reference tissue and to measure
SRB (peak strain index=SRB/SRA).
Normal tissue is typically present as a green area on the
elastogram (27,28), therefore only the green area was
selected, and not the blue or red merged areas, as the reference
tissue to avoid any effect on ROI calculation.
The accuracy of systematic biopsy for detecting PCa
varies depending upon the number of cores that are biopsied
(29,30). The sensitivity of the 12-core biopsy
that adds additional lateral and apical peripheral zone biopsies is
only 53% (4). The 18 or 24 core
‘saturation biopsy’ does not increase the PCa detection rate
(31). As the number of cores
increase, the potential risk, including pain, bleeding and
infection following biopsy, also increase (32,33)
RTE-targeted biopsy had a higher sensitivity with fewer biopsy
cores compared with the systematic biopsy (16,34).
In the present study, the rate of identifying
patients with prostate cancer using RTE-targeted biopsy combined
with peak strain index (45%, 63/141 cases) was similar compared
with systematic biopsy (44%, 62/141 cases). RTE failed to detect 9
patients with PCa (6%), of which7 cases (78%) had a Gleason score
<7 and 5 cases (56%) had multifocal and diffuse lesions in the
prostate gland. The possible hypotheses for the false-negative
findings are that low risk PCa may be less stiff, or due to a lack
of benign tissue for a reference. Notably, the majority of the
false-positive findings were potentially associated with chronic
inflammation or BPH with stromal hyperplasia and fibrosis. Junker
et al (35) identified that
the detection rate of PCa for RTE is dependent on tumor
localization and histological type.
The distribution of Gleason scores between
RTE-targeted biopsy and systematic biopsy were similar (P=0.539).
However, when the peak strain index was ≥5.97, RTE-targeted biopsy
detected a higher number of clinically significant PCa cases
compared with the systematic biopsy. This suggests that a positive
peak strain index may be an independent marker for the detection of
moderate and high-risk PCa, which requires timely treatment.
Detection using RTE-targeted biopsy in varying parts
of the prostate differs. Pelzer et al (36) indicated that RTE is effective in
detecting apex and mid-gland PCa. This is possibly as the size and
volume of the base area is too large for the probe to compress
adequately. Secondly, the total detection rate of RTE-targeted
biopsy on the right side was higher compared with that on the left
(61 vs. 39%). Salomon et al (37) theorized that this was due to the use
of the left decubitus position during RTE. However, Pelzer et
al (36) also demonstrated
similar results with patients examined in the lithotomy
position.
Magnetic resonance imaging (MRI) is an approach for
targeted prostate biopsy. The three techniques of MRI guidance that
are available (38,39) are as follows: a) Cognitive targeting
(physician performs a TRUS-guided biopsy following a review of the
previous prostate MRI revealing a lesion); b) MRI/TRUS fusion
(software co-registration of real-time TRUS with stored MRI); c)
direct MRI-guided biopsy (in-bore targeting). In-bore targeting is
a specific and direct targeting method, but its limitations include
a long procedure time, high costs and position difficulties. By
contrast, the advantages of RTE targeting combined with peak strain
index are obvious. The advantages include: Less time required,
cheaper and simpler for the patient to reach the left decubitus
position.
The current study has a number of limitations.
Firstly, the surrounding media stiffness region (green area) was
selected as the reference tissue and chronic inflammatory,
low-grade PCa, multifocal and diffuse PCa lesions may also be
displayed in green and, therefore, affect the peak strain index
value. Secondly, there are artefacts in the elastogram that affect
the calculation of the peak strain index, including lateral
stiffness artefacts that typically occur in cases of BPH. The
examination of the lateral suspicious region following the tilting
of the ultrasound probe is effective to identify these artefacts.
However, deep stiffness artefacts caused by the increasing depth of
ultrasound penetration are challenging to overcome. This may reduce
the ability to detect PCa in the transition zone and
anterior-localized PCa in enlarged prostates (40). However, Miyagawa et al
(41) demonstrated that a higher
number of lesions in the anterior prostate were detected using
elastography. Junker et al (42) indicated that between RTE and
multiparametric MRI, there was no significant difference in the
detection of anterior-localized PCa with a prostate volume of
<40 cm3. Thirdly, RTE has intra- and inter-observer
variability as elastograms were produced by manual compression and
the pressure and speed induced by manual compression maybe adjusted
using a visual indicator. An experienced examiner (performed
>500 examinations of patients) is required for performing
reliable elastograms used for diagnosis of PCa. Finally, the
pathological diagnosis was based on biopsy cores.
To the best of our knowledge, the current study is
the first to demonstrate that RTE-targeted biopsy combined with
peak strain index may improve the detection rate of clinically
significant PCa in the peripheral zone. The present study indicated
that the peak strain index may be an effective quantitative
parameter in RTE-targeted biopsy.
In conclusion, the results of the present study
demonstrated that peak strain index as a quantitative parameter is
an independent marker for PCa lesions in the prostate peripheral
zone. Transrectal RTE-targeted biopsy combined with peak strain
index may enhance the detection of clinically significant PCa with
a small number of biopsy cores. RTE-targeted biopsy combined with
systematic biopsy may provide an effective approach for the
diagnosis of patients with PCa and, particularly, for those with
clinically significant prostate cancer.
Acknowledgements
The present study was supported by the Suzhou
Society Development Program (grant no. SYSD2014093) and the
Superior Specialty Group Program of The Second Affiliated Hospital
of Soochow University (grant no. XKQ2015009). The authors would
like to thank Dr Jin Zhu, Dr Ya-Chen Zang (Department of Urology,
The Second Affiliated Hospital of Soochow University, Suzhou,
Jiangsu, P.R. China), Professor Yuan-Yuan Zhang (Wake Forest
Institute for Regenerative Medicine, Wake Forest School of Medicine
Center, Blvd, Winston-Salem, NC, USA) and Professor Zhou Wang
(Department of Urology, University of Pittsburgh School of
Medicine, Pittsburgh, PA, USA), for their assistance in manuscript
preparation.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen R, Ren SC; Chinese Prostate Cancer
Consortium, ; Yiu MK, Fai NC, Cheng WS, Ian LH, Naito S, Matsuda T,
Kehinde E, et al: Prostate cancer in Asia: A collaborative report.
Asian J Urol. 1:15–29. 2014. View Article : Google Scholar
|
|
3
|
Shao Q, Ouyang J, Fan Y, Xie J, Zhou J, Wu
J, Kader A Karim, Xu J, Liu G, Shan Y, et al: Prostate cancer in
the senior men from rural areas in east district of China:
Contemporary management and 5-year outcomes at multi-institutional
collaboration. Cancer Lett. 315:170–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haas GP, Delongchamps NB, Jones RF,
Chandan V, Serio AM, Vickers AJ, Jumbelic M, Threatte G, Korets R,
Lilja H and de la Roza G: Needle biopsies on autopsy prostates:
Sensitivity of cancer detection based on true prevalence. J Natl
Cancer Inst. 99:1484–1489. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campos-Fernandes JL, Bastien L, Nicolaiew
N, Robert G, Terry S, Vacherot F, Salomon L, Allory Y, Vordos D,
Hoznek A, et al: Prostate cancer detection rate in patients with
repeated extended 21-sample needle biopsy. Eur Urol. 55:600–606.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schröder FH, Carter HB, Wolters T, van den
Bergh RC, Gosselaar C, Bangma CH and Roobol MJ: Early detection of
prostate cancer in 2007. Part 1: PSA and PSA kinetics. Eur Urol.
53:468–477. 2008.
|
|
7
|
Wolters T, Roobol MJ, van Leeuwen PJ, van
den Bergh RC, Hoedemaeker RF, van Leenders GJ, Schröder FH and van
der Kwast TH: A critical analysis of the tumor volume threshold for
clinically insignificant prostate cancer using a data set of a
randomized screening trial. J Urol. 185:121–125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Phipps S, Yang TH, Habib FK, Reuben RL and
McNeill SA: Measurement oftissue mechanical characteristics to
distinguish between benign andmalignant prostatic disease. Urology.
66:447–450. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Good DW, Stewart GD, Hammer S, Scanlan P,
Shu W, Phipps S, Reuben R and McNeill AS: Elasticity as a biomarker
for prostate cancer: A systematic review. BJU Int. 113:523–534.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kapoor A, Kapoor A, Mahajan G and Sidhu
BS: Real-time elastography in the detection of prostate cancer in
patients with raised PSA level. Ultrasound Med Biol. 37:1374–1381.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Romagnoli A, Autieri G, Centrella D,
Gastaldi C, Pedaci G, Rivolta L, Pozzi E, Anghileri A, Cerabino M,
Bianchi CM and Roggia A: Real-time elastography in the diagnosis of
prostate cancer: Personal experience. Urologia. 77:248–253.
2010.(In Italian). PubMed/NCBI
|
|
12
|
Cochlin DL, Ganatra RH and Griffiths DF:
Elastography in the detection of prostatic cancer. Clin Radiol.
57:1014–1020. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferrari FS, Scorzelli A, Megliola A, Drudi
FM, Trovarelli S and Ponchietti R: Real-time elastography in the
diagnosis of prostate tumor. J Ultrasound. 12:22–31. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giurgiu CR, Manea C, Crişan N, Bungărdean
C, Coman I and Dudea SM: Real-time sonoelastography in the
diagnosis of prostate cancer. Med Ultrason. 13:5–9. 2011.PubMed/NCBI
|
|
15
|
Zhang Y, Tang J, Li YM, Fei X, Lv FQ, He
EH, Li QY and Shi HY: Differentiation of prostate cancer from
benign lesions using strain index of transrectal real-time tissue
elastography. Eur J Radiol. 81:857–862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
König K, Scheipers U, Pesavento A, Lorenz
A, Ermert H and Senge T: Initial experiences with real-time
elastography guided biopsies of the prostate. J Urol. 174:115–117.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carter HB, Albertsen PC, Barry MJ, Etzioni
R, Freedland SJ, Greene KL, Holmberg L, Kantoff P, Konety BR, Murad
MH, et al: Early detection of prostate cancer: AUA guideline. J
Urol. 190:419–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: EAU guidelines on prostate cancer. part 1:
Screening, diagnosis, and local treatment with curative intent
update 2013. Eur Urol. 65:124–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Na YQ, Ye ZQ, Sun YH, Sun G, Huang J, Kong
CZ, et al: Chinese guidelines for the diagnosis and treatment of
Urologic diseases. 67–75. 2014.(monograph).
|
|
20
|
Krouskop TA, Wheeler TM, Kallel F, Garra
BS and Hall T: Elastic moduli of breast and prostate tissues under
compression. Ultrason Imaging. 20:260–274. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scattoni V, Zlotta A, Montironi R,
Schulman C, Rigatti P and Montorsi F: Extended and saturation
prostatic biopsyin the diagnosis and characterisation of prostate
cancer: A critical analysis of the literature. Eur Urol.
52:1309–1322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aigner F, Pallwein L, Schocke M, Andrei L,
Junker D, Schäfer G, Mikuz G, Pedross F, Horninger W, Jaschke W, et
al: Comparison of real-time sonoelastography with T2-weighted
endorectal magnetic resonance imaging for prostate cancer
detection. J Ultrasound Med. 30:643–649. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nygård Y, Haukaas SA, Halvorsen OJ,
Gravdal K, Frugård J, Akslen LA and Beisland C: A positive
real-time elastography is an independent marker for detection of
high-risk prostate cancers in the primary biopsy setting. BJU Int.
113:E90–E97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salomon G, Drews N, Autier P, Beckmann A,
Heinzer H, Hansen J, Michl U, Schlomm T, Haese A, Steuber T, et al:
Incremental detection rate of prostate cancer by real-time
elastography targeted biopsies in combination with a conventional
10-core biopsy in 1024 consecutive patients. BJU Int. 113:548–553.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cho N, Moon WK, Kim HY, Chang JM, Park SH
and Lyou CY: Sonoelastographic strain index for differentiation of
benign and malignant nonpalpable breast masses. J Ultrasound Med.
29:1–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lyshchik A, Higashi T, Asato R, Tanaka S,
Ito J, Mai JJ, Pellot-Barakat C, Insana MF, Brill AB, Saga T, et
al: Thyroid gland tumor diagnosis at US elastography. Radiology.
237:202–211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kamoi K, Okihara K, Ochiai A, Ukimura O,
Mizutani Y, Kawauchi A and Miki T: The utility of transrectal
real-time elastography in the diagnosis of prostate cancer.
Ultrasound Med Biol. 34:1025–1032. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goddi A, Sacchi A, Magistretti G and
Almolla J: Transrectal real-time elastography of the prostate:
Normal patterns. J Ultrasound. 14:220–232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen ME, Troncoso P, Johnston DA, Tang K
and Babaian RJ: Optimization of prostate biopsy strategy using
computer based analysis. J Urol. 158:2168–2175. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Norberg M, Egevad L, Holmberg L, Sparen P,
Norlén BJ and Busch C: The sextant protocol for ultrasound-guided
core biopsies of the prostate underestimates the presence of
cancer. Urology. 50:562–566. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jones JS, Patel A, Schoenfield L, Rabets
JC, Zippe CD and Magi-Galluzzi C: Saturation technique does not
improve cancer detection as an initial prostate biopsy strategy. J
Urol. 175:485–488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jones JS, Patel A, Schoenfield L, Rabets
JC, Zippe CD and Magi-Galluzzi C: Saturation technique does not
improve cancer detection as an initial prostate biopsy strategy. J
Urol. 175:485–488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Naughton CK, Miller DC, Mager DE, Ornstein
DK and Catalona WJ: A prospective randomized trial comparing 6
versus 12 prostate biopsy cores: Impact on cancer detection. J
Urol. 164:388–392. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pallwein L, Mitterberger M, Struve P,
Horninger W, Aigner F, Bartsch G, Gradl J, Schurich M, Pedross F
and Frauscher F: Comparison of sonoelastography guided biopsy with
systematic biopsy: Impact on prostate cancer detection. Eur Radiol.
17:2278–2285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Junker D, Schäfer G, Aigner F, Schullian
P, Pallwein-Prettner L, Bektic J, Horninger W, Halpern EJ and
Frauscher F: Potentials and limitations of real-time elastography
for prostate cancer detection: A whole-mount step section analysis.
Scientific World Journal. 2012:1932132012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pelzer AE, Heinzelbecker J, Weiß C,
Frühbauer D, Weidner AM, Kirchner M, Stroebel P, Schoenberg SO and
Dinter DJ: Real-time sonoelastography compared to magnetic
resonance imaging using four different modalities at 3.0 T in the
detection of prostate cancer: Strength and weaknesses. Eur J
Radiol. 82:814–821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Salomon G, Köllerman J, Thederan I, Chun
FK, Budäus L, Schlomm T, Isbarn H, Heinzer H, Huland H and Graefen
M: Evaluation of prostate cancer detection with ultrasound
real-time elastography: A comparison with step section pathological
analysis after radical prostatectomy. Eur Urol. 54:1354–1362. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim CK: Magnetic resonance imaging-guided
prostate biopsy: Present and future. Korean J Radiol. 16:90–98.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tilak G, Tuncali K, Song SE, Tokuda J,
Olubiyi O, Fennessy F, Fedorov A, Penzkofer T, Tempany C and Hata
N: 3T MR-guided in-bore transperineal prostate biopsy: A comparison
of robotic and manual needle-guidance templates. J Magn Reson
Imaging. 42:63–71. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Junker D, de Zordo T, Quentin M, Ladurner
M, Bektic J, Horniger W, Jaschke W and Aigner F: Real-time
elastography of the prostate. Bio Med Res Int. 2014:1808042014.
|
|
41
|
Miyagawa T, Tsutsumi M, Matsumura T,
Kawazoe N, Ishikawa S, Shimokama T, Miyanaga N and Akaza H:
Real-time elastography for the diagnosis of prostate cancer:
Evaluation of elastographic moving images. Jpn J Clin Oncol.
39:394–398. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Junker D, Schäfer G, Kobel C, Kremser C,
Bektic J, Jaschke W and Aigner F: Comparison of real-time
elastography and multiparametric MRI for prostate cancer detection:
A whole-mount step-section analysis. AJR Am J Roentgenol.
202:W263–W269. 2014. View Article : Google Scholar : PubMed/NCBI
|