Introduction
Glioma is not only the most common primary
intracranial tumor (1), but also the
most intractable in the treatment of neurosurgery tumors. Incidence
rates of about 5 in 100,000 have been reported with a rising trend
(2). The treatment of glioma has
shown great improvement for patients who are diagnosed early with
excision often used as treatment approach (3). However, surgical treatment has a poor
effect for the malignant growth of tumor cells, leading to high
recurrence and mortality rates (4).
In order to decrease the recurrence and mortality rate, patients
receive antineoplastic drugs post-operatively (5). However, data show that patients
diagnosed with glioma, have a survival time of only 12–15 months,
and the 5-year survival rate <5% (6).
O6-methylguanine DNA methyltransferase
(MGMT) is a DNA repair protein, found in humans and many
prokaryotic organisms. Research has shown that for the patients
with glioma, antineoplastic drug treatments by alkylating agents
may result in drug resistance, and MGMT is the main reason and
therefore, not an ideal treatment for patients (7). Some reports indicate that the effect of
positive MGMT expression is inferior to that of negative expression
during chemotherapy for the patient with glioma (8). Thus, MGMT activity is higher, and the
drug resistance to antineoplastic drugs of alkylating agent class
is greater. If MGMT expression is lower, the alkylating class of
antineoplastic drugs is more sensitive, with a better efficacy. We
analyzed the expression of MGMT in newly diagnosed glioma patients
to increase the sensitivity of patients with glioma to
antineoplastic drugs of alkylating agent class so as to
significantly improve the prognosis and reduce the
reoccurrence.
Materials and methods
In the study, 62 patients with glioma, who were
admitted by neurosurgery from January 2011 to January 2013, were
selected. Post-surgical treatment was required and the pathology
results were diagnosed as glioma. A total of 33 males (aged 25–72
years; average 42.7 years) and 29 females (aged 23–71 years;
average 40.1 years) were included. There were 15 cases of grade I,
16 cases of grade II, 14 cases of grade III and 17 cases of grade
IV glioma. As a control group we used brain tissue from 12 patients
with cerebral hemorrhage caused by high blood pressure (aged 40–55
years; average 48.1 years). It was required that the time between
incidence and operative treatment was not more than 6 h.
This study was approved by the Ethics Committee of
Tongde Hospital of Zhejiang Province. Signed written informed
consents were obtained from all participants before the study.
Inclusion criteria
In the study were: ⅰ) Age ≥18 years old and
Karnofsky performance status (KPS) >60 scores; ⅱ) women of
non-gestation or non-lactation; ⅲ) normal heart, brain, liver, lung
and kidney; ⅳ) all patients received surgical treatment of tumor
resection, and the postoperative pathological diagnosis results
could be confirmed; ⅴ) complications such as intracranial infection
and intracranial hematoma did not occur after operation; ⅵ)
favorable paraffin-embedded tissue; and ⅶ) patients with good
compliance, complete data of cases and coordination of
follow-up.
Therapeutic regimen
All patients had surgical treatment, and the tumor
was resected as much as possible on the precondition of retaining
neurological function. In the study, 62 patients were given
chemotherapy combining with radiotherapy after operation. After 2–3
weeks, the patients were given conformal radiotherapy of photon
knife (Elekta, Stockholm, Sweden), with at least one course of
treatment. On the third day of radiotherapy, 42-day chemotherapy by
the dose of 75 mg/m2 temozolomide (TMZ) (Biosharp,
Hefei, China) was started. After 4 weeks of combination of
chemotherapy and radiotherapy, for 2-periods a single-drug
chemotherapy of trimethylamine (TMA) was carried out. During the
process of chemoradiotherapy, if the patients had encephaledema,
intracranial pressure was decreased by dehydration and diuresis
treatments. Blood routine examination was reviewed once a week, and
hepatorenal function was tested at fixed period. Symptomatic
treatment such as liver protection also was conducted according to
the test situation and clinical symptoms.
Immunohistochemical methods
SP immunohistochemical method was applied and the
steps proceeded according to instructions. In cell nucleus and/or
intracytoplasm, the yellow or claybank indicated positive MGMT. The
specific judgement methods of positive MGMT were as follows: under
the scope of high power lens (400 times), 10 representative areas
that gathered glioma cells were selected, the rate of positive
cells was observed, which was divided into four grades: i) Negative
(−), without positive cells or <10%; ii) weakly positive (+),
positive cells among 10 scopes of 10–24%; iii) intermediately
positive (++), positive cells of 25–50%; and iv) strongly positive
(+++), positive cells >50%.
Observation methods
The time from initial chemotherapy to the last
return visit, or death was tracked, namely, the overall survival
(OS). Related data were recorded and Kaplan-Meier survival curve
was drawn. The level of myelosuppression was determined by WHO
grade. According to the subjective feelings and clinical
manifestation of patients, gastrointestinal symptoms were
judged.
Statistical analysis
SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) was used to
analyze the data. The χ2 test was applied to detect the
difference of positive rate for different grades of glioma.
P<0.05 meant that the difference had statistical significance.
Fishers exact probability test was used to judge the relation
between MGMT expression and gender and age for the patients with
glioma. Spearmans correlation was taken to analyze the expression
of MGMT in different grades of glioma; Kaplan-Meier survival curve
presented the relation between the survival time and of the patient
tissues, and log-rank detection was also conducted at the same
time.
Results
MGMT expression in different grades of
glioma
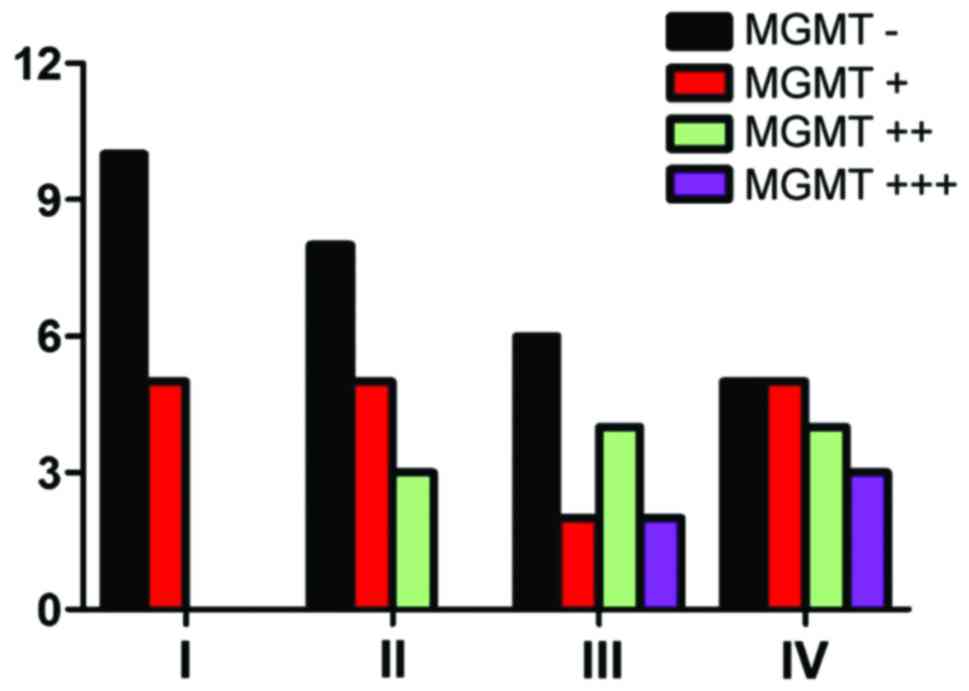
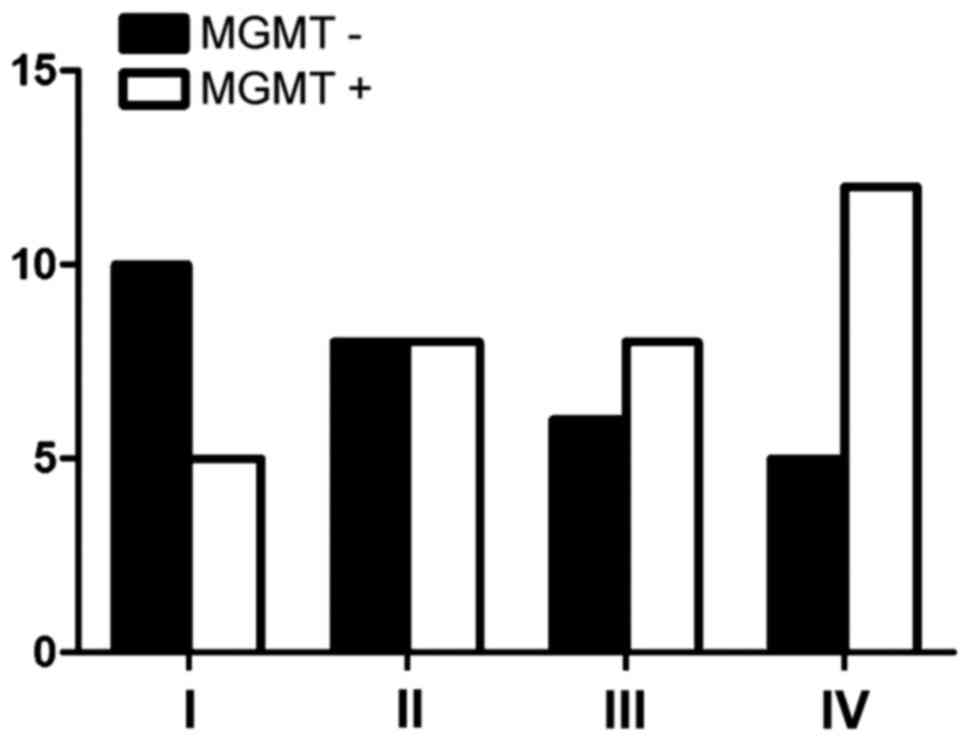
As shown in Table I
and Fig. 1, the negative expression
of MGMT reached 66.7% in grade I, 50% in grade II, 42.9% in grade
III and 29.4% in grade IV. MGMT expression increased in high-grade
glioma, and the expression gradually decreased in low-grade glioma.
By statistical analysis, it was confirmed that the expression of
MGMT had no relation with WHO grade (P>0.05).
 | Table I.MGMT distribution in different grades
of glioma. |
Table I.
MGMT distribution in different grades
of glioma.
| MGMT expression | I | II | III | IV | Total |
|---|
| − | 10 | 8 | 6 | 5 | 29 |
| + | 5 | 5 | 2 | 5 | 17 |
| ++ | 0 | 3 | 4 | 4 | 11 |
| +++ | 0 | 0 | 2 | 3 | 5 |
| Total | 15 | 16 | 14 | 17 | 62 |
MGMT expression and biological
characteristics
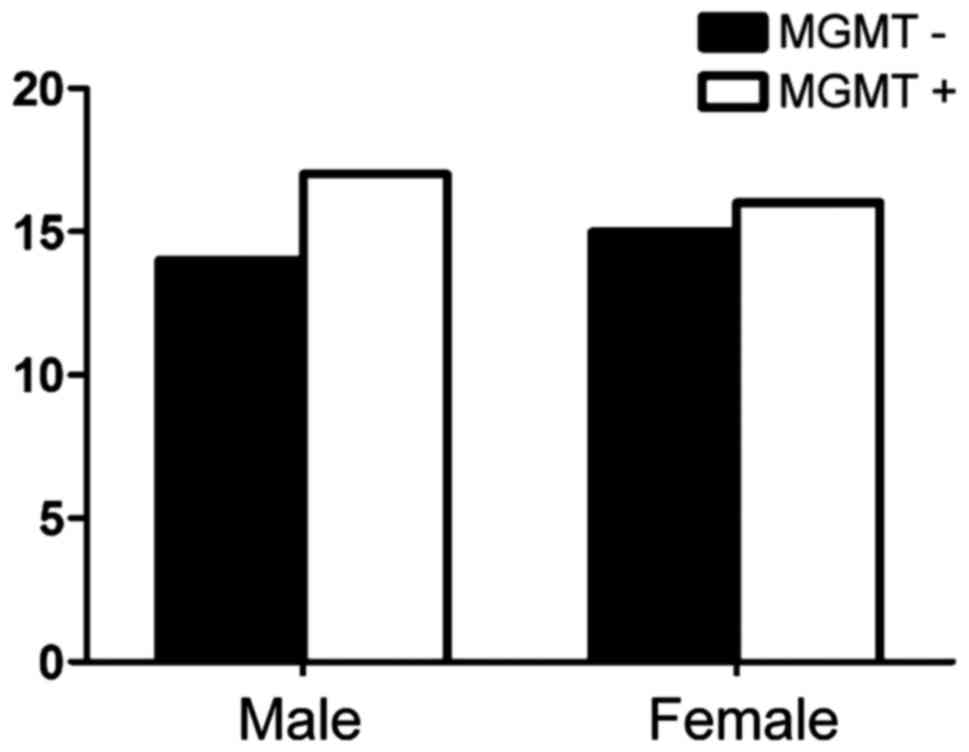
Fig. 2 shows the
expression of MGMT among males was 52.9%, while the positive
expression of MGMT among females was 53.6% (P>0.05) and no
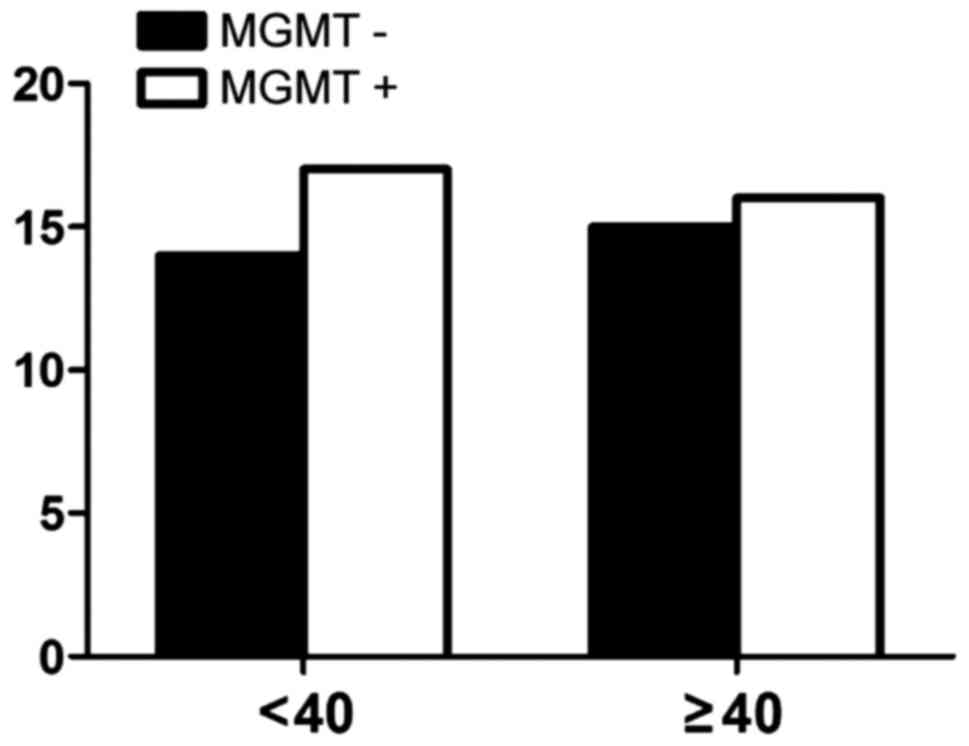
correlation with age (Fig. 3). There
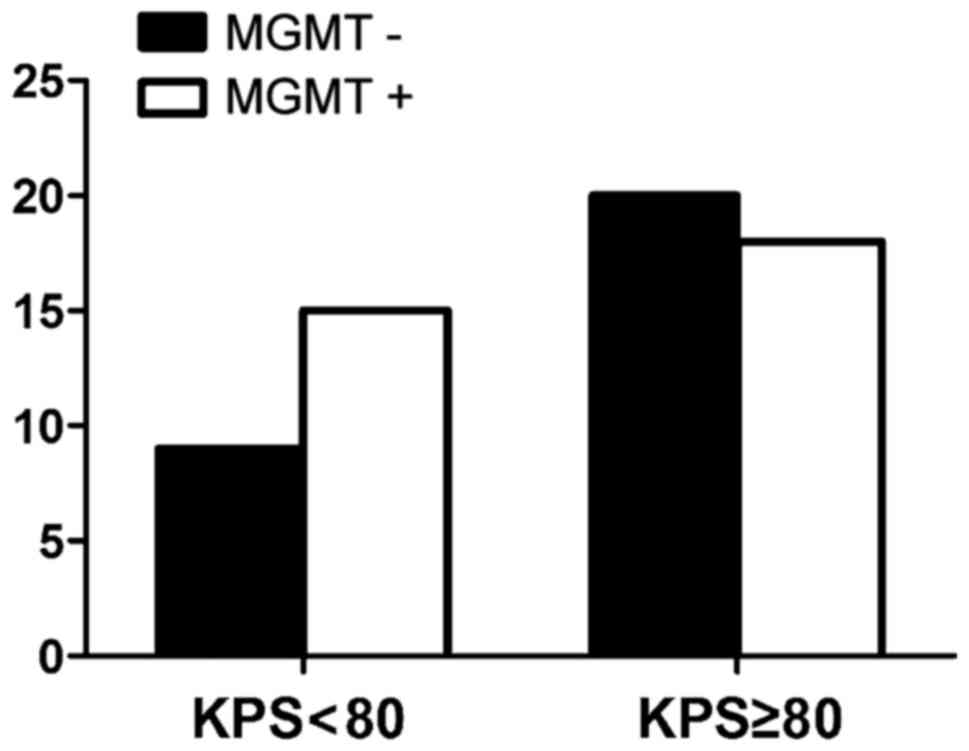
were nine cases with negative expression of MGMT in patients whose
KPS score was <80 and 20 cases in patients whose score was
>80 (P>0.05) (Fig. 4). The
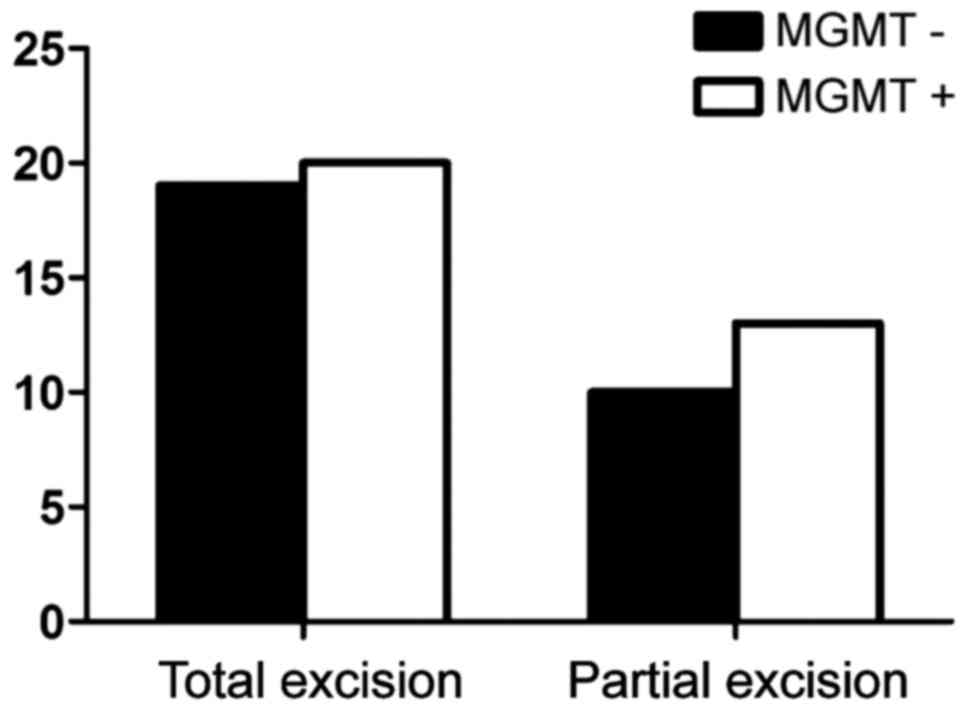
expression of MGMT was not related to the method of excision
(P>0.05) (Fig. 5). In patients
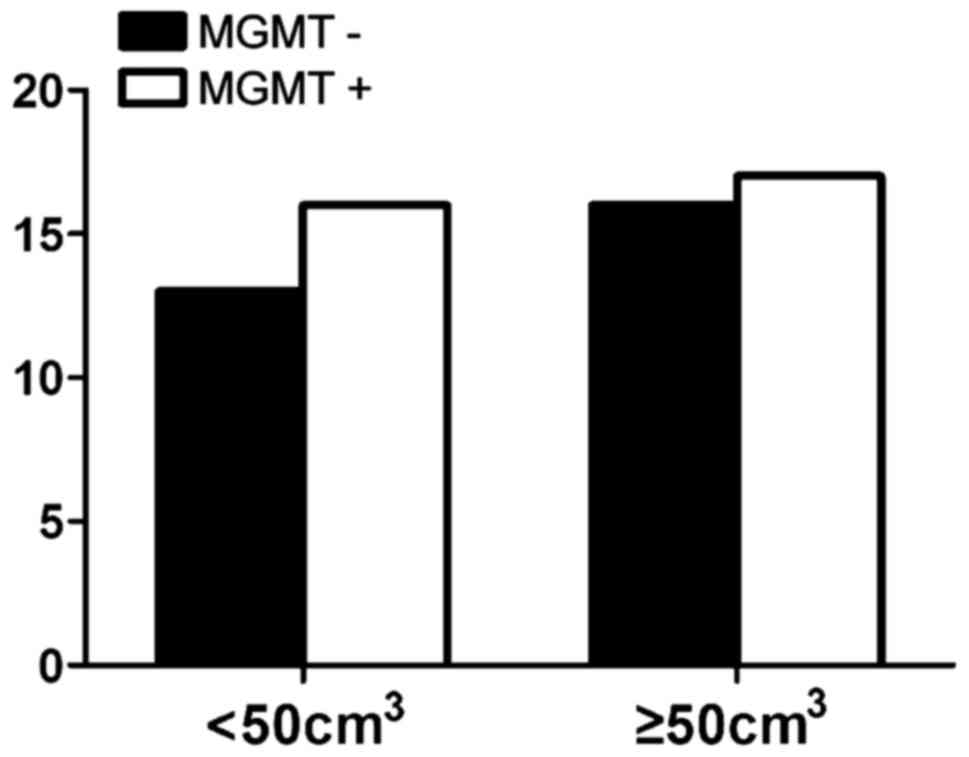
whose tumor volume was <50 cm3, 44.8% had negative
and 55.2% had positive expression rate and the difference had no
statistical significance (P>0.05) (Fig. 6). The negative expression in low-grade
glioma (grade I and II) was 58.1% and the positive rate was 41.9%,
while the negative expression in high-grade (grade III and IV) was
35.5% and the positive expression was 54.5% (Fig. 7). The positive expression of MGMT in
low-grade glioma was significantly lower than that of high-grade
(P<0.05). However, gender, age, tumor size, surgical method and
KPS score had no statistical difference with MGMT among the 62
patients (P>0.05).
Western blotting
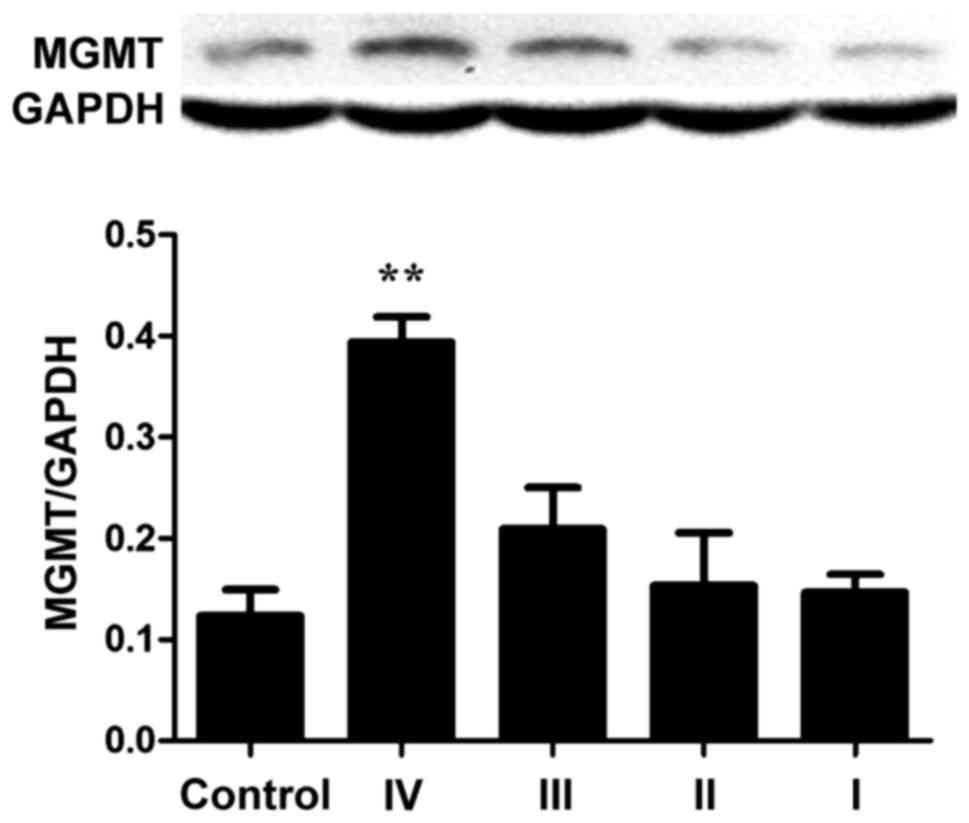
Protein expression of MGMT in normal controls was
the lowest, while protein expression increased with increasing
glioma grade especially for grade IV (Fig. 8). The expression of MGMT was 3.2-fold
that of the control group, and the difference had statistical
significance (P<0.01), but the expression of each tumor grade
showed no relation with WHO tumor grade (P>0.05).
Evaluation of short-term
remission
After treatment, the patients were divided into
complete remission and partial remission as according to the
short-term evaluation standard of WHO. In this experiment, the
objective efficacy for patients with negative MGMT protein
expression was better than that of MGMT-positive expression group.
In MGMT negative expression group, the effective rate reached 72.4%
(21/29), while the effective rate of MGMT-positive expression group
was only 18.2% (5/33), with statistical significance
(P<0.05).
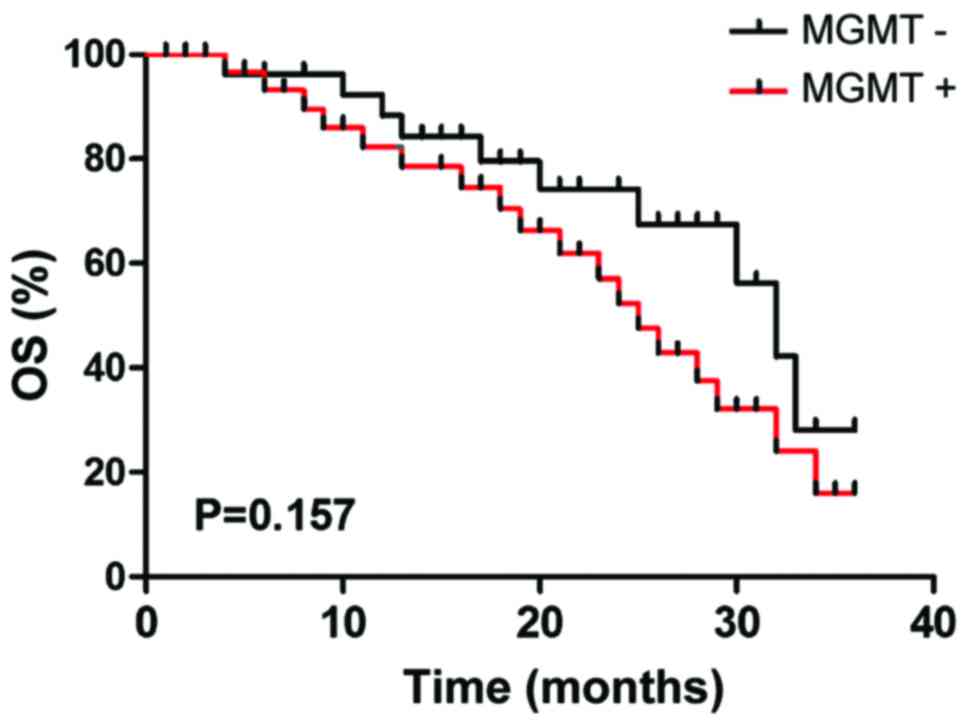
Survival time
The 3-year Kaplan-Meier survival of patients with
MGMT-negative expression was 65.5% (19 cases), while the survival
rate of patients with MGMT-positive expression was 45.5% (15
cases), but the difference had no statistical significance by
log-rank detection (P>0.05) (Fig.
9).
Discussion
Glioma often occurs in the neurogliocyte of
neuroderm, which is a most common malignant tumor with high
recurrence, fatality and low recovery rates. The tumor cell has
obvious effect, high invasiveness and infiltrative growth. Tumor
cell proliferation is fast and expands to other brain tissue.
Currently, the main therapy for glioma is a comprehensive treatment
including neurosurgical operation, chemotherapy and radiotherapy.
However, the prognosis is not ideal (9–11).
Radiotherapy is a vital step in the process of
glioma treat-ment, and the main chemotherapeutic agent is from the
alkylating class of drugs. These drugs can alkylate DNA and form
crosslinks, leading to high cytotoxicity, effect on DNA replication
leading to tumor cell death. MGMT is distributed in different
tissues, with brain tissue among the lowest. The expression of MGMT
in normal tissue is lower than that of tumor tissue, whose activity
is often taken as an important index for observing the prognosis of
patients. MGMT can act on DNA crosslinks and restore DNA
alkylation, reducing the toxic effect of alkane chemotherapy drugs
and causing MGMT inactivation, so that patients develop drug
resistance. As a result, the expression level of MGMT can influence
the drug resistance of an alkylating agent in tumor cells (12). However, besides individual variation,
the main reason that influences the chemotherapeutic effect for
tumor also depends on drug resistance (13). The survival time of the patients with
glioma is usually only 12–15 months, up to 90% of patients die due
to drug tolerance of the tumor cells (14). Therefore, before the treatment on
glioma, it is important to identify high expression of MGMT for the
chemotherapy prognosis of patients. For the patients with glioma,
the current first line of chemotherapeutics is alkylating agent TMZ
and new nitrosourea drugs. Some clinical research has confirmed
that the treatment effect of radiotherapy combined with TMZ was
better than that of single radiotherapy for patients with glioma
who received tumor excision, which can increase the survival rate
of patients from 10 to 26% and improve disease prognosis (15).
In this study, gender, age, tumor size, surgical
method and KPS score had no statistical difference in MGMT
expression. This suggests that MGMT could not be used as an
independent reference for the individual chemotherapy regimen in
clinic. At the same time, it was confirmed that the difference
between positive expression of MGMT and WHO grade of glioma had
statistical significance. The expression of MGMT in glioma tissue
was higher than that of normal tissue and with an increase in WHO
grade, the positive expression of MGMT was higher. Research by Yuan
et al showed that expression of MGMT in the patients with
lower level malignancy increased, while the expression was
decreased in the patients with high-grade malignancy (16–18). Other
research has also confirmed that there was no obvious correlation
between the positive expression of MGMT and WHO grade (18). However, the conclusions were based on
limited cases.
Our results show that the survival time of the
MGMT-negative expression group was longer than that of
MGMT-positive expression group, but the difference showed no
statistical significance by log-rank detection. It suggests that
for glioma patients with positive expression of MGMT,
antineoplastic drugs of alkylating agent class should be avoided,
so as to provide patients with a better personalized treatment
(19–21).
References
|
1
|
Blumenthal DT, Dvir A, Lossos A,
Tzuk-Shina T, Lior T, Limon D, Yust-Katz S, Lokiec A, Ram Z, Ross
JS, et al: Clinical utility and treatment outcome of comprehensive
genomic profiling in high grade glioma patients. J Neurooncol.
130:211–219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Hegi ME, van den Bent MJ, Mason
WP, Weller M, Mirimanoff RO and Cairncross JG: European
Organisation for Research and Treatment of Cancer Brain Tumor and
Radiotherapy Groups; National Cancer Institute of Canada Clinical
Trials Group: Changing paradigms - an update on the
multidisciplinary management of malignant glioma. Oncologist.
11:165–180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaina B, Christmann M, Naumann S and Roos
WP: MGMT: Key node in the battle against genotoxicity,
carcinogenicity and apoptosis induced by alkylating agents. DNA
Repair (Amst). 6:1079–1099. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsu CY, Lin SC, Ho HL, Chang-Chien YC, Hsu
SP, Yen YS, Chen MH, Guo WY and Ho DM: Exclusion of
histiocytes/endothelial cells and using endothelial cells as
internal reference are crucial for interpretation of MGMT
immunohistochemistry in glioblastoma. Am J Surg Pathol. 37:264–271.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jacinto FV and Esteller M: MGMT
hypermethylation: A prognostic foe, a predictive friend. DNA Repair
(Amst). 6:1155–1160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bocangel D, Sengupta S, Mitra S and Bhakat
KK: p53-mediated down-regulation of the human DNA repair gene
O6-methylguanine-DNA methyltransferase (MGMT) via
interaction with Sp1 transcription factor. Anticancer Res.
29:3741–3750. 2009.PubMed/NCBI
|
|
7
|
Molnár J, Engi H, Hohmann J, Molnár P,
Deli J, Wesolowska O, Michalak K and Wang Q: Reversal of multidrug
resitance by natural substances from plants. Curr Top Med Chem.
10:1757–1768. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tugcu B, Postalci LS, Gunaldi O,
Tanriverdi O and Akdemir H: Efficacy of clinical prognostic factors
on survival in patients with glioblastoma. Turk Neurosurg.
20:117–125. 2010.PubMed/NCBI
|
|
9
|
Yoshino A, Ogino A, Yachi K, Ohta T,
Fukushima T, Watanabe T, Katayama Y, Okamoto Y, Naruse N, Sano E,
et al: Gene expression profiling predicts response to temozolomide
in malignant gliomas. Int J Oncol. 36:1367–1377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lombardi G, Pace A, Pasqualetti F, Rizzato
S, Faedi M, Anghileri E, Nicolotto E, Bazzoli E, Bellu L, Villani
V, et al: Predictors of survival and effect of short (40 Gy) or
standard-course (60 Gy) irradiation plus concomitant temozolomide
in elderly patients with glioblastoma: A multicenter retrospective
study of AINO (Italian Association of Neuro-Oncology). J
Neurooncol. 125:359–367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suryadevara CM, Verla T, Sanchez-Perez L,
Reap EA, Choi BD, Fecci PE and Sampson JH: Immunotherapy for
malignant glioma. Surg Neurol Int. 6 Suppl 1:S68–S77. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen ZP, Malapetsa A, McQuillan A,
Marcantonio D, Bello V, Mohr G, Remack J, Brent TP and Panasci LC:
Evidence for nucleotide excision repair as a modifying factor of
O6-methylguanine-DNA methyltransferase-mediated innate
chloroethylnitrosourea resistance in human tumor cell lines. Mol
Pharmacol. 52:815–820. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stupp R, Hegi ME, Gilbert MR and
Chakravarti A: Chemoradiotherapy in malignant glioma: Standard of
care and future directions. J Clin Oncol. 25:4127–4136. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bush ZM, Longtine JA, Cunningham T, Schiff
D, Jane JA Jr, Vance ML, Thorner MO, Laws ER Jr and Lopes MB:
Temozolomide treatment for aggressive pituitary tumors: Correlation
of clinical outcome with O6-methylguanine
methyltransferase (MGMT) promoter methylation and expression. J
Clin Endocrinol Metab. 95:E280–E290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nitta M, Muragaki Y, Maruyama T, Ikuta S,
Komori T, Maebayashi K, Iseki H, Tamura M, Saito T, Okamoto S, et
al: Proposed therapeutic strategy for adult low-grade glioma based
on aggressive tumor resection. Neurosurg Focus. 38:E72015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan Q, Matsumoto K, Nakabeppu Y and Iwaki
T: A comparative immunohistochemistry of
O6-methylguanine-DNA methyltransferase and p53 in
diffusely infiltrating astrocytomas. Neuropathology. 23:203–209.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Esteller M, Garcia-Foncillas J, Andion E,
Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB and Herman JG:
Inactivation of the DNA-repair gene MGMT and the clinical response
of gliomas to alkylating agents. N Engl J Med. 343:1350–1354. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rimel BJ, Huettner P, Powell MA, Mutch DG
and Goodfellow PJ: Absence of MGMT promoter methylation in
endometrial cancer. Gynecol Oncol. 112:224–228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lavon I, Zrihan D, Zelikovitch B, Fellig
Y, Fuchs D, Soffer D and Siegal T: Longitudinal assessment of
genetic and epigenetic markers in oligodendrogliomas. Clin Cancer
Res. 13:1429–1437. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bobola MS, Blank A, Berger MS and Silber
JR: O6-methylguanine-DNA methyltransferase deficiency in
developing brain: Implications for brain tumorigenesis. DNA Repair
(Amst). 6:1127–1133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rapkins RW, Wang F, Nguyen HN, Cloughesy
TF, Lai A, Ha W, Nowak AK, Hitchins MP and McDonald KL: The MGMT
promoter SNP rs16906252 is a risk factor for MGMT methylation in
glioblastoma and is predictive of response to temozolomide. Neuro
Oncol. 17:1589–1598. 2015. View Article : Google Scholar : PubMed/NCBI
|