Introduction
Angiopoietin (Ang)-1 and −2 are ligands of the Tie-2
receptor, which is a receptor tyrosine kinase expressed by
endothelial cells during vascular development. Ang-1 stabilizes the
mature vasculature by promoting the recruitment of pericytes and
smooth muscle cells. By contrast, Ang-2 destabilizes the
vasculature so that it assumes a flexible state more amenable to
sprouting under the influence of the angiogenic inducer vascular
endothelial growth factor (VEGF) (1).
Ang-1 participates in the regulation of angiogenesis and
lymphangiogenesis by competing with Ang-2 in binding to the Tie-2
receptor, which blocks Ang-1-mediated receptor phosphorylation and
vascular maturation and stabilization (1). However, this function may be
context-dependent; Ang-2 may induce endothelial cell apoptosis with
consequent vascular regression by loosening cell-matrix contacts in
the absence of VEGF while stimulating endothelial cell migration
and proliferation, thereby serving as a proangiogenic signal for
tumors in cooperation with VEGF and Ang-1 through Tie-2-dependent
and/or -independent signaling pathways (2). In addition, Ang-2 is upregulated in
various types of tumor, including breast (3), non-small cell lung (4), and gastric cancer (5), as well as hepatocellular carcinoma
(6) and melanoma (7).
Colorectal cancer (CRC) is the third most common
malignancy and the fourth highest cause of cancer mortality
worldwide (8). It had the second
highest incidence in 2010 and the highest rate of increase among
cancer types in South Korea (9). CRC
progression may lead to the dissemination of tumor cells to other
tissues including the bone, brain, liver and lungs, where new
tumors may arise. As metastatic colon cancer is associated with
high mortality (10,11), progression to metastasis is a turning
point for CRC development, which depends on an angiogenic network
(12). Although anti-metastatic
molecule-based therapies, including the use of monoclonal
antibodies, have been widely investigated (13), numerous patients with CRC still
succumb to disease recurrence and metastasis. Therefore, insight
into the association between the expression of angiogenic molecules
and clinicopathological factors relating to the progression and
prognosis of CRC is essential for developing novel therapeutic
strategies to treat this disease.
Among the various studies investigating the
expression of Ang-2 and its role in CRC, research has primarily
focused on tumor angiogenesis (14),
and the link between Ang-2 expression and the biological
characteristics of CRC has seldom been investigated. The oncogenic
function of Ang-2 has not yet been studied and it remains to be
elucidated. It was previously revealed that Ang-2 overexpression is
associated with the invasive phenotype of CRC cells in vitro
(15). The clinical significance of
Ang-2 expression in CRC and its effects on tumor growth remain
unclear. The current study investigated the malignant biological
functions of Ang-2 as an oncogene using short hairpin (sh) RNA
knockdown of the endogenous transcript in the LoVo colon cancer
cell line. Furthermore, endogenous Ang-2 expression in CRC tumor
cells was correlated with clinicopathological parameters. The in
vitro results demonstrate that Ang-2 may be an oncogene, and
its expression may serve as a prognostic marker and a potential
therapeutic target for CRC.
Materials and methods
Tissue specimens and patient
information
A total of 415 CRC tissue specimens containing tumor
and adjacent normal tissue were collected from patients who were
diagnosed and underwent colectomy or Miles' operation at
Soonchunhyang University Cheonan Hospital (Cheonan, Korea) between
January 2002 and December 2009. Specimens obtained from surgical
resection were fixed in 10% neutral buffered formalin for 24 h at
RT prior to being processed in paraffin. Patients did not receive
any treatment prior to surgery. Patient data are presented in
Table I. Informed consent was
provided by all patients and the study protocol was approved by the
Ethics Committee of Soonchunhyang University Cheonan Hospital.
 | Table I.Comparison of clinicopathological
factors and Ang-2 expression. |
Table I.
Comparison of clinicopathological
factors and Ang-2 expression.
|
| Ang-2 |
|
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
factors | High Exp.
(N=190) | Low Exp. (N=225) | Total (N=415) | P-value |
|---|
| Age, years (SD) | 63.3±12.9 | 62.4±12.3 | 62.8±12.6 | 0.488 |
| Sex, N (%) |
|
|
| 0.764 |
| Male | 107 (56.3) | 130 (57.8) | 237 (57.1) |
|
|
Female | 83 (43.7) | 95 (42.2) | 178 (42.9) |
|
| pT stage, N (%) |
|
|
| 0.048 |
| 1 | 7 (3.7) | 14 (6.4) | 21 (5.1) |
|
| 2 | 25 (13.2) | 38 (16.9) | 63 (15.2) |
|
| 3 | 122 (64.2) | 148 (65.8) | 270 (65.1) |
|
| 4 | 36 (18.9) | 25 (11.1) | 61 (14.7) |
|
| pN stage, N
(%) |
|
|
| <0.001 |
| 0 | 86 (45.3) | 140 (62.2) | 226 (54.5) |
|
| 1 | 56 (29.5) | 63 (28.0) | 119 (28.7) |
|
| 2 | 48 (25.3) | 22 (9.8) | 70 (16.9) |
|
| Venous
invasion |
|
|
| 0.023 |
| 0 | 142 (74.7) | 187 (83.1) | 329 (79.3) |
|
| 1 | 48 (25.3) | 38 (16.9) | 86 (20.7) |
|
| Lymphatic
invasion |
|
|
| <0.001 |
| 0 | 117 (61.6) | 184 (81.8) | 301 (72.5) |
|
| 1 | 73 (38.4) | 41 (18.2) | 114 (27.5) |
|
| TNM stage |
|
|
| 0.022 |
| I | 24 (12.6) | 38 (16.9) | 62 (14.9) |
|
| II | 63 (33.2) | 98 (43.6) | 161 (38.8) |
|
|
III | 91 (47.9) | 82 (36.4) | 173 (41.7) |
|
| IV | 12 (6.3) | 7 (3.1) | 19 (4.6) |
|
Tissue microarray construction and
immunohistochemistry
Tissue microarrays were produced from
formalin-fixed, paraffin-embedded (FFPE) CRC blocks. Two cores of
FFPE CRC block (2 mm in diameter) were embedded in a recipient
block using a 2-mm puncher (Unitech Science, Seoul, South Korea).
The paraffin tissue microarray (TMA) blocks were sectioned at 4-µm
thickness and transferred on the glass slide. The slides were baked
for 1 h at 60°C, deparaffinized with 100% zylene at 3 times for 5
min, hydrated in gradient ethanol (100–70%) and washed in 0.01 M
PBS at pH 7.2±7.4. The antigens were retrieved by heating the
slides in 0.01 M citrate buffer (pH 6.0, Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) in a pressure cooker for 15 min.
Sections were incubated overnight at 4°C with human Ang-2
polyclonal antibody (dilution, 1:20; cat. no. GTX100991, GeneTex,
Inc., Irvine, CA, USA). Incubation with secondary antibody was
performed using an EnVision Horseradish Peroxidase-Labeled Polymer
(cat. no. K400311, Dako; Agilent Technologies, Inc., Santa Clara,
CA, USA) for 30 min, at room temperature followed by treatment with
100 µl 3,3′-diaminobenzidene (Dako; Agilent Technologies, Inc.).
The TMA slides were counterstained with Harris's hematoxylin for 20
sec, dehydrated in gradient ethanol (80–100%), cleared with 100%
zylene and mounted with Canada balsam (cat. no. C1795,
Sigma-Aldrich; Merck Millipore). Immunostaining was scored by
counting 5 random fields using a light microscope for each stained
tissue section according to a semi-quantitative optical analysis by
three independent investigators blinded to the clinical data, and a
consensus score was determined for each specimen.
Immunohistochemistry data
analysis
The expression of Ang-2 was assessed in terms of
staining intensity and the percentage of positive cells by light
microscopy, as previously described (7). Staining intensity was scored as follows:
0) no detectable staining; 1) faint staining; 2) moderate staining;
3) intense staining. The percentage of positive cells was also
classified as one of four categories: 0) (0%), 1) (1–10%), 2)
(11–50%), and 3) (51–100%). The final score, calculated as the
product of the intensity and percentage scores, was grouped into
low and high expression (low; scores ≤3 and high; scores ≥4).
Cell lines and culture
Five human CRC cell lines (HCT116, SW480, LoVo, HT29
and colo205) were obtained from the Korean cell line bank (Seoul,
Korea). The CCD841 CoN normal colon epithelial cells and HUVEC
cells were obtained from the American Type Culture Collection
(Manassas, VA, USA). The CRC cell lines (HCT116, SW480, LoVo, HT29
and Colo205) were screened to select a high Ang-2-expressing line.
The CCD841 CoN normal colon epithelial cell line was used as Ang-2
expressing negative control and the vascular endothelial HUVEC cell
line was used as Ang-2 expressing positive control. Cells were
cultured in RPMI 1640 medium supplemented with 10% fetal bovine
serum and 1% penicillin-streptomycin (all from Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C in an atmosphere
containing 5% CO2. Ang-2 expression levels were evaluated by
reverse transcription-polymerase chain reaction (RT-PCR) and
western blot analysis. LoVo cells expressed the highest levels of
Ang-2 and were therefore used in the following assays.
RT-PCR
CRC HCT116, SW480, LoVo, HT29, Colo205, CCD-841, and
HUVEC cell lines were lysed using 1 ml of QIAzol in the RNeasy kit
(Qiagen, Inc., Valencia, CA, USA) to extract RNAs. The total RNA of
shRNA-transfected LoVo cells was extracted 4 days after
transfection. A total of 1 µg RNA was reverse transcribed using the
ReverTra Ace qPCR kit (Toyobo Co., Ltd., Osaka, Japan).
Semi-quantitative PCR was performed using the Maxime PCR PreMix kit
(Intron Biotechnology, Inc., Sungnam, Korea) and the following
primer pairs: Ang-2 forward, 5′-GGATCTGGGGAGAGAGGAAC-3′; reverse,
5′-CTCTGCACCGAGTCATCGTA-3′; GAP DH forward,
5′-ACCACTTTGTCAAGCTCATT-3′ and reverse, 5′-AGGAAGAGAGAGACCCTCAC-3′.
PCR amplification of Ang-2 and GAPDH was performed in 35 cycles
(Ang-2) or 38 cycles (GAPDH) as follows: 30 sec of denaturing at
95°C, 60 sec (Ang-2) or 30 sec (GAPDH) of annealing at 56°C (Ang-2)
and 58°C (GAPDH), and 30 sec of extension at 72°C. PCR products
were analyzed and visualized by capillary electrophoresis (Qiagen,
Inc.).
Western blotting
CRC HCT116, SW480, LoVo and HT29 cells were
collected in buffer (62.5 mmol/l Tris-HCl, pH 6.8; 10% glycerol; 2%
sodium dodecyl sulfate) and heated for 10 min at 100°C. Protein
concentration was determined by the bicinchoninic acid assay using
an xMark Microplate Absorbance Spectrophotometer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Equal quantities (30 µg/20
µl) of protein were separated by electrophoresis on Mini-PROTEAN
TGX precast gels (Bio-Rad Laboratories, Inc.), using β-actin as a
loading control, and were transferred onto an Immobilon
polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA),
which was probed with anti-human Ang-2 polyclonal antibody
(dilution, 1:2,000; GeneTex, Inc.). The signal was detected using
an enhanced chemiluminescence kit (Advansta, Menlo Park, CA, USA)
and a Molecular Imager ChemiDoc XRS+ system (Bio-Rad Laboratories,
Inc.).
RNA interference
LoVo cells (1×106) were grown overnight
in RPMI 1640 medium in six-well culture plates to 60–70% confluence
prior to transfection with Ang-2 shRNA lentiviral particles
(TRCN000005923, TRCN000005924, TRCN000005925, TRCN000005926, and
TRCN000005927), which were purchased from Sigma-Aldrich (Merck
Millipore) along with a non-target negative control shRNA
(SHC002V). The negative controls consisted of shRNA untransfected
LoVo cells and non-target negative control shRNA (HSC002V)
transfected LoVo cells. Lentiviral transfection was performed
according to the manufacturer's protocol. Transfected cells were
cultured for 2 additional days prior to puromycin selection (1
mg/ml; Sigma-Aldrich; Merck Millipore). Ang-2 levels were
determined by RT-PCR and western blotting.
Cell proliferation assay
The negative controls and Ang2-target shRNA
transfected LoVo cells were seeded (1×104 cells/well) in
96-well culture plates and incubated for 48 h. An MTT assay was
performed by adding 10 µl MTT (5 mg/ml; Sigma-Aldrich; Merck
Millipore) to each well prior to incubation for 4 h at 37°C,
followed by the addition of 100 µl dimethyl sulfoxide to each well
prior to overnight incubation. The following day, cell
proliferation/viability was measured as the absorbance at 570
nm.
Invasion assay
The negative controls and Ang2-target shRNA
transfected LoVo cells were cultured for 48 h in serum-free RPMI
1640 with penicillin-streptomycin (5 U/ml); 6.5-mm polycarbonate
filters (8-µm pore size, EMD Millipore, Billerica, MA, USA) were
coated with growth factor-reduced Matrigel (BD Biosciences, San
Jose, CA, USA) and incubated for 1 h at 37°C prior to use. Cells
(1×105/well) were resuspended in the top chamber while
700 µl culture medium with 10% FBS was added to the bottom chamber
as a chemoattractant. After 48 h incubation at 37°C, the medium in
the bottom chamber was removed, and cells were fixed with 3.5%
paraformaldehyde and their nuclei stained with methyl green.
The number of cells that had migrated through the
pores of the filter and into the bottom chamber was counted under a
phase contrast microscope. Cells in five random visual fields at
×20 magnification were counted for each well and used to determine
an average value. The experiment was performed in triplicate.
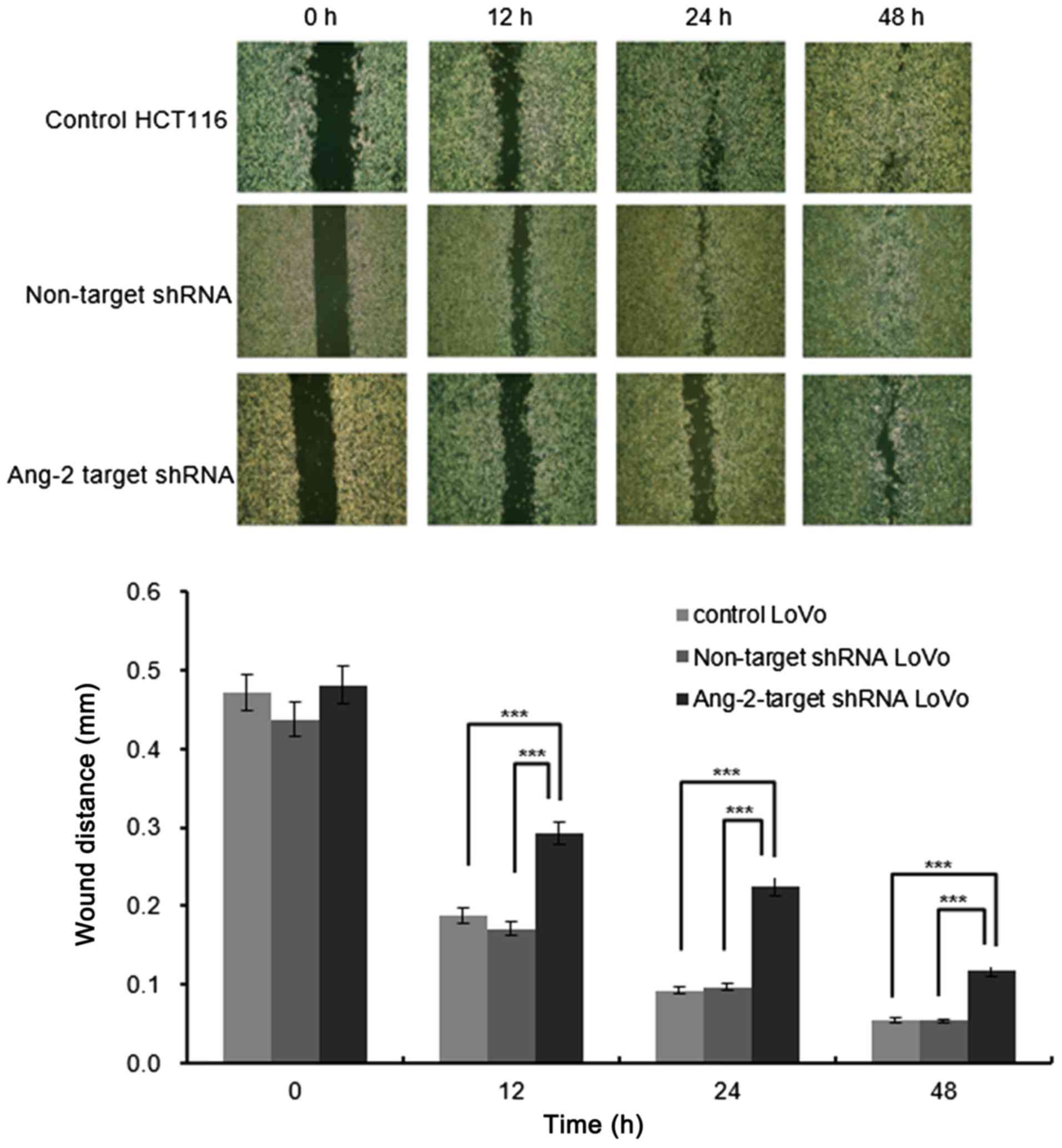
Cell migration assay
A wound healing cell migration assay was performed
using the 24-well cell culture insert system (Ibidi GmbH, München,
Germany). The negative controls or Ang2-target shRNA transfected
LoVo cells were resuspended in RPMI 1640 containing 10% FBS and
were seeded (1×105 cells) into each well of the insert.
After 48 h, the inserts were removed and cells were cultured in
fresh medium. Images were captured using a phase contrast
microscope with an AxioCam camera (Zeiss, Jena, Germany). The size
of the cell-free gap was monitored for 12, 24 and 48 h and measured
in five random visual fields to determine an average value.
Statistical analysis
Data were analyzed using SPSS version 18.0 (SPSS,
Inc., Chicago, IL, USA). The clinical and pathological factors that
were evaluated included primary tumors (pT; 1, 2, 3 and 4), primary
nodes (pN; 0, 1 and 2), vascular and lymphatic invasion and TNM
stage classification (I, II, III and IV). The association between
Ang-2 expression and clinicopathological factors was evaluated with
the χ2 test and a two-sided t-test. A survival analysis
using the Kaplan-Meier method was performed based on clinical and
pathological variables and Ang-2 expression, and the statistical
significance at P<0.05 was assessed by the log-rank test. A
multivariate Cox regression analysis in which all covariates were
controlled was performed to evaluate the association between
clinicopathological factors and Ang-2 expression, with hazard
ratios (HR) and 95% confidence intervals (CI) estimated from Cox
proportional hazard models. P<0.05 was considered to indicate a
statistically significant difference.
Results
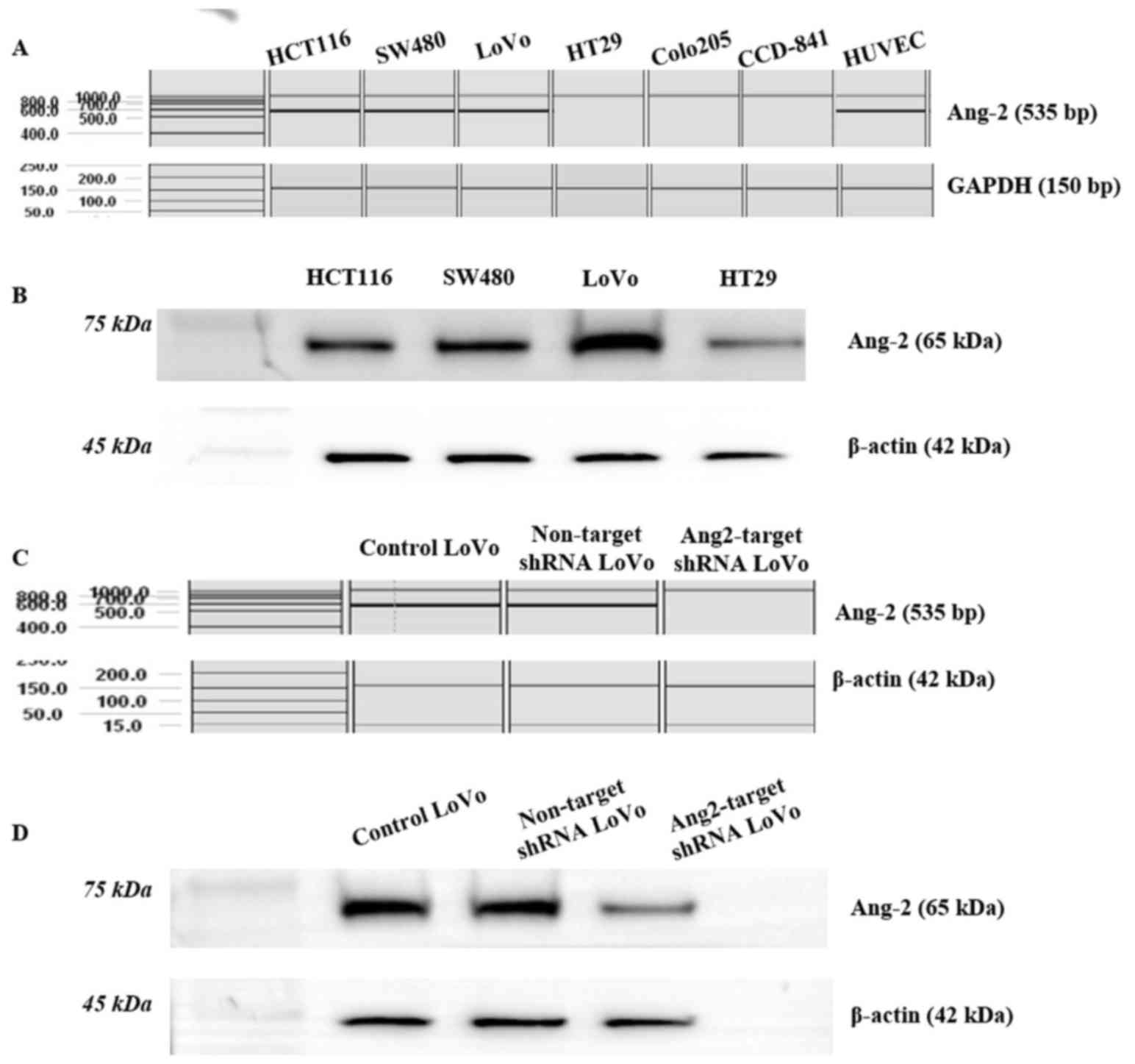
Ang-2 is expressed in CRC cell lines
and HUVECs
HUVECs expressed a high level of Ang-2 and were used
as a positive control. Three of the five colorectal cancer cell
lines screened (HCT116, SW480 and LoVo) had Ang-2 levels that were
comparable with those of HUVECs, whereas the CCD-841CoN cell line
did not express Ang-2 (Fig. 1A). LoVo
cells had the highest Ang-2 level as determined by western blot
analysis (Fig. 1B) and they were used
for shRNA knockdown studies. The shRNA-mediated knockdown of Ang-2
in LoVo cells was confirmed by RT-PCR (Fig. 1C) and by western blotting (Fig. 1D). The Ang-2 transcript and protein
were detected in untransfected control LoVo cells and non-target
shRNA transfected LoVo cells, but they were not expressed in
Ang-2-target shRNA transfected LoVo cells, indicating that
endogenous Ang-2 expression was effectively suppressed (Fig. 1C and D).
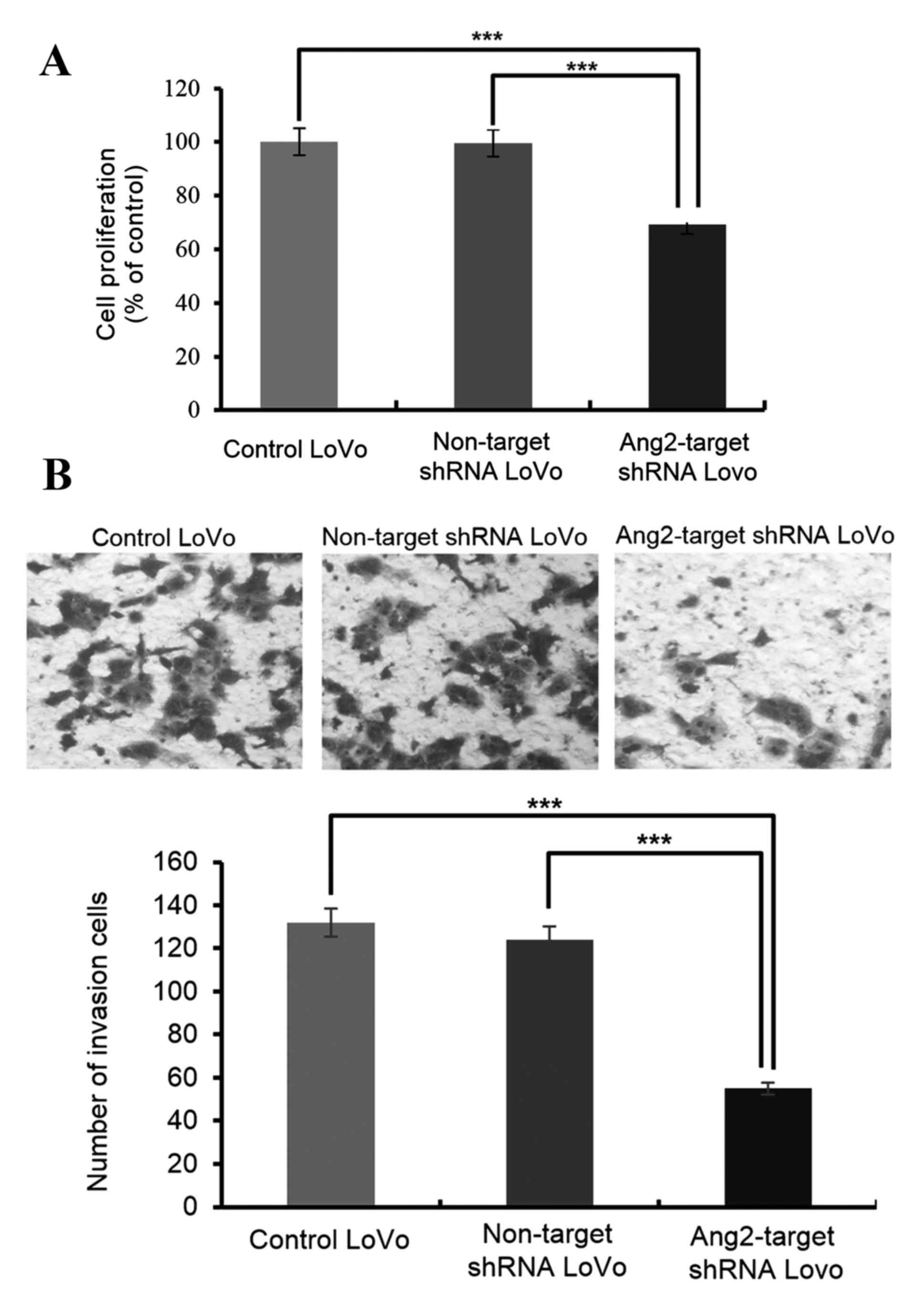
Ang-2 knockdown leads to the loss of
malignant features in CRC cells
Malignant cells are characterized by the capacity to
proliferate, migrate and invade tissues. Ang-2 knockdown resulted
in a 35% decrease in cell proliferation compared with untransfected
controls or non-target shRNA transfected LoVo cells, as determined
by the MTT assay (Fig. 2A).
Similarly, cell invasion was markedly decreased by Ang-2 knockdown
(Fig. 2B). Furthermore, compared with
control cells, the cell migration at all the time points examined
(12, 24 and 48 h) was reduced upon knockdown of Ang-2 (Fig. 3).
Ang-2 overexpression is associated
with clinical features of CRC
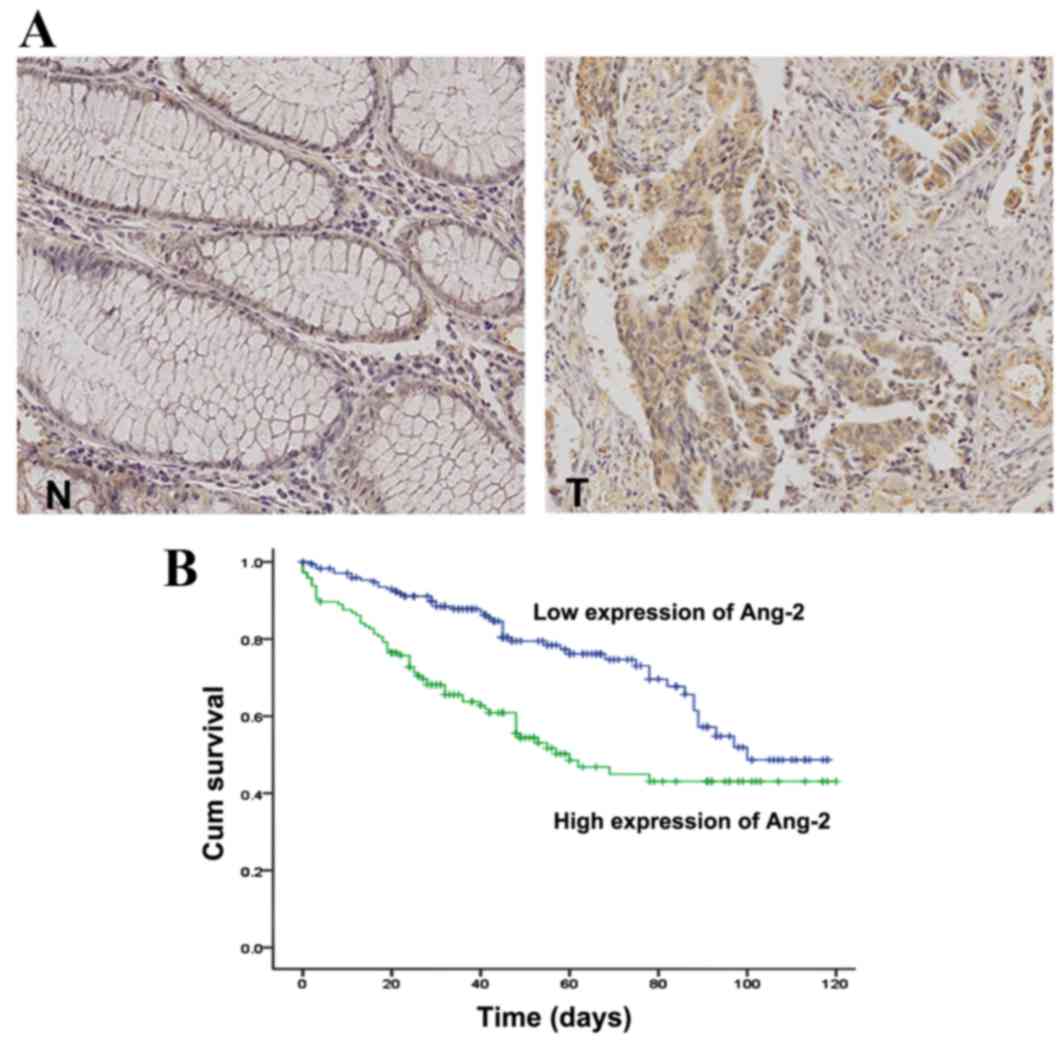
Ang-2 expression in tissue specimens from patients
with CRC was examined by immunohistochemistry. Ang-2 was rarely
observed in normal colorectal epithelial cells but was intensely
stained in the cytoplasm of tumor cells (Fig. 4A). Elevated Ang-2 expression was
observed in 190/415 cases (46%) of CRC. The association between
Ang-2 expression and clinicopathological parameters of 415 patients
with CRC is presented in Table I.
Although there were no correlations with patient age or sex, Ang-2
expression was associated with pT and pN stages (P<0.05 and
P<0.001, respectively), venous and lymphatic invasion (P=0.023
and P<0.001, respectively), and clinical stage (P=0.022).
The multivariate Cox proportional hazards regression
analysis revealed that pN stages 1 and 2 were significantly
association with high mortality, with HRs of 5.17 (95% CI,
1.36–19.59; P=0.016) and 7.01 (95% CI, 1.76–27.96; P=0.006),
respectively. Furthermore, Ang-2 expression in patients with CRC
was associated with high mortality, with an HR of 6.78 (95% CI,
4.16–11.05; P<0.001), indicating that Ang-2 expression is an
independent prognostic factor for CRC progression (Table II). The mean survival time was
markedly diminished in patients with high Ang-2 expression levels
compared with those with low Ang-2 expression levels (P=0.001;
Fig. 4B).
 | Table II.Cox regression analysis of
clinicopathological factors. |
Table II.
Cox regression analysis of
clinicopathological factors.
| Clinicopathological
factors | Hazard ratio (95%
CI) | P-value |
|---|
| Age, years | 1.03
(1.01–1.04) | 0.003 |
| Sex |
|
|
|
Female |
|
|
|
Male | 0.90
(0.62–1.31) | 0.569 |
| pT stage |
| 0.291 |
| 1 | 1.00 |
|
| 2 | 0.67
(0.14–3.19) | 0.618 |
| 3 | 0.46
(0.09–2.50) | 0.371 |
| 4 | 0.77
(0.13–4.47) | 0.770 |
| pN stage |
|
|
| 0 | 1.00 |
|
| 1 | 5.17
(1.36–19.59) | 0.016 |
| 2 | 7.01
(1.76–27.96) | 0.006 |
| Venous
invasion |
|
|
| 0 |
|
|
| 1 | 1.27
(0.72–2.26) | 0.410 |
| Lymphatic
invasion |
|
|
| 0 |
|
|
| 1 | 0.90
(0.52–1.57) | 0.719 |
| TNM stage |
|
|
| I | 1.00 |
|
| II | 1.83
(0.68–4.99) | 0.234 |
|
III | 0.43
(0.12–1.59) | 0.206 |
| IV | 1.24
(0.31–5.00) | 0.761 |
| Ang-2
expression |
|
|
| 0 |
|
|
| 1 | 6.78
(4.16–11.05) | <0.001 |
Discussion
Rapidly proliferating tumor cells require a greater
oxygen supply than normal cells, creating a relatively hypoxic
microenvironment surrounding the tumor; this triggers the secretion
of hypoxia-inducible factors from endothelial or tumor cells that
induce cytokines, such as angiopoietin, VEGF, and Tie (16–19).
Constitutive, low-level expression of Ang-1 by normal tissue
stabilizes existing blood vessels, whereas Ang-2 overexpression in
newly formed tumors leads to vessel destabilization and hypoxia;
therefore, cancer progression depends on the dysregulation of the
balance between normal and pathological neoangiogenesis (14).
The current study revealed that endogenous Ang-2
expression in cancer cell lines is associated with its aggressive
phenotype as a potential oncogene, including processes such as
proliferation, migration and invasion. The inhibition of Ang-2 in
the LoVo CRC cell line, which has a high endogenous level of Ang-2,
resulted in decreased proliferation, migration and invasion, which
may indicate that Ang-2 affects these aspects of the tumorigenic
phenotype as a potential oncogene in CRC.
The tumor tissue is composed of various types of
cell, including tumor parenchymal cells, stromal cells, immune
inflammatory cells and vascular cells. The origin of Ang-2 in tumor
tissue is not certain. Although early studies indicated that Ang-2
was primarily expressed in endothelial cells, other studies now
point to its expression in various tumor parenchymal cells,
including breast, gastric and lung cancer as well as melanoma and
hepatocellular carcinoma (3–7); however, the role of Ang-2 in CRC is
controversial. It has been reported that Ang-2 is almost
exclusively produced by endothelial cells themselves, suggesting an
autocrine mode of neoangiogenic action (20). One study reported high Ang-2
expression in tumor cells (21),
whilst another reported high Ang-2 expression in the stromal
compartment of CRC tissues but not in tumor cells (22).
In the current study, Ang-2 overexpression was
significantly associated with an aggressive tumor phenotype, as
evidenced by pT, pN and TNM stages and venous and lymphatic
invasion. Ang-2 overexpression in CRC was an independent prognostic
factor for decreased survival in pN stages 1 and 2, which is
concordant with the findings of a previous study, which
demonstrated that the upregulation of Ang-2 expression in CRC was
associated with lymph node metastasis (19).
Ang-2 inhibition decreases tumor size as well as
lumen formation in tumor-associated vessels in vivo
(20). Although the precise
underlying mechanism of Ang-2 function in CRC remains unknown, in
triple-negative breast cancer Ang-2 secreted by tumor cells
breaches the blood brain barrier, leading to brain metastasis
(23). Ang-2 promotes metastasis by
inducing epithelial-mesenchymal transition through β1
integrin-mediated signaling. Structurally, Ang-2 has a highly
conserved N-terminal fibrinogen-like receptor-binding domain that
may interact with specific integrin receptors (24). The association between Ang-2
expression and aggressive tumor phenotype has been studied in U87MG
glioma cells, in which upregulation of Ang-2 expression leads to
increased invasion (25).
To the best of our knowledge, this study is the
first to report a major role for Ang-2 in tumor progression that
involves stimulatory effects on angiogenesis through the paracrine
function of Ang-2 secreted by tumor cells. It remains to be
established whether Ang-2 is expressed at the early stages of the
tumor. The growth rates of tumors derived from lung cancer and
melanoma cell lines differ for wild-type and Ang-2-deficient mice
during the early stages of tumor development, whereas the growth
rates were similar during the later stages of primary tumor
progression (26). However, in the
present study, 83.2% of pT3 and 4 cases exhibited Ang-2
overexpression, and only 16.8% of pT1 and 2 cases had Ang-2
overexpression, which may indicate that Ang-2 expression exerts
fewer effects in the early stages of colorectal cancer compared
with the late stage as a function of tumor progression. This
difference may be due to the variability of cancer tissues. In one
previous study, Ang-2 expression was significantly upregulated in
CRC and associated with lymph node metastases (27), consistent with the observations of the
present study, in which Ang-2 overexpression was associated with
TNM stages III and IV and lymph node metastasis.
The diverse roles of Ang-2 in tumor progression have
been extensively studied. However, the focus has been on its
paracrine effect on angiogenesis through a Tie2-dependent pathway
in endothelial cells (28), rather
than through its aberrant overexpression in tumor cells and
autocrine effects, which is closely associated with tumor
progression. For instance, in gastric cancer, endogenous Ang-2
expression in tumor cells has a critical role in tumor metastasis
and is correlated with Ang-2 expression in endothelial cells
(29).
In conclusion, the present study revealed that
production of Ang-2, functioning as a potential oncogene in
vitro, in cancer cell lines resulted in aggressive cell
phenotypes. Ang-2 expression in tumor parenchymal cells may be a
marker of CRC progression that is associated with the clinical
outcome of patients. Given its involvement in a wide range of
tumor-specific processes, Ang-2 is a potential therapeutic target
for CRC treatment. Additional studies are underway to investigate
the factors downstream of Ang-2 that are responsible for modulating
these various cellular processes by promoting invasion and
metastasis.
Acknowledgements
The present study was supported by the Soonchunhyang
University Research Fund.
References
|
1
|
Fiedler U, Krissl T, Koidl S, Weiss C,
Koblizek T, Deutsch U, Martiny-Baron G, Marmé D and Augustin HG:
Angiopoietin-1 and angiopoietin-2 share the same binding domains in
the Tie-2 receptor involving the first Ig-like loop and the
epidermal growth factor-like repeats. J Biol Chem. 278:1721–1727.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu B and Cheng SY: Angiopoietin-2:
Development of inhibitors for cancer therapy. Curr Oncol Rep.
11:111–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rykala J, Przybylowska K, Majsterek I,
Pasz-Walczak G, Sygut A, Dziki A and Kruk-Jeromin J: Angiogenesis
markers quantification in breast cancer and their correlation with
clinicopathological prognostic variables. Pathol Oncol Res.
17:809–817. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang ZL, Liu ZS and Sun Q: Expression of
angiopoietins, Tie2 and vascular endothelial growth factor in
angiogenesis and progression of hepatocellular carcinoma. World J
Gastroenterol. 12:4241–4245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanaka F, Ishikawa S, Yanagihara K,
Miyahara R, Kawano T, Li M, Otake Y and Wada H: Expression of
angiopoietins and its clinical significance in non-small cell lung
cancer. Cancer Res. 62:7124–7129. 2002.PubMed/NCBI
|
|
6
|
Moom WS, Park HS, Yu KH, Jang KY, Kang MJ,
Park H and Tarnawski AS: Expression of angiopoietin 1, 2 and their
common receptor Tie2 in human gastric carcinoma: Implication for
angiogenesis. J Korean Med Sci. 21:272–278. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Helfrch I, Edler L, Sucker A, Thomas M,
Christian S, Schadendorf D and Augustin HG: Angiopoietin-2 levels
are associated with disease progression in metastatic malignant
melanoma. Clin Cancer Res. 15:1384–1392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2008. CA Cancer J Clin. 58:71–96. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG
and Lee JS: Cancer statistics in Korea: Incidence, mortality,
survival and prevalence in 2010. Cancer Res Treat. 45:1–14. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldberg RM: Therapy for metastatic
colorectal cancer. Oncologist. 11:981–987. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Haddad AJ, Hani M Bani, Pawlik TM and
Cunningham SC: Colorectal liver metastases. Int J Surg Oncol.
2011:2858402011.PubMed/NCBI
|
|
12
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scott AM, Wolchok JD and Old LJ: Antibody
therapy of cancer. Nat Rev Cancer. 12:278–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahmad SA, Liu W, Jung YD, Fan F, Wilson M,
Reinmuth N, Shaheen RM, Bucana CD and Ellis LM: The effects of
angiopoietin-1 and -2 on tumor growth and angiogenesis in human
colon cancer. Cancer Res. 61:1255–1259. 2001.PubMed/NCBI
|
|
15
|
Rigamonti N, Kadioglu E, Keklikoglou I,
Rmili C Wyser, Leow CC and De Palma M: Role of angiopoietin-2 in
adaptive tumor resistance to VEGF Signaling Blockade. Cell Rep.
8:696–706. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rankin EB and Giaccia AJ: The role of
hypoxia-inducible factors in tumorigenesis. Cell Death Differ.
15:678–685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peters KG: Vascular endothelial growth
factor and the angiopoietins: Working together to build a better
blood vessel. Circ Res. 83:342–343. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li C, Sun CJ, Fan JC, Geng N, Li CH, Liao
J, Mi K, Zhu GQ, Ma H, Song YF, et al: Angiopoietin-2 expression is
correlated with angiogenesis and overall survival in oral squamous
cell carcinoma. Med Oncol. 30:5712013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ochiumi T, Tanaka S, Oka S, Hiyama T, Ito
M, Kitadai Y, Haruma K and Chayama K: Clinical significance of
angiopoietin-2 expression at the deepest invasive tumor site of
advanced colorectal carcinoma. Int J Oncol. 24:539–547.
2004.PubMed/NCBI
|
|
20
|
Scharpfenecker M, Fiedler U, Reiss Y and
Augustin HG: The Tie-2 ligand angiopietin-2 destabilizes quiescent
endothelium through an internal autocrine loop mechanism. J Cell
Sci. 15:771–780. 2005. View Article : Google Scholar
|
|
21
|
Ahmad SA, Liu W, Jung YD, Fan F, Reinmuth
N, Bucana CD and Ellis LM: Differential expression of
angiopoietin-1 and angiopoietin-2 in colon carcinoma. A possible
mechanism for the initiation of angiogenesis. Cancer. 92:1138–1143.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kahlert C, Pecqueux M, Halama N, Dienemann
H, Muley T, Pfannschmidt J, Lasitschka F, Klupp F, Schmidt T,
Rahbari N, et al: Tumour-site-dependent expression profile of
angiogenic factors in tumour-associated stroma of primary
colorectal cancer and metastases. Br J Cancer. 110:441–449. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Avraham HK, Jiang S, Fu Y, Nakahatri H,
Ovadia H and Avraham S: Angiopoietin-2 mediates blood-brain barrier
impairment and colonization of triple-negative breast cancer cells
in brain. J Pathol. 232:369–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shim WS, Ho IA and Wong PE: Angiopoietin:
A TIE(d) balance in tumor angiogenesis. Mole Cancer Res. 5:655–665.
2007. View Article : Google Scholar
|
|
25
|
Hu B, Jarzynka MJ, Guo P, Imanishi Y,
Schlaepfer DD and Cheng SY: Angiopoietin 2 induces glioma cell
invasion by stimulating matrix metalloprotease 2 expression through
the alphavbeta1 integrin and focal adhesion kinase signaling
pathway. Cancer Res. 66:775–783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nasarre P, Thomas M, Kruse K, Helfrich I,
Wolter V, Deppermann C, Schadendorf D, Thurston G, Fiedler U and
Augustin HG: Host-derived angiopoietin-2 affects early stages of
tumor development and vessel maturation but is dispensable for
later stages of tumor growth. Cancer Res. 69:1324–1333. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang HL, Deng CS, Lin J, Pan Dy, Zou Zy
and Zhou XY: Expression of angiopoietin-2 is correlated with
vascularization and tumor size in human colorectal adenocarcinoma.
Tohoku J Exp Med. 213:33–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yancopoulos GD, Davis S, Gale NW, Rudge
JS, Wiegand SJ and Holash J: Vascular-specific growth factors and
blood vessel formation. Nature. 407:242–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Etoh T, Inoue H, Tanaka S, Barnard GF,
Kitano S and Mori M: Angiopoietin-2 is related to tumor
angiogenesis in gastric carcinoma: Possible in vivo regulation via
induction of proteases. Cancer Res. 61:2145–2153. 2001.PubMed/NCBI
|