Introduction
The incidence of primary central nervous system
(CNS) lymphoma (PCNSL), defined as diffuse large B cell lymphoma
(DLBCL) confined to the CNS, has reportedly increased in the
immunocompetent population (1). It is
not possible to morphologically distinguish PCNSL and peripheral
DLBCL. High-dose methotrexate-based chemotherapy is the standard
therapy for PCNSL. Chemotherapy with whole brain radiation therapy
has produced response rates up to 80–90%, and median overall
survival up to 5 years. However, PCNSL has a poor prognosis
compared with that of DLBCL (2) and
the reason for difference in prognosis between PCNSL and DLBCL has
not been elucidated.
Multiple leukocytes, including macrophages, are
known to be present in tumor tissues. Among the inflammatory cells,
tumor-associated macrophages (TAMs) are thought to directly or
indirectly promote tumor progression (3–5). TAMs are
divided into two categories: M1 macrophages, also known as
classically activated macrophages; and M2 macrophages, frequently
referred to as alternatively activated macrophages. In malignant
tumors, TAMs that have differentiated into M2 macrophages act as
protumoral macrophages and contribute to disease progression
(6). Previous studies have
demonstrated that higher levels of M2-polarized TAMs are associated
with a worse prognosis in various tumors, including glioma
(7,8),
renal cell cancer (9) and T-cell
leukemia/lymphoma (10). Certain
reports concerning DLBCL have indicated that TAMs are associated
with a worse clinical course (11),
but others have suggested that TAMs are not associated with overall
survival (12,13). On the other hand, TAM infiltration has
no prognostic value in PCNSL (14,15). The
reason for this remains unclear.
Monocyte chemoattractant protein (MCP-1), also known
as C-C motif chemokine ligand 2, is a member of a superfamily of
cytokines known as chemokines. C-C motif chemokine receptor 2
(CCR2) is the major receptor for MCP-1, and its binding activates a
series of downstream signaling pathways including p42/44
mitogen-activated protein kinase (MAPK), phospholipase C-γ and
protein kinase C through G protein-dependent mechanisms to regulate
cellular adhesion and motility in macrophages (16–18). This
binding recruits TAMs (19). In
several types of cancer, MCP-1 has been demonstrated to be an
important determinant of tumor growth (20–22). MCP-1
is primarily produced by neoplastic B cells, and less frequently by
lesional reactive astrocytes in primary CNS lymphoma (23). However, the involvement of MCP-1 has
not been elucidated in PCNSL.
The aim of the present study was to determine
whether MCP-1 expression and TAM infiltration differ between PCNSL
and DLBCL. The relationship between MCP-1 expression and the
proliferation of tumor cells in PCNSL was also investigated.
Materials and methods
Patients
The present study included 19 patients with PCNSL
and 16 patients with DLBCL. Patients were treated at Hokuto
Hospital (Obihiro, Japan) and Kumamoto University Hospital
(Kumamoto, Japan) between January 2008 and December 2013.
Laboratory examinations were negative for acquired immunodeficiency
syndrome in all patients. Informed written consent was obtained
from all patients from each hospital, and the present study was
approved by the Ethics Committee of Hokuto Hospital (Obihiro,
Japan). Histopathological diagnosis of malignant lymphoma (diffuse
large B cell type) was confirmed with each author. The analyses of
each patient in the present study are summarized in Table I.
 | Table I.Summary of analyses of patients with
PCNSL and DLBL. |
Table I.
Summary of analyses of patients with
PCNSL and DLBL.
|
|
|
|
|
| Immunostaining
score |
|---|
|
|
|
|
|
|
|
|---|
| Type of cancer | Case | Age | Sex | RT-qPCR (MCP-1;
arbitrary unit) | MCP-1 | CD14 | CD68 |
|---|
| PCNSL | P1 | 71 | F | 18.6 | 3 | 3 | 2 |
|
| P2 | 62 | M | 4.3 | 2 | 3 | 2 |
|
| P3 | 73 | F | 29.3 | 3 | 3 | 1 |
|
| P4 | 84 | M | 9.9 | 1 | 2 | 2 |
|
| P5 | 83 | M | 3.2 | 3 | 3 | 2 |
|
| P6 | 66 | M | 7.1 | 3 | 3 | 2 |
|
| P7 | 71 | M | NA | 2 | 2 | 2 |
|
| P8 | 85 | F |
5.4 | 2 | 3 | 1 |
|
| P9 | 50 | M |
6.5 | 3 | 1 | NA |
|
| P10 | 64 | F | NA | 2 | 3 | NA |
|
| P11 | 49 | M |
4.5 | 0 | 0 | NA |
|
| P12 | 80 | F | 39.5 | 3 | 0 | NA |
|
| P13 | 71 | F | 29.1 | 1 | 1 | NA |
|
| P14 | 80 | F | NA | 3 | 1 | NA |
|
| P15 | 44 | F | 57.6 | 1 | 1 | NA |
|
| P16 | 64 | M | 18.5 | NA | NA | NA |
|
| P17 | 49 | M |
1.1 | 3 | 3 | NA |
|
| P18 | 76 | M | 17.4 | 3 | 3 | NA |
|
| P19 | 81 | F | 12.1 | 3 | 3 | NA |
| DLBCL | D1 | 71 | M |
4.6 | 1 | 3 | 2 |
|
| D2 | 77 | M |
5.0 | 1 | 3 | 3 |
|
| D3 | 65 | M | NA | 1 | 0 | 1 |
|
| D4 | 75 | F |
1.0 | 2 | 1 | 1 |
|
| D5 | 69 | M |
4.9 | 2 | 1 | 2 |
|
| D6 | 79 | M | NA | 0 | 1 | 3 |
|
| D7 | 61 | F |
3.6 | 1 | 0 | 2 |
|
| D8 | 86 | M |
4.1 | 0 | 1 | 1 |
|
| D9 | 56 | F |
2.8 | NA | 1 | NA |
|
| D10 | 66 | M |
1.2 | NA | 1 | NA |
|
| D11 | 69 | M |
1.3 | NA | 2 | NA |
|
| D12 | 76 | M | NA | NA | 3 | NA |
|
| D13 | 83 | M |
4.5 | NA | 3 | NA |
|
| D14 | 92 | M |
2.9 | NA | 3 | NA |
|
| D15 | 82 | M |
4.2 | NA | 2 | NA |
|
| D16 | 58 | F | 1.1 | NA | 3 | NA |
RNA extraction, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
In total, 16 specimens with PCNSL and 13 specimens
with DLBCL were adequate for RNA extraction. Formalin-fixed
paraffin-embedded sections were deparaffinized using Paraffin
Dissolver (Machery-Nagel GmbH, Düren, Germany; cat. no. 740968.25),
according to the manufacturer's instruction. For preparation of
total RNA from tumors, the MasterPure RNA Purification kit
(Epicentre; Illumina, Inc., San Diego, CA, USA; cat. no. MCR85102)
was used according to the manufacturer's protocol. cDNA was
synthesized from total RNA using the High-Capacity cDNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The cDNA (50–60 ng) was used for qPCR
analyses via TaqMan Gene Expression Assays (Applied Biosystems;
Thermo Fisher Scientific, Inc.) for MCP-1 (prove ID: Hs0023410_m1;
Applied Biosystems; Thermo Fisher Scientific, Inc.). PCR was
conducted using the Applied Biosystems 7900HT Fast Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
real-time PCR conditions were 5 min at 95°C followed by 40 cycles
at 95°C for 15 sec and 60°C for 1 min. Ribosomal RNA (prove ID:
Hs99999901_s1; Applied Biosystems; Thermo Fisher Scientific, Inc.)
served as the reference gene. The relative quantification (RQ)
method was applied to determine gene expression levels. Values of
RQ within the range (RQ ± 2 standard deviations) of the
corresponding reference group were accepted as normal. Threshold
cycle (Cq) values were automatically calculated for each
replicate and used to determine the expression of the gene of
interest relative to that of the reference gene for treated and
untreated samples using the 2−ΔΔCq method (24).
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissues were
sectioned (4-µm-thick). Antigen epitopes were heat-retrieved in
ImmunoSaver™ (Nissin EM Ltd., Tokyo, Japan) at 98°C for 45 min. The
samples were stained using the following antibodies: MCP-1 (1:200;
Abcam, Cambridge, UK; cat. no. ab7202); cluster of differentiation
(CD)14 (1:100; Abcam; cat. no. ab183322); and CD68 (supplied at
working dilution; Nichirei Biosciences Inc. Tokyo, Japan; cat. no.
413791). The samples were incubated with each antibody at room
temperature for 1 h. The intensity scores were as follows: 0,
negative; 1, weakly positive; 2, moderately positive and 3,
strongly positive. The proportional scores were as follows: 0%; 1,
1–10%; 2, 11–50% and 3, 51–100%. Using a total score (intensity
score + proportional score), immunohistochemical positivity was
classified as negative (0), total score=0; weakly positive (+1),
total score=1, 2; moderately positive (+2), total score=3, 4; or
strongly positive (+3), total score=5, 6. These criteria were
determined by one of the co-authors who was blinded with respect to
the clinical data. The relative number of positive cells and the
intensity of staining were assessed in five random fields under a
light microscope and magnification ×200.
CCR2 and CD20, a B cell marker, were examined by
immunofluorescent double staining. For double staining with two
monoclonal antibodies, the cells were first incubated in CD20
antibody (1:400; Dako; Agilent Technologies, Inc., Santa Clara, CA,
USA; cat. no. M0755) at room temperature for 1 h, followed by the
CCR2 antibody (1:100; Abcam; cat. no. ab32144 at room temperature
for 1 h. Anti-mouse immunoglobulin (Ig)G (heavy and light chain;
H+L), F(ab)2 fragment (Alexa FluorR 555
conjugate; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA,
USA; cat. no. 4409) and anti-rabbit IgG (H+L), F(ab)2
Fragment (Alexa FluorR 488 conjugate; 1:1,000; Cell
Signaling Technology, Inc.; cat. no. 4412) were used as secondary
antibodies at room temperature for 1 h. Confocal fluorescence
images (magnification, ×400) were confirmed on a Carl Zeiss
confocal system (Zeiss GmbH, Jena, Germany) in 10 random fields by
two of the co-authors.
Cell culture
The human brain-derived lymphoma cell line, HKBML
(Riken BioResource Center, Tsukuba, Japan), was maintained in Ham's
nutrient mixture F-12 medium (Sigma-Aldrich; KGaA, Darmstadt,
Germany; cat. no. N6658) supplemented with 15% fetal bovine serum
and penicillin-streptomycin, and incubated in a 37°C, 5%
CO2 and a humidified atmosphere. Prior to stimulation
with 0, 250, 500 or 1,000 ng/ml MCP-1, the cells were preincubated
in F-12 medium containing 0.5% fetal bovine serum overnight. HKBML
proliferation activity was measured by 5-bromo-2′-deoxyuridine
(BrdU) incorporation into the cells, which were plated as floating
single cells at 2×104 cells per well onto 96 well
plates. Following a 3 day incubation with MCP-1, BrdU (10 µM) was
added for 2 h at 37°C and in a 5% CO2 humidified
atmosphere, using the Cell Proliferation ELISA kit (Roche
Diagnostics, Basel, Switzerland; cat. no. 11647229001), according
to the manufacturer's instruction. The media and no cells was used
as a negative control.
Western blotting
Cells were lysed for western blotting following
stimulation with 300 ng/ml MCP-1. The lysis buffer contained 50 mM
HEPES buffer, 1% Triton X-100, 5 mM EDTA, 50 mM sodium chloride, 10
mM sodium pyrophosphate, 50 mM sodium fluoride, 1 mM sodium
orthovanadate, and protease inhibitors (1 mM
phenylmethylsulfonylfluoride/1 µM leupeptin/1 µM pepstatin A).
Protein was quantified using BCA protein assay (Pierce; Thermo
Fisher Scientific, Inc.; cat. no. 23227) and crude lysates (25 µg)
were fractionated by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis, blotted onto polyvinylidene difluoride (PVDF)
membranes, incubated with primary antibodies against
mitogen-activated protein kinase (MAPK; Cell Signaling Technology,
Inc.; cat. no. 9101) and phosphorylated (p-)MAPK (Cell Signaling
Technology, Inc.; cat. no. 9102) or β-tubulin (Cell Signaling
Technology, Inc.; cat. no. 2182) as a loading control, followed by
incubation with horseradish peroxidase (HRP)-conjugated secondary
antibody (goat anti-rabbit IgG-HRP; Santa Cruz Biotechnology, Santa
Cruz, CA, USA; cat. no. cs-2004). For immunoblotting analysis,
membranes were incubated with diluted antibodies at a dilution of
1:2,000 in TBS buffer including 0.1% Tween-20 (TBST) with 5% w/v
bovine serum albumin and agitated at 4°C overnight. Incubation with
TBST including 5% non-fat dry milk for 1 h at room-temperature was
performed as the blocking step. Membranes were washed with TBST for
10 min three times and incubated with 1:4,000 diluted secondary
antibody for 1 h at room-temperature with agitation, followed by
washing with TBST three times. Signals were detected using
Immobilon Western Chemiluminescent HRP Substrate (EMD Millipore,
Billerica, MA, USA; cat. no. WBKLS0500).
Statistical analysis
The differences between two groups was analyzed
using two-tailed Student's t-test. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using StatView 5.0 software (SAS Institute
Inc., Cary, NC, USA).
Results
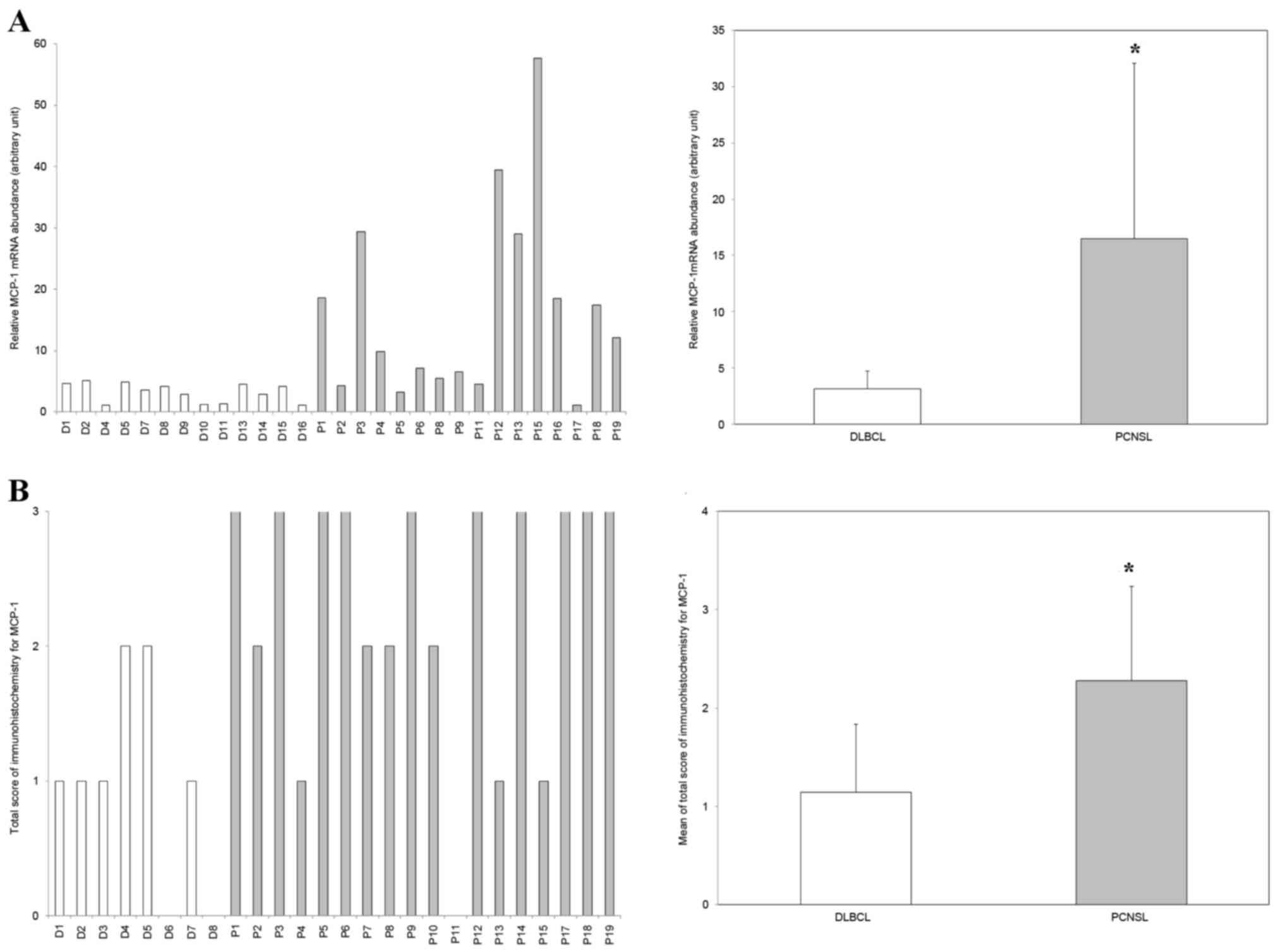
MCP-1 expression is upregulated in
PCNSL compared with DLBCL
MCP-1-specific RT-qPCR analysis revealed that
MCP-1 mRNA expression was upregulated >5-fold in PCNSL
compared with DLBCL (P<0.002; Fig.
1A). Immunohistochemistry also revealed that MCP-1 expression
levels trended toward upregulation in PCNSL compared with DLBCL
(P=0.002; Fig. 1B).
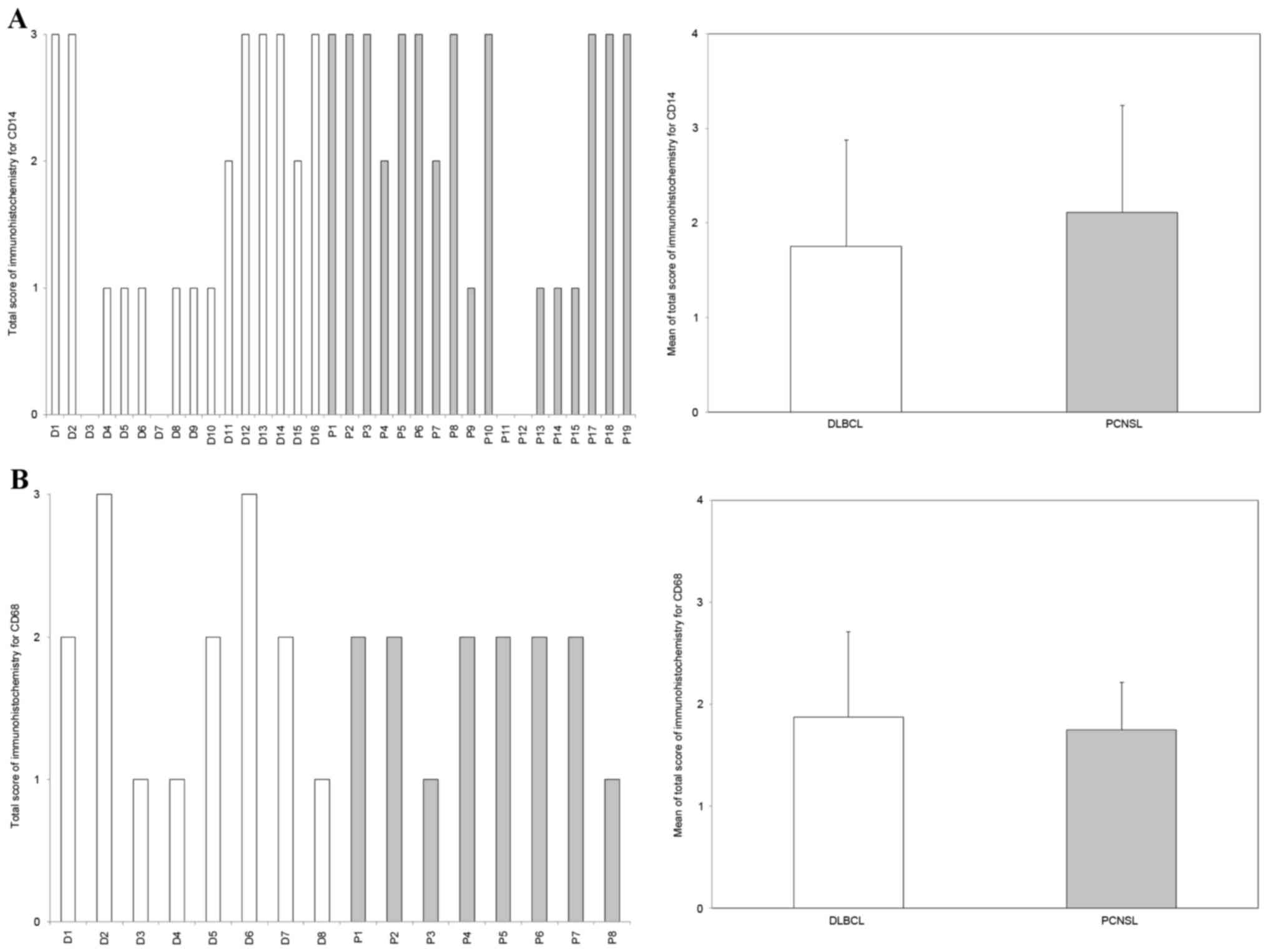
TAM infiltration levels are similar in
PCNSL and DLBCL
Immunohistochemistry revealed that CD14-positive and
CD68-positive TAMs infiltrated into multiple cases of PCNSL and
DLBCL. However, there was no difference in the ratio of
CD14-positive and CD68-positive TAMs between PCNSL and DLBCL
(P=0.18 and P=0.36, respectively; Fig.
2).
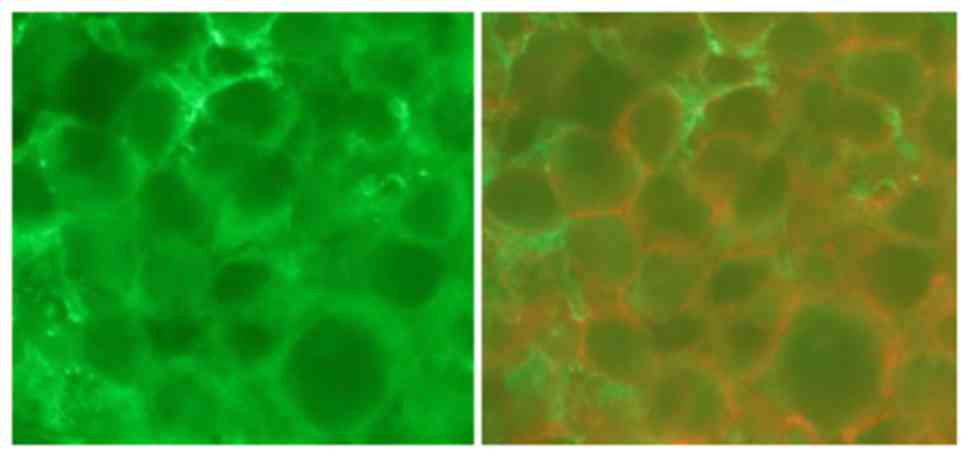
CCR2, a receptor of MCP-1, was present
in neoplastic PCNSL cells
Immunohistochemical staining with CD20 confirmed
that all cases were B cell type lymphoma. Immunofluorescence double
staining revealed that the CCR2 signal was concomitant with the
cells expressing B cell markers (Fig.
3).
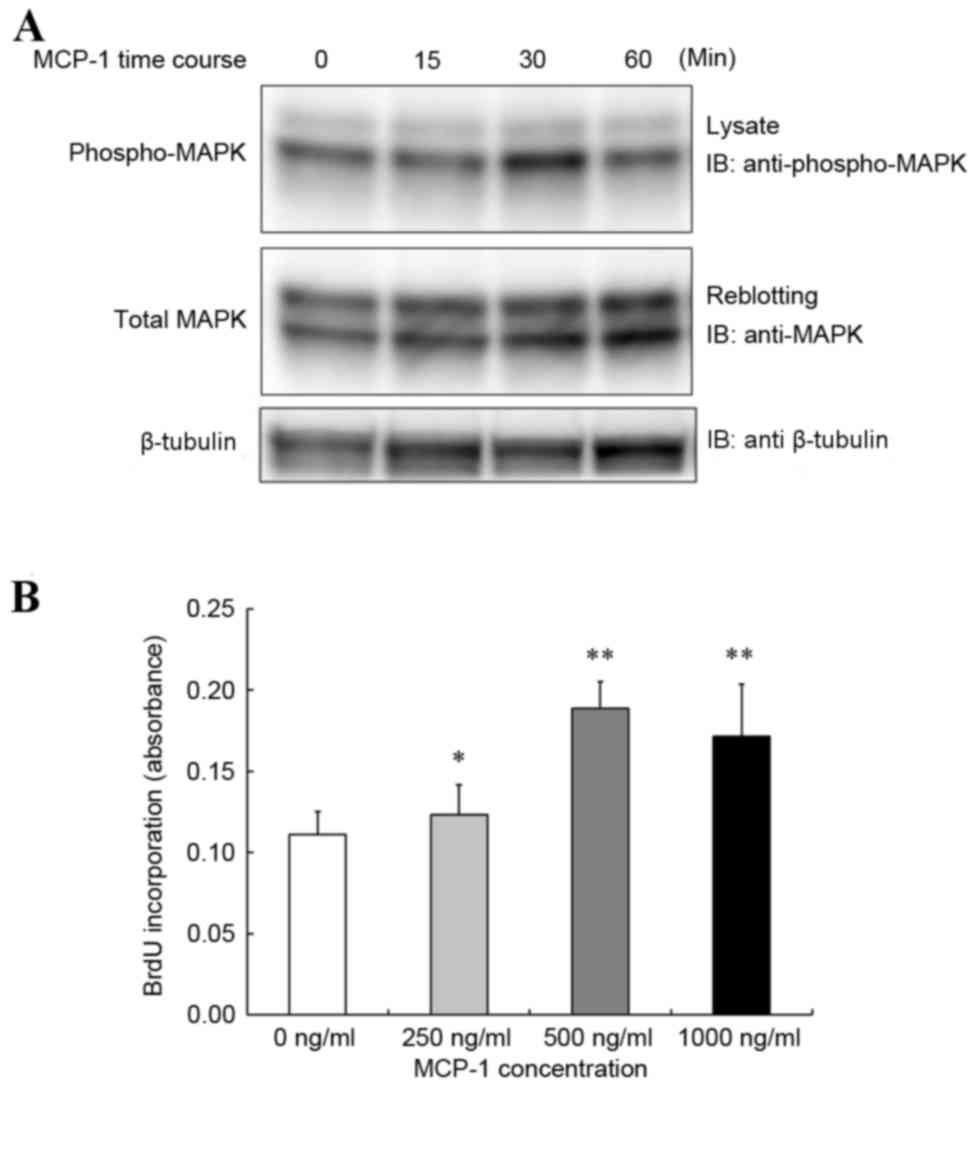
MCP-1 induces MAPK activation in a
PCNSL cell model
HKBML cells were stimulated with a dose of MCP-1
(300 ng/ml). MCP-1 induced tyrosine phosphorylation of MAPK, as
assessed by western blot analysis with a maximum activation
observed at 30 min (Fig. 4A).
MCP-1 increased cell proliferation in
PCNSL model
To assess the effect of MCP-1 on PCNSL cell
proliferation, HKBML cells were stimulated with increasing
concentrations of MCP-1. HKBML cells demonstrated increased
proliferation in response to MCP-1 compared with the untreated
control, with maximum proliferation increase observed at a dose of
500 ng/ml (Fig. 4B).
Discussion
In the present study, MCP-1 mRNA and protein
expression was demonstrated to be significantly upregulated in
PCNSL compared with DLBCL. To the best of our knowledge, there are
no previous reports comparing MCP-1 expression between these
lymphomas. Although the number of cases used in the present study
was limited, the results may be associated with the differences in
biological characteristics between PCNSL and DLBCL.
Because a well-known function of MCP-1 is TAM
recruitment, the degree of TAM infiltration was confirmed in each
type of lymphoma by immunohistochemistry, using CD14 and CD68 as
TAM markers. Notably, the degree of TAM infiltration did not differ
between the types of lymphoma. In multiple tumor types, the level
of CD68-positive TAM infiltration has been demonstrated to be of
significant prognostic value (21,25–27). On
the other hand, the results of studies concerning the prognostic
correlation between the level of infiltration of TAMs and DLBCL was
not consistent. Hasselblom et al (11) demonstrated that it was not possible to
predict clinical outcomes in DLBCL by evaluating CD68-positive TAM
concentration (11), whereas other
studies revealed that high TAM density was significantly associated
with poor prognosis (13) and worse
response to therapy (12). Two
additional studies demonstrated that TAMs in PCNSL were not
correlated with overall survival (14,15). Thus,
there is a possibility that MCP-1 serves a function other than
infiltrating TAMs in PCNSL.
Hence, the present study hypothesized that
MCP-1-upregulated lymphoma, PCNSL, may produce and maintain MCP-1.
The MCP-1 receptor, CCR2, has been reported to mediate the action
of the mitogen-activated protein kinase kinase/extracellular
signal-regulated kinase and phosphoinositide 3-kinase-protein
kinase B pathways when activated by MCP-1, which has been
implicated in cell proliferation and survival (28). There are no reports supporting an
autocrine function for MCP-1 in PCNSL. To evaluate the hypothesis,
PCNSL was confirmed to express CCR2 by double immunohistochemistry.
The results implied that the MCP-1/CCR2 axis functioned in an
autocrine manner in PCNSL, which appeared to worsen the
prognosis.
Therefore, the autocrine signaling mechanisms by
which MCP-1 acts in HKBML cells derived from PCNSL were
investigated. MAPK signal transduction pathways are involved in the
regulation of cell proliferation. The present study confirmed that
MCP-1 induced MAPK phosphorylation in HKBML cells, and further
demonstrated that MCP-1 increased MAPK phosphorylation in HKBML
cells. Next, to assess the effects of MCP-1 on PCNSL cell
proliferation, HKBML cells were stimulated with MCP-1. HKBML cell
proliferation increased in response to increasing doses of MCP-1.
These results implied that upregulation of MCP-1 expression
mediated the augmentation of tumor cell proliferation in PCNSL in
an autocrine manner, which appeared to worsen prognosis.
There were several limitations to the present study.
First, the number of patients with PCNSL and DLBCL enrolled in the
present study was small. PCNSL is rare disease and the amount of
tumor tissue obtained by biopsy or surgery is limited. Cooperation
among multiple hospitals was therefore necessary for the present
study. Second, the association between patient survival and MCP-1
expression was not investigated because the therapies used to treat
patients were different between the hospitals. Third, the
relationships between signaling pathways other than MAPK and MCP-1
were not evaluated.
In future studies, the effects of abolishing MCP-1
activity using MCP-1 antibodies should be confirmed in a PCNSL cell
line. In addition, the association between prognosis and MCP-1
expression should be investigated in a larger number of patients.
By further understanding the function of MCP-1 in PCNSL, it may be
possible to develop novel strategies to treat patients with
PCNSL.
In conclusion, the results of the present study
indicated that MCP-1 expression in PCNSL promoted cell
proliferation in an autocrine manner.
Acknowledgements
The human brain-derived lymphoma cell line, HKBML
was provided by Riken BioResource Center (Tsukuba, Japan). The
authors would like to thank Mrs. Masayo Obata (Department of
Neurosurgery, Graduate School of Medical Science, Kumamoto
University) for assisting with the immunohistochemistry.
References
|
1
|
Olson JE, Janney CA, Rao RD, Cerhan JR,
Kurtin PJ, Schiff D, Kaplan RS and O'Neill BP: The continuing
increase in the incidence of primary central nervous system
non-Hodgkin lymphoma: A surveillance, epidemiology, and end results
analysis. Cancer. 95:1504–1510. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prodduturi P and Bierman PJ: Current and
emerging pharmacotherapies for primary CNS lymphoma. Clin Med
Insights Oncol. 6:219–231. 2012.PubMed/NCBI
|
|
3
|
Bingle L, Brown NJ and Lewis C: The role
of tumour-associated macrophages in tumour progression:
Implications for new anticancer therapies. J Pathol. 196:254–265.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Komohara Y, Jinushi M and Takeya M:
Clinical significance of macrophage heterogeneity in human
malignant tumors. Cancer Sci. 105:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ding P, Wang W, Wang J, Yang Z and Xue L:
Expression of tumor-associated macrophage in progression of human
glioma. Cell Biochem Biophys. 70:1625–1631. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Komohara Y, Horlad H, Ohnishi K, Fujiwara
Y, Bai B, Nakagawa T, Suzu S, Nakamura H, Kuratsu J and Takeya M:
Importance of direct macrophage-tumor cell interaction on
progression of human glioma. Cancer Sci. 103:2165–2172. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Komohara Y, Hasita H, Ohnishi K, Fujiwara
Y, Suzu S, Eto M and Takeya M: Macrophage infiltration and its
prognostic relevance in clear cell renal cell carcinoma. Cancer
Sci. 102:1424–1431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Komohara Y, Niino D, Saito Y, Ohnishi K,
Horlad H, Ohshima K and Takeya M: Clinical significance of
CD163+ tumor-associated macrophages in patients with
adult T-cell leukemia/lymphoma. Cancer Sci. 104:945–951. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hasselblom S, Hansson U, Sigurdardottir M,
Nilsson-Ehle H, Ridell B and Andersson PO: Expression of CD68+
tumor-associated macrophages in patients with diffuse large B-cell
lymphoma and its relation to prognosis. Pathol Int. 58:529–532.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cai QC, Liao H, Lin SX, Xia Y, Wang XX,
Gao Y, Lin ZX, Lu JB and Huang HQ: High expression of
tumor-infiltrating macrophages correlates with poor prognosis in
patients with diffuse large B-cell lymphoma. Med Oncol.
29:2317–2322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wada N, Zaki MA, Hori Y, Hashimoto K,
Tsukaguchi M, Tatsumi Y, Ishikawa J, Tominaga N, Sakoda H, Take H,
et al: Tumour-associated macrophages in diffuse large B-cell
lymphoma: A study of the osaka lymphoma study group.
Histopathology. 60:313–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Komohara Y, Horlad H, Ohnishi K, Ohta K,
Makino K, Hondo H, Yamanaka R, Kajiwara K, Saito T, Kuratsu J and
Takeya M: M2 macrophage/microglil cells induce activation of Stat3
in primary central nervous system lymphoma. J Clin Exp Hematop.
51:93–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sasayama T, Tanaka K, Mizowaki T,
Nagashima H, Nakamizo S, Tanaka H, Nishihara M, Mizukawa K, Hirose
T, Itoh T and Kohmura E: Tumor-associated macrophages associate
with cerebrospinal fluid interleukin-10 and survival in primary
central nervous system lymphoma (PCNSL). Brain Pathol. 26:479–487.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiménez-Sainz MC, Fast B, Mayor F Jr and
Aragay AM: Signaling pathways for monocyte chemoattractant protein
1-mediated extracellular signal-regulated kinase activation. Mol
Pharmacol. 64:773–782. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnson Z, Power CA, Weiss C, Rintelen F,
Ji H, Ruckle T, Camps M, Wells TN, Schwarz MK, Proudfoot AE and
Rommel C: Chemokine inhibition - why, when, where, which and how?
Biochem Soc Trans. 32:366–377. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mellado M, Rodríguez-Frade JM, Aragay A,
Del Real G, Martín AM, Vila-Coro AJ, Serrano A, Mayor F Jr and
Martínez-A C: The chemokine monocyte chemotactic protein 1 triggers
Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B
receptor. J Immunol. 161:805–813. 1998.PubMed/NCBI
|
|
19
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Conti I and Rollins BJ: CCL2 (monocyte
chemoattractant protein-1) and cancer. Semin Cancer Biol.
14:149–154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fujiwara T, Fukushi J, Yamamoto S,
Matsumoto Y, Setsu N, Oda Y, Yamada H, Okada S, Watari K, Ono M, et
al: Macrophage infiltration predicts a poor prognosis for human
ewing sarcoma. Am J Pathol. 179:1157–1170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Loberg RD, Day LL, Harwood J, Ying C, St
John LN, Giles R, Neeley CK and Pienta KJ: CCL2 is a potent
regulator of prostate cancer cell migration and proliferation.
Neoplasia. 8:578–586. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kitai R, Ishisaka K, Sato K, Sakuma T,
Yamauchi T, Imamura Y, Matsumoto H and Kubota T: Primary central
nervous system lymphoma secretes monocyte chemoattractant protein
1. Med Mol Morphol. 40:18–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Steidl C, Lee T, Shah SP, Farinha P, Han
G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, et al:
Tumor-associated macrophages and survival in classic Hodgkin's
lymphoma. N Engl J Med. 362:875–885. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takayama H, Nishimura K, Tsujimura A,
Nakai Y, Nakayama M, Aozasa K, Okuyama A and Nonomura N: Increased
infiltration of tumor associated macrophages is associated with
poor prognosis of bladder carcinoma in situ after intravesical
bacillus Calmette-Guerin instillation. J Urol. 181:1894–1900. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan KL, Scott DW, Hong F, Kahl BS, Fisher
RI, Bartlett NL, Advani RH, Buckstein R, Rimsza LM, Connors JM, et
al: Tumor-associated macrophages predict inferior outcomes in
classic Hodgkin lymphoma: A correlative study from the E2496
Intergroup trial. Blood. 120:3280–3287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yao H, Peng F, Dhillon N, Callen S,
Bokhari S, Stehno-Bittel L, Ahmad OS, Wang JQ and Buch S:
Involvement of TRPC channels in CCL2-mediated neuroprotection
against tat toxicity. J Neurosci. 29:1657–1669. 2009. View Article : Google Scholar : PubMed/NCBI
|