Introduction
Pancreatic cancer, which has a 5-year survival rate
of <5%, is a major cause of cancer- associated mortality
worldwide (1,2). Complete resection is essential for the
cure of pancreatic cancer. However, only 10–20% of patients with
pancreatic cancer are candidates for curative resection (3,4).
Furthermore, due to the high rate of recurrence, the postoperative
5-year survival rate is only 10–20% when curative resection is
performed (3–5).
Numerous randomized controlled studies of adjuvant
chemotherapy following pancreatic cancer resection have been
conducted (6–8). Recently, the European Study Group for
Pancreatic Cancer-1 and −3 trials and the Charite Onkologic 001
(CONKO-001) trial demonstrated that treatment with gemcitabine or
fluorouracil (FU) plus folinic acid could significantly increase
overall survival (OS) time following surgical resection in patients
with pancreatic cancer, as compared with surgery alone (6–8). Based on
these results, adjuvant chemotherapy with gemcitabine is now
considered to be a standard treatment and is routinely recommended
following curative resection for pancreatic cancer. However, even
adjuvant chemotherapy with gemcitabine is unable to completely
prevent the development of recurrence. The selection of patients
who can derive a true benefit from gemcitabine may be an important
step towards improving the clinical outcomes associated with
pancreatic cancer.
Human equilibrative nucleoside transporter-1
(hENT-1) is a nucleoside transporter protein that mediates the
entry of cytotoxic chemotherapies into cells, including gemcitabine
(9). In vitro studies have
demonstrated that the expression of hENT-1 is associated with
sensitivity to nucleoside analogues (10). Specifically, the overexpression of
hENT-1 can enhance response to gemcitabine in human pancreatic
cancer, whereas cells in which hENT-1 expression is absent are
resistant to gemcitabine (11,12).
Previous studies of patients with pancreatic cancer treated with
gemcitabine indicate that there is an association between hENT-1
expression and treatment outcome (13,14). In
addition, clinical studies of adjuvant chemoradiation therapy for
resected pancreatic cancer have revealed that, compared with low
hENT-1 expression, a high tumor expression level of hENT-1 is
associated with a longer patient survival time following
gemcitabine chemotherapy (15,16).
However, few published studies have evaluated the predictive value
of hENT-1 expression in patients with pancreatic cancer treated by
resection and gemcitabine-only adjuvant chemotherapy, and no
definite conclusions can be made regarding the predictive value of
hENT-1 in such patients.
The characterization of genes associated with
sensitivity or resistance to antitumor agents, using cancer tissues
from individual patients, serves a critical role in the development
and provision of individualized adjuvant chemotherapy treatments.
In the present study, hENT-1 expression was investigated in
consecutive patients who underwent curative resection followed by
adjuvant chemotherapy with gemcitabine, and the associations
between hENT-1 expression, clinicopathological parameters and
survival were investigated.
Materials and methods
Patient selection
The patients were selected from the medical records
of 201 consecutive patients who underwent pancreatic surgery at
Kanagawa Cancer Center (Yokohama, Japan) between April 2005 and
December 2014. The following inclusion criteria were applied: The
patients had a pathologically common type of pancreatic
adenocarcinoma according to the definitions of the International
Union Against Cancer (UICC) TNM 7th edition (17); the patients initially underwent
curative resection and the resected specimens were archived; and
the patients received adjuvant chemotherapy with gemcitabine. The
resected specimens were examined histopathologically and were
staged according to the UICC TNM 7th edition (17). Patients with other pancreatic and
periampullary neoplasms, including intraductal papillary mucinous
neoplasm, cystadenocarcinoma and endocrine tumors, as well as
patients who underwent R2 resection, were excluded from the present
study. The present study was approved by the Institutional Review
Board of the Kanagawa Cancer Center.
In total, 101 patients were eligible for inclusion
in the present study, including 57 men and 44 women, with a median
age of 66 years.
Surgical procedure
All pancreatic surgeries were performed in
accordance with standardized procedures previously described
(18–20). Briefly, in cases of distal
pancreatectomy, lymph node dissection was performed in the region
of the celiac trunk and the superior mesenteric artery and vein, as
well as behind the pancreas along the left side of the renal vein
and the left adrenal gland. In each case, intraperitoneal drains
were placed close to the pancreatic anastomosis and stump. In cases
of pancreaticoduodenectomy, subtotal stomach-preserving
pancreaticoduodenectomy was performed as the standard procedure.
Lymph node dissection along the hepatoduodenal ligament, common
hepatic artery, vena cava, superior mesenteric vein and the right
side of the superior mesenteric artery were included in the
procedure as standard. Multiple intraperitoneal drains were placed,
with the first being posterior to the hepaticojejunostomy and the
second on the anterior surface of the pancreaticojejunostomy or the
closed remnant of the pancreas.
Adjuvant chemotherapy
Gemcitabine treatment was initiated within 8 weeks
following surgery. The patients received a weekly dose of 1,000
mg/m2 for 3 weeks, followed by 1 week of rest. The
gemcitabine treatment was continued for 6 months.
Follow-up
Patients were followed up at outpatient clinics.
Hematological tests and physical examinations were performed a
minimum of every 2 weeks during adjuvant chemotherapy, and at least
every 3 months for 5 years after course of adjuvant chemotherapy
had been completed. The levels of the tumor markers
carcinoembryonic antigen and carbohydrate antigen 19-9 were
assessed at least every 3 months for 5 years.
Patients underwent a computed tomography examination
every 3 months during the first 3 years following surgery, and then
every 6 months until 5 years following surgery. Peritoneal
recurrence was defined as positive when at least one of the
following findings was identified on imaging studies: Massive
ascites, ascites confirmed by cytology, enhanced abdominal nodules,
abnormal intestinal wall thickness, increased fat density of the
intestinal mesentery, diffuse hydronephrosis or an intraabdominal
mass. Imaging studies were checked by one radiologist and two staff
physicians. When liver metastasis was suspected based on imaging
studies,
gadolinium-ethoxybenzyl-diethylenetriamine-pentaacetate-enhanced
magnetic resonance imaging or contrast-enhanced ultrasonography was
performed to confirm the diagnosis.
Immunohistochemical analysis of hENT-1
expression
Hematoxylin and eosin-stained slides containing
specimens from each pancreatic adenocarcinoma were reviewed, and a
representative tumor region and the corresponding formalin-fixed,
paraffin-embedded tissue block were selected for use in a tissue
microarray. The hENT-1 expression was evaluated using a rabbit
anti-hENT-1 monoclonal antibody (MBL International Co., Woburn, MA,
USA). The immunohistochemical staining procedure is described
elsewhere (21).
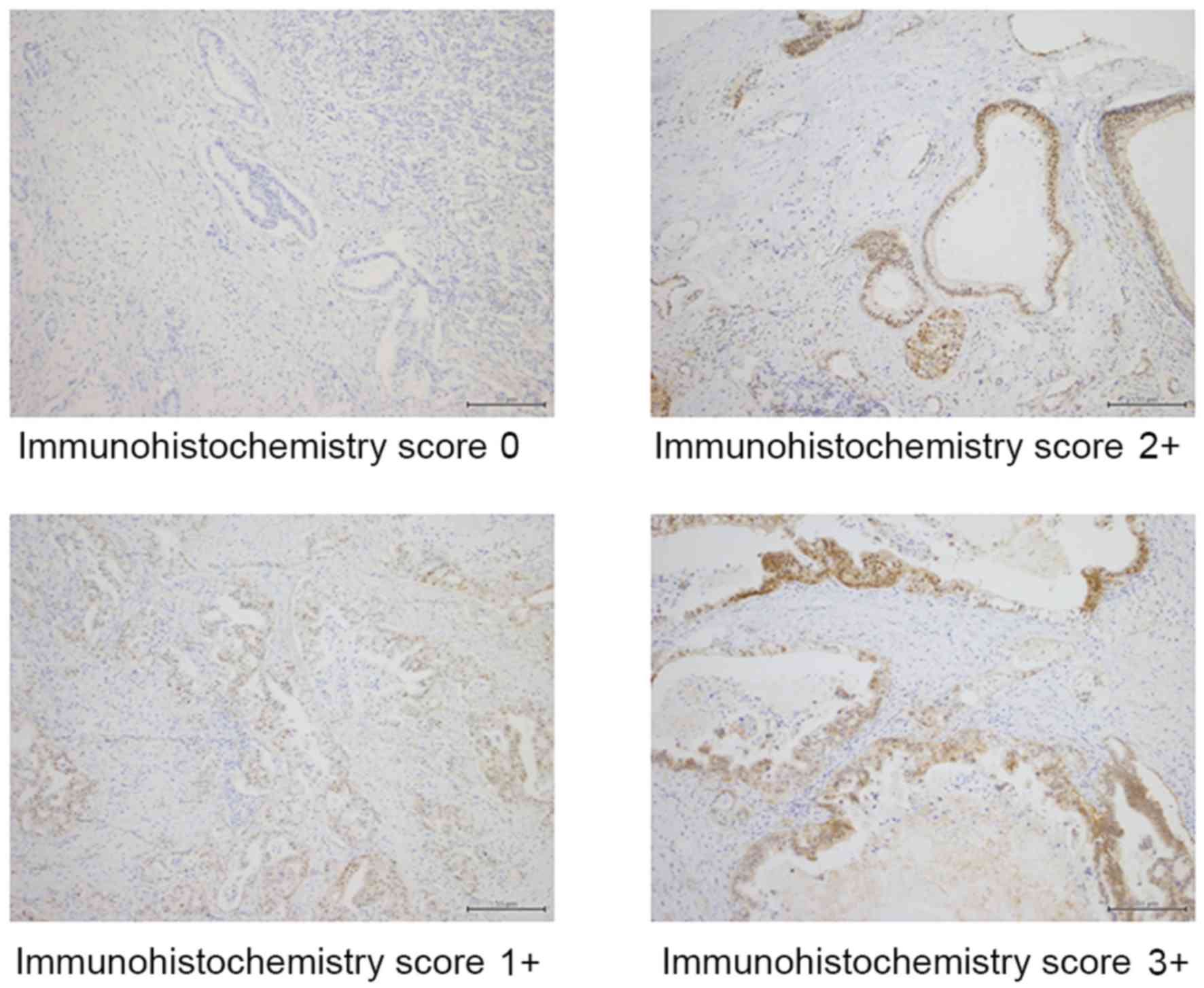
The staining intensity for the hENT-1 protein and
the percentage of positive tumor cells were scored, and a composite
score (hENT-1 score) was obtained by calculating the sum of these
two scores. The staining intensity for the hENT-1 protein was
assigned a score ranging from 0 to 3: 0+, no staining; 1+, weakly
positive; 2+, moderately positive; and 3+, strongly positive. The
percentage of positive tumor cells was scored as follows: 0+, no
positive tumor cells; 1+, <50% positive cells; 2+, 50–80%
positive cells; and 3+, ≥81% positive cells. Tumors with composite
hENT-1 scores of 0–3 were classified as having low hENT-1
expression and tumors with scores of 4–6 as having high hENT-1
expression.
Evaluations and statistical
analysis
The significance of the associations between hENT-1
expression and clinicopathological parameters were determined using
Fisher's exact test or χ2 test. OS was defined as the
period between surgery and mortality. Recurrence-free survival
(RFS) was defined as the period between surgery and recurrence or
mortality, whichever came first. The data of the patients who had
not experienced an event were censored at the date of the final
observation. The OS and RFS were evaluated by univariate and
multivariate analyses. OS and RFS curves were calculated using the
Kaplan-Meier method and compared using the log-rank test. The
univariate and multivariate survival analyses were performed using
Cox's proportional hazards model. P<0.05 was considered to
indicate a statistically significant difference. The survival data
were obtained from hospital records or from the city registry
system. SPSS software (v11.0J Win; SPSS, Inc., Chicago, IL, USA)
was used for all of the statistical analyses.
Results
Patients
A total of 201 patients underwent surgical resection
between 2005 and 2014. Of these patients, 101 were eligible for
inclusion in the present study. The ages of the patients ranged
from 40–78 years (median, 66 years); 57 patients were male and 44
were female. A total of 28 patients underwent distal
pancreatectomy, 70 underwent pancreaticoduodenectomy and 3
underwent total pancreatic resection. In total, 85 patients
achieved R0 resection and 16 patients achieved R1 resection. The
median follow-up period was 67.3 months (range, 22.2–122.7
months).
Immunohistochemical analyses
Representative results from the immunohistochemical
staining for hENT-1 in pancreatic adenocarcinoma tissue sections
are shown in Fig. 1. Immunoreactivity
was observed in the cytoplasm of cancer cells. Among the 101 tumor
samples, 38 samples exhibited negative staining, 11 samples
exhibited weak staining, 32 samples exhibited moderate staining and
20 samples exhibited strong staining. Of the 63 tumor samples that
showed positive staining, 3 samples had <50% positive cells, 10
samples had 50–80% positive cells and 50 samples had ≥81% positive
cells. According to the hENT-1 scoring system, 41 patients were
assigned to the low hENT-1 expression group and 60 patients to the
high hENT-1 expression group.
Association between
clinicopathological factors and hENT-1 expression
A total of eight clinicopathological factors were
compared between the patients with high and low hENT-1 expression.
Although a significant difference was observed in sex distribution,
no differences were observed between the two groups for all other
clinicopathological parameters (Table
I).
 | Table I.Associations between patient
characteristics and hENT-1 expression. |
Table I.
Associations between patient
characteristics and hENT-1 expression.
| Factor | High hENT-1 group, n
(%) | Low hENT-1 group, n
(%) | P-value |
|---|
| Total | 60 | 41 |
|
| Sex |
|
| 0.017 |
| Male | 28 (46.7) | 29 (70.7) |
|
|
Female | 32 (53.3) | 12 (29.3) |
|
| Age, years |
|
| 0.725 |
|
<65 | 27 (45.0) | 17 (41.5) |
|
| ≥65 | 33 (55.0) | 24 (58.5) |
|
| Tumor location |
|
| 0.536 |
|
Pancreatic head | 42 (70.0) | 31 (75.6) |
|
|
Pancreatic body/tail | 18 (30.0) | 10 (24.4) |
|
| Pathological
differentiation |
|
| 0.370 |
| Well
differentiated | 51 (85.0) | 32 (78.0) |
|
|
Moderately/poorly
differentiated | 9 (15.0) | 9 (22.0) |
|
| UICC pT factor |
|
| 0.142 |
|
T1/T2 | 6 (10.0) | 1 (2.4) |
|
| T3 | 54 (90.0) | 40 (97.6) |
|
| Lymph node
metastasis |
|
| 0.871 |
| N0 | 14 (23.3) | 9 (22.0) |
|
| N1 | 46 (76.7) | 32 (78.0) |
|
| Lymphatic
invasion |
|
| 0.496 |
| No | 29 (48.3) | 17 (41.5) |
|
| Yes | 31 (51.7) | 24 (58.5) |
|
| Vascular
invasion |
|
| 0.590 |
| No | 16 (26.7) | 9 (22.0) |
|
|
Yes | 44 (73.3) | 32 (78.0) |
|
Survival analysis
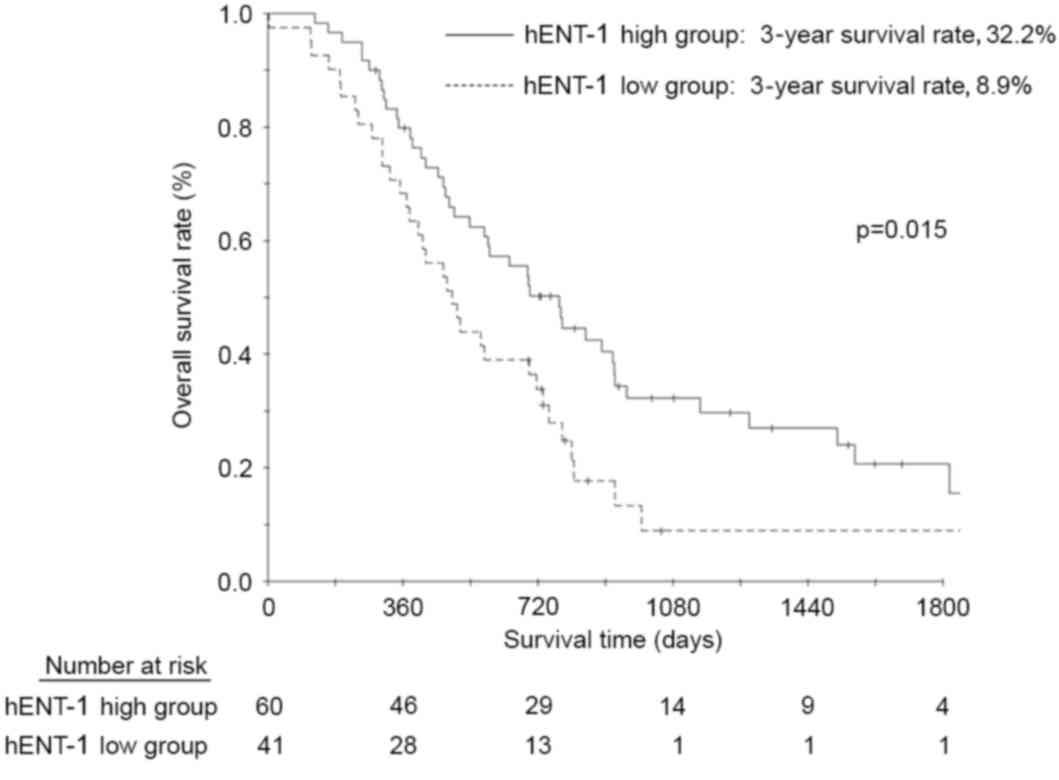
The OS rates at 3 and 5 years following surgery in
the patients with high hENT-1 expression were 32.2 and 20.6%,
respectively; and 8.9 and 8.9% in the patients with low hENT-1
expression (Fig. 2); this result was
also statistically significant (P=0.015). The multivariate analyses
demonstrated that hENT-1 expression status and lymphatic invasion
were significant risk factors for OS (Table II).
 | Table II.Univariate and multivariate analyses
of risk factors for overall survival. |
Table II.
Univariate and multivariate analyses
of risk factors for overall survival.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factor | n | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Sex |
|
|
| 0.561 |
|
| – |
|
Female | 44 | 1.000 | – |
| – | – |
|
|
Male | 57 | 1.143 | 0.728–1.795 |
| – | – |
|
| Age, years |
|
|
| 0.740 |
|
| – |
|
<65 | 44 | 1.000 | – |
| – | – |
|
|
≥65 | 57 | 1.081 | 0.683–1.709 |
| – | – |
|
| Resection
status |
|
|
| 0.041 |
|
| – |
| R0 | 85 | 1.000 | – |
| – | – |
|
| R1 | 16 | 1.850 | 1.026–3.336 |
| – | – |
|
| Tumor location |
|
|
| 0.024 |
|
| 0.057 |
|
Pancreatic body/tail | 28 | 1.000 | – |
| 1.000 | – |
|
|
Pancreatic head | 73 | 1.840 | 1.085–3.120 |
| 1.667 | 0.984–2.825 |
|
| Pathological
differentiation |
|
|
| 0.892 |
|
| – |
| Well
differentiated | 83 | 1.000 | – |
| – | – |
|
|
Moderately/poorly
differentiated | 18 | 1.042 | 0.572–1.898 |
| – | – |
|
| UICC pT factor |
|
|
| 0.035 |
|
| – |
|
T1/T2 | 7 | 1.000 | – |
| – | – |
|
| T3 | 94 | 4.545 | 1.113–18.559 |
| – | – |
|
| Lymph node
metastasis |
|
|
| 0.038 |
|
| – |
| N0 | 23 | 1.000 | – |
| – | – |
|
| N1 | 78 | 1.802 | 1.034–3.140 |
| – | – |
|
| Lymphatic
invasion |
|
|
| 0.001 |
|
| 0.001 |
| No | 46 | 1.000 | – |
| 1.000 | – |
|
|
Yes | 55 | 2.192 | 1.374–3.498 |
| 2.250 | 1.405–3.603 |
|
| Vascular
invasion |
|
|
| 0.032 |
|
| – |
| No | 38 | 1.000 | – |
| – | – |
|
|
Yes | 63 | 1.678 | 1.044–2.695 |
| – | – |
|
| hENT-1 status |
|
|
| 0.021 |
|
| 0.019 |
|
High | 60 | 1.000 | – |
| 1.000 | – |
|
|
Low | 41 | 1.713 | 1.083–2.707 |
| 1.740 | 1.095–2.766 |
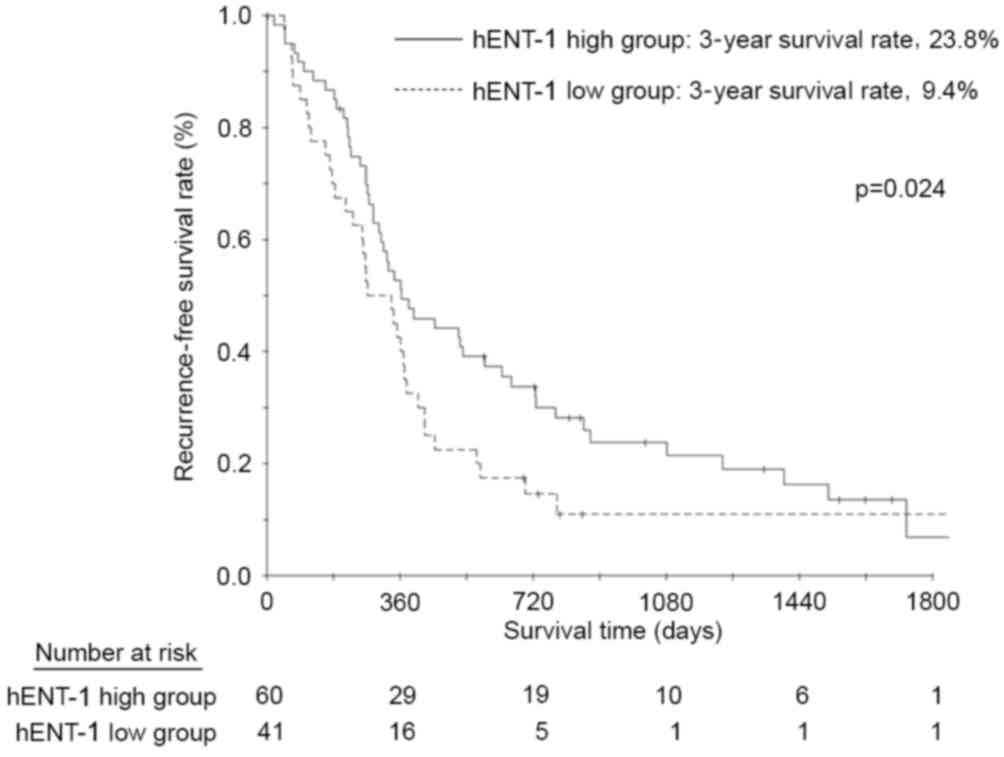
The RFS rates at 3 and 5 years following surgery in
the patients with high hENT-1 expression were 23.8 and 6.8%,
respectively; and 9.4 and 9.4% in the patients with low hENT-1
expression (Fig. 3). The difference
in both 3 and 5-year survival rates was statistically significantly
(P=0.024). The multivariate analyses demonstrated that hENT-1
expression status, resection status and lymphatic invasion were
significant risk factors for RFS (Table
III).
 | Table III.Univariate and multivariate analyses
of risk factors for recurrence-free survival. |
Table III.
Univariate and multivariate analyses
of risk factors for recurrence-free survival.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factor | n | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Sex |
|
|
| 0.874 |
|
| – |
|
Female | 44 | 1.000 | – |
| – | – |
|
|
Male | 57 | 1.035 | 0.674–1.590 |
| – | – |
|
| Age, years |
|
|
| 0.293 |
|
| – |
|
<65 | 44 | 1.000 | – |
| – | – |
|
|
≥65 | 57 | 1.264 | 0.817–1.954 |
| – |
| – |
| Resection
status |
|
|
| 0.001 |
|
| 0.036 |
| R0 | 85 | 1.000 | – |
| 1.000 | – |
|
| R1 | 16 | 2.668 | 1.469–4.845 |
| 1.931 | 1.044–3.572 |
|
| Tumor location |
|
|
| 0.016 |
|
| – |
|
Pancreatic body/tail | 28 | 1.000 | – |
| – | – |
|
|
Pancreatic head | 73 | 1.816 | 1.118–2.949 |
| – | – |
|
| Pathological
differentiation |
|
|
| 0.775 |
|
| – |
| Well
differentiated | 83 | 1.000 | – |
| – | – |
|
|
Moderately/poorly | 18 | 1.083 | 0.627–1.869 |
| – | – |
|
|
differentiated |
| UICC pT factor |
|
|
| 0.148 |
|
| – |
|
T1/T2 | 7 | 1.000 | – |
| – | – |
|
| T3 | 94 | 1.778 | 0.814–3.883 |
| – | – |
|
| Lymph node
metastasis |
|
|
| 0.074 |
|
| – |
| N0 | 23 | 1.000 | – |
| – | – |
|
| N1 | 78 | 1.597 | 0.956–2.669 |
| – | – |
|
| Lymphatic
invasion |
|
|
| 0.001 |
|
| 0.001 |
| No | 46 | 1.000 | – |
| 1.000 | – |
|
|
Yes | 55 | 2.238 | 1.438–3.482 |
| 2.199 | 1.395–3.467 |
|
| Vascular
invasion |
|
|
| 0.204 |
|
| – |
| No | 38 | 1.000 | – |
| – | – |
|
|
Yes | 63 | 1.330 | 0.856–2.065 |
| – | – |
|
| hENT-1 status |
|
|
| 0.046 |
|
| 0.049 |
|
High | 60 | 1.000 | – |
| 1.000 | – |
|
|
Low | 41 | 1.563 | 1.003–2.462 |
| 1.574 | 1.002–2.472 |
|
Discussion
The present study evaluated hENT-1 expression status
in patients with pancreatic adenocarcinoma who underwent curative
resection followed by adjuvant chemotherapy with gemcitabine, and
identified 60% of these patients as exhibiting high hENT-1
expression. The OS and RFS rates of the patients differed
significantly based on their hENT-1 status. The present results
indicated that gemcitabine alone was insufficient as an adjuvant
therapy, particularly in the patients with low hENT-1 expression.
These patients may be a target group for clinical trials of novel
treatments.
A predictive role of hENT-1 in pancreatic cancer has
been reported in previous studies. Giovannetti et al
(13) characterized the expression
patterns of genes involved in gemcitabine activity in pancreatic
tumor specimens from surgical or biopsy samples of 102 patients
with pancreatic cancer treated with gemcitabine, and evaluated
their associations with treatment outcomes. The authors reported
that the patients with increased levels of hENT-1 expression had
significantly longer OS and disease-free survival (DFS) times and
times-to-progression compared with those with lower transcriptional
hENT-1 levels. In addition, Spratlin et al (14) reported that patients who had uniformly
detectable hENT-1 immunostaining in their pancreatic adenocarcinoma
samples had a significantly longer survival time following
gemcitabine chemotherapy compared with hENT-1 status. Similar
results were observed in the adjuvant setting (21).
A prognostic role of hENT-1 in pancreatic cancer has
been also reported in previous studies. One of the largest
retrospective studies demonstrating the predictive and prognostic
value of hENT-1 used 229 specimens from the RTOG 9704 trial
(15). The study randomized patients
to gemcitabine or 5-FU treatment arms following pancreatic surgical
resection; patients in both arms received concurrent chemotherapy
with 5-FU and radiation. The results indicated that hENT-1
expression predicted OS and DFS in patients with pancreatic cancer
who received gemcitabine, but not in those who received 5-FU, and
the authors concluded that hENT-1 is a useful predictive biomarker
of response to gemcitabine treatment, but not a prognostic
biomarker (15). By contrast, Kim
et al (22) investigated the
prognostic value of hENT-1 and ribonucleoside reductase subunit M1
expression in 48 resected pancreatic cancer patients. The authors
found that low expression of hENT-1 was associated with decreased
OS and progression-free survival times in patients with resected
pancreatic adenocarcinoma, independently of gemcitabine therapy.
Therefore, future studies should focus on whether hENT-1 may have
prognostic value as well as tpredictive value for sensitivity to
gemcitabine.
Numerous studies have examined the presence and
impact of hENT-1 protein overexpression or gene amplification in
patients with pancreatic adenocarcinoma (14,15). These
studies have reported that hENT-1 is highly expressed in 40–80% of
patients. The explanation for the variation in hENT-1 expression in
the previous studies may be due to a number of reasons. First,
different methods were used to examine the expression of hENT-1 in
the previous studies: Spratlin et al (14) and Farrell et al (15) used immunohistochemistry performed on
formalin-fixed, paraffin-embedded pancreatic tissue microarrays,
whereas Giovannetti et al (13) used reverse transcription-polymerase
chain reaction (RT-PCR) performed on laser-captured malignant cells
from frozen biopsy or resection specimens, with results normalized
to a single housekeeping gene (GAPDH), and the authors used RT-PCR
performed on paraffin-embedded tissue with results normalized to
two housekeeping genes (hydroxymethylbilane synthase and ribosomal
protein L13a). Second, studies have investigated different stages
of pancreatic cancer: Resectable pancreatic cancer was investigated
in the present cohort and the Farrell et al study (15), whereas the study by Giovannetti et
al (13) used a mixture of
resectable cases and unresectable or recurrent cases of pancreatic
cancer, and unresectable or recurrent pancreatic cancer cases were
used in the study by Spratlin et al (14).
Regarding the associations between hENT-1 expression
and clinicopathological factors in pancreatic cancer, the analysis
by Farrell et al (15) of 538
patients who were assigned randomly to gemcitabine or 5-FU
treatment groups following surgical resection indicated that tumor
location in the pancreatic head (vs. all other locations) was the
only baseline characteristic to have a positive statistical
association between absent or positive hENT-1 expression in the
gemcitabine treatment arm (P=0.02). By contrast, no positive
statistical associations existed for this grouping between absent
or positive hENT-1 expression in the 5-FU treatment arm. In the
current study, a significant difference was observed only for sex,
and there were no differences in any of the other
clinicopathological parameters, including UICC pT factor and lymph
node status, between the high and low hENT-1 expression groups;
expression of hENT-1 appeared to be independent of other
clinicopathological factors.
Caution is required when interpreting the current
results, since the present study has several potential limitations.
First, the present study was a retrospective analysis that was
performed in a single institution. Therefore, there is a
possibility that the present findings were observed by chance.
Second, there was a selection bias in the patients in this series:
Surgeons often avoid performing pancreatectomy in certain patients
as the procedure is associated with high rates of morbidity and
mortality (40–60% and 1–1.5%, respectively) (23–26); thus,
the fact that certain patients in the present study received
pancreatectomy may be considered to be a potential bias. In
addition, Kanagawa Cancer Center is a specialized cancer center
treating only cancer patients, and therefore, there is a
possibility that only patients with good status were selected.
Third, the evaluation of hENT-1 expression was not standardized,
and the appropriate hENT-1 cutoff value is unclear. Considering
these limitations, the results must be confirmed in another cohort
or in a prospective multicenter study.
In summary, the OS and RFS rates of patients with
pancreatic cancer who underwent curative resection followed by
adjuvant gemcitabine chemotherapy differed significantly based on
their hENT-1 expression. The present results indicate that
gemcitabine may not be sufficient as a treatment, particularly for
patients with low hENT-1 expression. These patients should be a
target group for clinical trials of novel treatments.
Acknowledgements
The present study was supported, in part, by the
Uehara Memorial Foundation (grant no. 201510145) and the Takeda
Science Foundation.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, ٢٠١٠. CA Cancer J Clin.
|
|
2
|
Nakao A, Fujii T, Sugimoto H, Kanazumi N,
Nomoto S, Kodera Y, Inoue S and Takeda S: Oncological problems in
pancreatic cancer surgery. World J Gastroenterol. 12:4466–4472.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsuno S, Egawa S, Fukuyama S, Motoi F,
Sunamura M, Isaji S, Imaizumi T, Okada S, Kato H, Suda K, et al:
Pancreatic cancer registry in Japan: 20 years of experience.
Pancreas. 28:219–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carpelan-Holmström M, Nordling S, Pukkala
E, Sankila R, Lüttges J, Klöppel G and Haglund C: Does anyone
survive pancreatic ductal adenocarcinoma? A nationwide study
re-evaluating the data of the Finnish Cancer Registry. Gut.
54:385–387. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wagner M, Redaelli C, Lietz M, Seiler CA,
Friess H and Büchler MW: Curative resection is the single most
important factor determining outcome in patients with pancreatic
adenocarcinoma. Br J Surg.
|
|
6
|
Neoptolemos JP, Stocken DD, Friess H,
Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C,
Lacaine F, et al: A randomized trial of chemoradiotherapy and
chemotherapy after resection of pancreatic cancer. N Engl J Med.
350:1200–1210. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neoptolemos JP, Stocken DD, Bassi C,
Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger
S, Mariette C, et al: Adjuvant chemotherapy with fluorouracil plus
folinic acid vs gemcitabine following pancreatic cancer resection:
A randomized controlled trial. JAMA. 304:1073–1081. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oettle H, Post S, Neuhaus P, Gellert K,
Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C,
et al: Adjuvant chemotherapy with gemcitabine vs observation in
patients undergoing curative-intent resection of pancreatic cancer:
A randomized controlled trial. JAMA. 297:267–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J, Visser F, King KM, Baldwin SA,
Young JD and Cass CE: The role of nucleoside transporters in cancer
chemotherapy with nucleoside drugs. Cancer Metastasis Rev.
|
|
10
|
Mackey JR, Mani RS, Selner M, Mowles D,
Young JD, Belt JA, Crawford CR and Cass CE: Functional nucleoside
transporters are required for gemcitabine influx and manifestation
of toxicity in cancer cell lines. Cancer Res. 58:4349–4357.
1998.PubMed/NCBI
|
|
11
|
Pérez-Torras S, García-Manteiga J, Mercadé
E, Casado FJ, Carbó N, Pastor-Anglada M and Mazo A:
Adenoviral-mediated overexpression of human equilibrative
nucleoside transporter 1 (hENT-1) enhances gemcitabine response in
human pancreatic cancer. Biochem Pharmacol. 76:322–329. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mori R, Ishikawa T, Ichikawa Y, Taniguchi
K, Matsuyama R, Ueda M, Fujii Y, Endo I, Togo S, Danenberg PV and
Shimada H: Human equilibrative nucleoside transporter 1 is
associated with the chemosensitivity of gemcitabine in human
pancreatic adenocarcinoma and biliary tract carcinoma cells. Oncol
Rep. 17:1201–1205. 2007.PubMed/NCBI
|
|
13
|
Giovannetti E, Del Tacca M, Mey V, Funel
N, Nannizzi S, Ricci S, Orlandini C, Boggi U, Campani D, Del Chiaro
M, et al: Transcription analysis of human equilibrative nucleoside
transporter-1 predicts survival in pancreas cancer patients treated
with gemcitabine. Cancer Res. 66:3928–3935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spratlin J, Sangha R, Glubrecht D, Dabbagh
L, Young JD, Dumontet C, Cass C, Lai R and Mackey JR: The absence
of human equilibrative nucleoside transporter 1 is associated with
reduced survival in patients with gemcitabine-treated pancreas
adenocarcinoma. Clin Cancer Res. 10:6956–6961. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Farrell JJ, Elsaleh H, Garcia M, Lai R,
Ammar A, Regine WF, Abrams R, Benson AB, Macdonald J, Cass CE, et
al: Human equilibrative nucleoside transporter 1 levels predict
response to gemcitabine in patients with pancreatic cancer.
Gastroenterology. 136:187–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marechal R, Mackey JR, Lai R, Demetter P,
Peeters M, Polus M, Cass CE, Young J, Salmon I, Devière J and Van
Laethem JL: Human equilibrative nucleoside transporter 1 and human
concentrative nucleoside transporter 3 predict survival after
adjuvant gemcitabine therapy in resected pancreatic adenocarcinoma.
Clin Cancer Res. 15:2913–2919. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sobin LH and Wittekind CH: TNM
Classification of Malignant Tumors. ٧th. John Wiley & Sons; New
York:
|
|
18
|
Büchler MW, Friess H, Wagner M, Kulli C,
Wagener V and Z'Graggen K: Pancreatic fistula after pancreatic head
resection. Br J Surg. ٨٧: ٨٨٣-٨٨٩, ٢٠٠٠.
|
|
19
|
Wagner M, Z'graggen K, Vagianos CE,
Redaelli CA, Holzinger F, Sadowski C, Kulli C, Zimmermann H, Baer
HU and Büchler MW: Pylorus-preserving total pancreatectomy. Early
and late results. Dig Surg. 18:188–195. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Andrén-Sandberg A, Wagner M, Tihanyi T,
Löfgren P and Friess H: Technical aspects of left-sided pancreatic
surgery for cancer. Dig Surg.
|
|
21
|
Morinaga S, Nakamura Y, Watanabe T,
Mikayama H, Tamagawa H, Yamamoto N, Shiozawa M, Akaike M, Ohkawa S,
Kameda Y and Miyagi Y: Immunohistochemical analysis of human
equilibrative nucleoside transporter-1 (hENT-1) predicts survival
in resected pancreatic cancer patients treated with adjuvant
gemcitabine monotherapy. Ann Surg Oncol. 19 Suppl 3:S558–S564.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim R, Tan A, Lai KK, Jiang J, Wang Y,
Rybicki LA and Liu X: Prognostic roles of human equilibrative
transporter ١ (hENT-١) and ribonucleoside reductase subunit M١
(RRM١) in resected pancreatic cancer. Cancer.
|
|
23
|
Povoski SP, Karpeh MS Jr, Conlon KC,
Blumgart LH and Brennan MF: Association of preoperative biliary
drainage with postoperative outcome following
pancreaticoduodenectomy. Ann Surg. PubMed/NCBI
|
|
24
|
Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA,
Campbell KA, Sauter PK, Coleman J, Abrams RA and Hruban RH:
Pancreaticoduodenectomy with or without distal gastrectomy and
extended retroperitoneal lymphadenectomy for periampullary
adenocarcinoma, part 2: Randomized controlled trial evaluating
survival, morbidity, and mortality. Ann Surg. 236:355–368. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawai M, Tani M, Terasawa H, Ina S, Hirono
S, Nishioka R, Miyazawa M, Uchiyama K and Yamaue H: Early removal
of prophylactic drains reduces the risk of intra-abdominal
infections in patients with pancreatic head resection: Prospective
study for 104 consecutive patients. Ann Surg. 244:1–7. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Munoz-Bongrand N, Sauvanet A, Denys A,
Sibert A, Vilgrain V and Belghiti J: Conservative management of
pancreatic fistula after pancreaticoduodenectomy with
pancreaticogastrostomy. J Am Coll Surg.
|