Introduction
The most critical and ultimately life-threatening
characteristic of malignant cancers is the ability to metastasize
to other organs (1). Between 4 and 7%
of patients with colon cancer present with peritoneal
carcinomatosis at the time of diagnosis, despite advances in early
detection of the disease (2).
Systemic chemotherapeutic agents, including 5-fluorouracil (5FU),
5FU derivatives, irinotecan (CPT-11) and oxaliplatin, as well as
targeted agents including bevacizumab, cetuximab, panitumumab and
regorafenib are currently used for the treatment of unresectable
metastatic colorectal cancer, and the survival time of patients
with unresectable metastatic colorectal cancer has been improved
(3,4).
However, as almost all patients involved in these studies are
patients with liver or lung metastases, the effects of these agents
on peritoneal dissemination remain unclear (5,6). In Phase
III trials (7,8), systemic chemotherapy, including the
5FU-based combination therapy folinic acid-5-FU-oxaliplatin, was
not identified to be effective against peritoneal dissemination and
failed to significantly improve survival (9). Peritoneal metastasis of gastric cancer
also leads to poor clinical outcomes, particularly with serosal
exposure and undifferentiated histology (10). For unresectable or recurrent gastric
cancer, only a limited number of Phase II studies have examined the
efficacy of these regimens for peritoneal metastasis (11,12). In
spite of recent advances in systemic chemotherapy, peritoneal
dissemination due to colorectal or gastric cancer remains a dismal
disease with a markedly low survival rate in patients.
Trifluridine/tipiracil (TFTD), formerly known as
TAS-102, is an antitumor therapy (13,14). It
comprises a mixture of two distinct chemicals, trifluridine and
tipiracil (TPI), at a molar ratio of 1:0.5. Trifluridine, an analog
of thymidine, exerts its antitumor activity through two mechanisms.
It inhibits thymidylate synthase (15) and is incorporated into DNA (16). TPI enhances the bioavailability of
trifluridine by inhibiting its in vivo degradation by
thymidine phosphorylase. TPI is therefore beneficial by producing a
more durable and sustainable response to trifluridine (17).
The antitumor effects of TFTD on colon cancer
xenograft in models refractory to 5FU are hypothesized to primarily
involve trifluridine incorporation into DNA (18). At the twice-daily oral dosing, the
clinically used administration schedule, the primary TFTD cytotoxic
mechanism is also hypothesized to be mediated by DNA incorporation
of trifluridine (19). TFTD
significantly improved the overall survival (OS) period [median OS,
7.1 months; 95% confidence interval (CI), 6.5–7.8 months vs. median
OS, 5.3 months; 95% CI, 4.6–6 months for placebo) and
progression-free survival (PFS) in patients with metastatic
colorectal cancer refractory to standard chemotherapies as
demonstrated by an international multi-center randomized
double-blind Phase III study (20).
The same study also demonstrated that TFTD exhibits a favorable
safety profile. These results led to the regulatory approval of the
drug in the USA and, recently, in Europe. With regard to gastric
cancer, a multicenter Phase II study demonstrated that TFTD
exhibited a positive efficacy and an acceptable toxicity profile in
patients with advanced gastric cancer following progression on one
or two prior systemic chemotherapies (21). A randomized double-blind Phase III
study evaluating TFTD plus best supportive care (BSC) compared with
placebo plus BSC in patients with metastatic gastric cancer
refractory to standard treatments is currently ongoing (22).
In the present study, the effects of TFTD on the
survival times of mice inoculated with colorectal or gastric cancer
cells into the peritoneal cavity were evaluated in comparison with
other drugs. The present study may provide useful information to
improve and/or expand options for the treatment of human colorectal
and gastric cancers.
Materials and methods
Reagents
TFTD, and tegafur, gimeracil and potassium oxonate
(S-1) were obtained from Taiho Pharmaceutical Co., Ltd. (Tokyo,
Japan). CPT-11 was purchased from Yakult Honsha Co., Ltd. (Tokyo,
Japan). 5FU was purchased from Kyowa Hakko Kirin Co., Ltd. (Tokyo,
Japan). Cisplatin (CDDP) was purchased from Nippon Kayaku Co., Ltd.
(Tokyo, Japan). Hydroxypropyl methylcellulose (HPMC) was obtained
from Shin-Etsu Chemical Co., Ltd. (Tokyo, Japan).
Cancer cell lines
The human colon cancer cell line HT-29 was purchased
from the American Type Culture Collection (Manassas, VA, USA).
Human colorectal carcinoma DLD-1 cells and HCT116 cells were
purchased from Dainippon Sumimoto Pharma Co., Ltd. (Osaka, Japan).
The 5FU-resistant cell line DLD-1/5FU was established using
long-term culture in the presence of 5FU in vitro (23). The human gastric cancer cell line
MKN45 was purchased from RIKEN BioResource Center Cell Bank
(Tsukuba, Japan) (24).
The cell lines were cultured in RPMI-1640 medium
(HT-29, DLD-1, DLD-1/5FU and MKN45) or Dulbecco's modified Eagle's
medium (HCT116) supplemented with 10% fetal bovine serum (FBS) at
37°C in a humidified atmosphere containing 5% CO2.
Culture media and FBS were obtained from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). The Kirsten rat sarcoma viral oncogene
homolog (KRAS) mutation status of HT-29 and MKN45 was wild-type,
whereas DLD-1 and HCT116 are KRAS mutants. The v-Raf murine sarcoma
viral oncogene homolog B (BRAF) mutation status of DLD-1, HCT116
and MKN45 was wild-type, whereas HT-29 is a BRAF mutant. The p53
mutation status of DLD-1, HT-29 and MKN45 was wild-type, whereas
HCT116 is a p53 mutant.
Animals
Five-week-old male nude mice (BALB/c nu/nu; body
weight range, 26–31 g, ~50 mice in each experiment) were purchased
from CLEA Japan, Inc. (Tokyo, Japan) and housed under specific
pathogen-free conditions. Food and water were provided ad libitum.
Testing rooms were maintained on a 12 h light/dark cycle (lights on
at 06:00 a.m., lights out at 06:00 p.m.) at a temperature of
23±3°C. All animal studies were performed according to the
guidelines and with the approval of the Institutional Animal Care
and Use Committee of Taiho Pharmaceutical Co., Ltd.
In vivo antitumor activity
Four colorectal cancer cell and one gastric cancer
cell suspensions were prepared from in vitro cell culture.
Each group consisted of 10 animals, into the peritoneal cavity of
which were injected 2×107 cells for the DLD-1, DLD-1/5FU
and HT-29 colorectal cancer cell lines, and MKN45 gastric cancer
cell line, or 5×106 cells for the HCT116 colorectal
cancer cell line. The drug treatment began 3 or 4 days later and
was considered as day 1. For the control group, vehicle (0.5% HPMC
solution, 10 ml/kg) was orally administered once daily for 5
consecutive days followed by 2 drug-free days for 6 weeks.
TFTD (200 mg/kg/day) was orally administered twice
daily for 5 consecutive days followed by 2 drug-free days for 6
weeks (18) and S-1 (10 mg/kg/day)
was orally administered once daily for 5 consecutive days followed
by 2 drug-free days for 6 weeks (25). 5FU (12 mg/kg) was injected three times
a week every 3 weeks for the 6-week period (days 3, 4, 5, 25, 26
and 27) (26). CPT-11 (100 mg/kg) was
injected intravenously once-weekly into the tail vein of mice
during the 6-week period for evaluating the effect on the
colorectal cancer cell lines (27).
CDDP (7 mg/kg) was injected intravenously once every 3 weeks into
the tail vein of mice (days 3 and 31) for evaluating the effect on
the gastric cancer MKN45 cell line (28).
The median increase in lifespan (ILS) was calculated
as a survival index according to the following formula: ILS
(%)=[(median survival time of treated group)/(median survival time
of control group)-1]x100.
Measurement of carcinoembryonic
antigen (CEA) levels in peritoneal dissemination
On days 10, 20, 30, 40 and 50, the mice were
sacrificed by cervical dislocation, and 0.5 ml saline was injected
into the peritoneal cavity. After 5 min, 0.1 ml peritoneal fluid
was recovered by abdominal puncture with a 21 G needle. Following
centrifugation at 2,300 × g for 5 min, CEA levels were measured in
the supernatant of each sample using a CEA ELISA kit (DRG
Instruments GmbH, Marburg, Germany).
Statistical analysis
The difference in the survival period distribution
among groups was analyzed using the log-rank test. All statistical
analyses were performed using EXSUS (version 8.1; CAC Exicare
Corp., Osaka, Japan). P<0.01 was considered to indicate a
statistically significant difference.
Results
Establishment of human colorectal and
gastric tumor intraperitoneal xenograft models
Mice inoculated with DLD-1, HT-29, HCT116 and MKN45
cell suspensions were sacrificed for macroscopic examination to
determine the distribution of the intraperitoneal dissemination at
50, 40, 30 and 30 days, respectively. Bloody ascites were observed
in the mice from the control group following intraperitoneal
inoculation with DLD-1 and HT-29 cells, but were almost
undetectable following intraperitoneal inoculation with HCT116 and
MKN45 cells (Fig. 1A). Numerous
metastatic nodules were detected in the mesentery of all mice
(Fig. 1B).
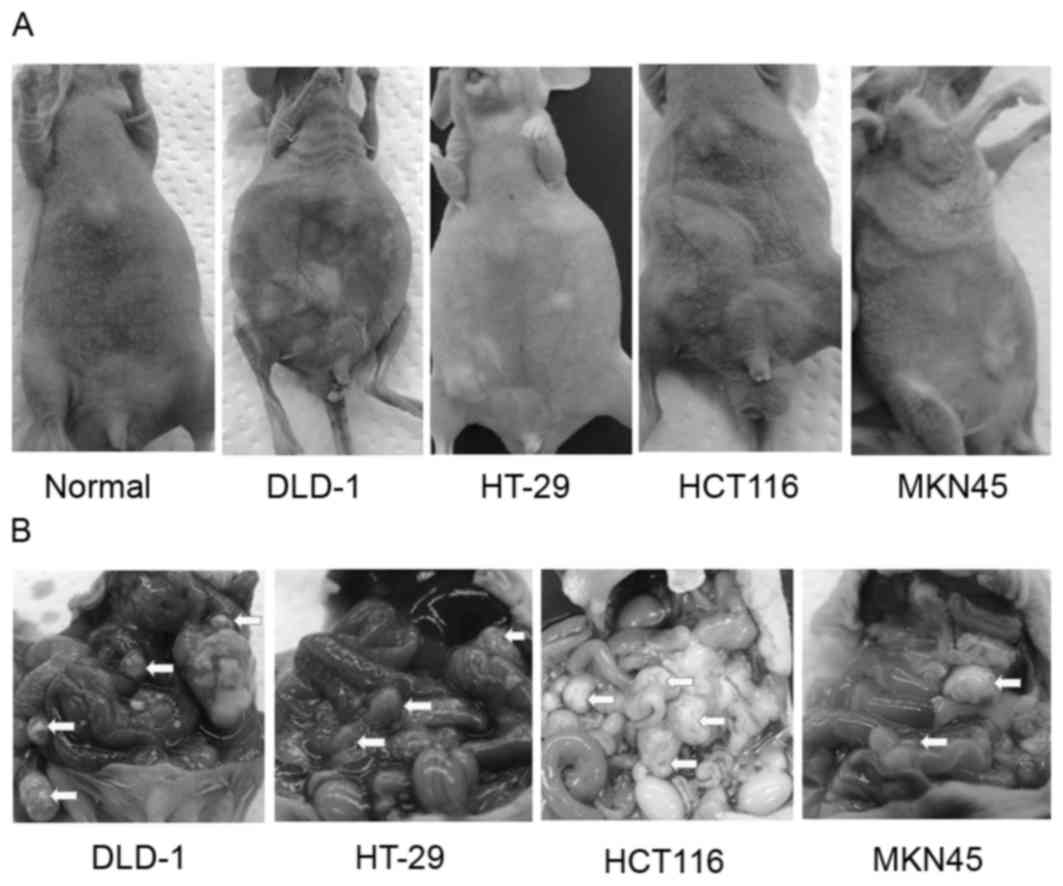 | Figure 1.Evaluation of human colorectal and
gastric tumor intraperitoneal xenograft models. DLD-1, HT-29 and
MKN45 cells (2×107 cells/mouse), and HCT116 cells
(5×106 cells/mouse) were injected intraperitoneally to
generate peritoneal dissemination. (A) General appearance of the
mice following intraperitoneal inoculation of DLD-1, HT-29, HCT116
and MKN45 cells at 50, 40, 30 and 30 days, respectively.
Carcinomatous peritonitis with an increased amount of bloody
ascites for DLD-1 and HT-29 cells or with a limited amount of
bloody ascites for HCT116 and MKN45 cells was observed. (B)
Intra-abdominal appearance of the mice following intraperitoneal
inoculation of DLD-1, HT-29, HCT116 and MKN45 cells at 50, 40, 30
and 30 days, respectively. Arrowheads indicate disseminated tumors
throughout the murine peritoneal cavity. |
Antitumor activity of TFTD in the
human colorectal intraperitoneal xenograft model
Fig. 2A and B presents
the survival curve and body weight of nude mice intraperitoneally
inoculated with DLD-1 cells, respectively. Untreated control mice
succumbed to peritoneal dissemination after ~7 weeks with bloody
ascites. The mice treated with 5FU, S-1, CPT-11 and TFTD exhibited
a significantly longer survival time compared with untreated mice
(P<0.01; Fig. 2A and Table I). No significant difference in mean
body weight of mice treated with these drugs compared with that of
untreated mice was identified (Fig.
2B). The results presented in Table
I indicate that, in the DLD-1 model, the mice treated with TFTD
or CPT-11 exhibited the most improved survival. The median ILS of
mice treated with TFTD and CPT-11 was 85 days and 87 days,
respectively. The mice treated with TFTD and CPT-11 exhibited
significantly increased survival times compared with the mice
treated with 5FU or S-1 (P<0.01; Table
I).
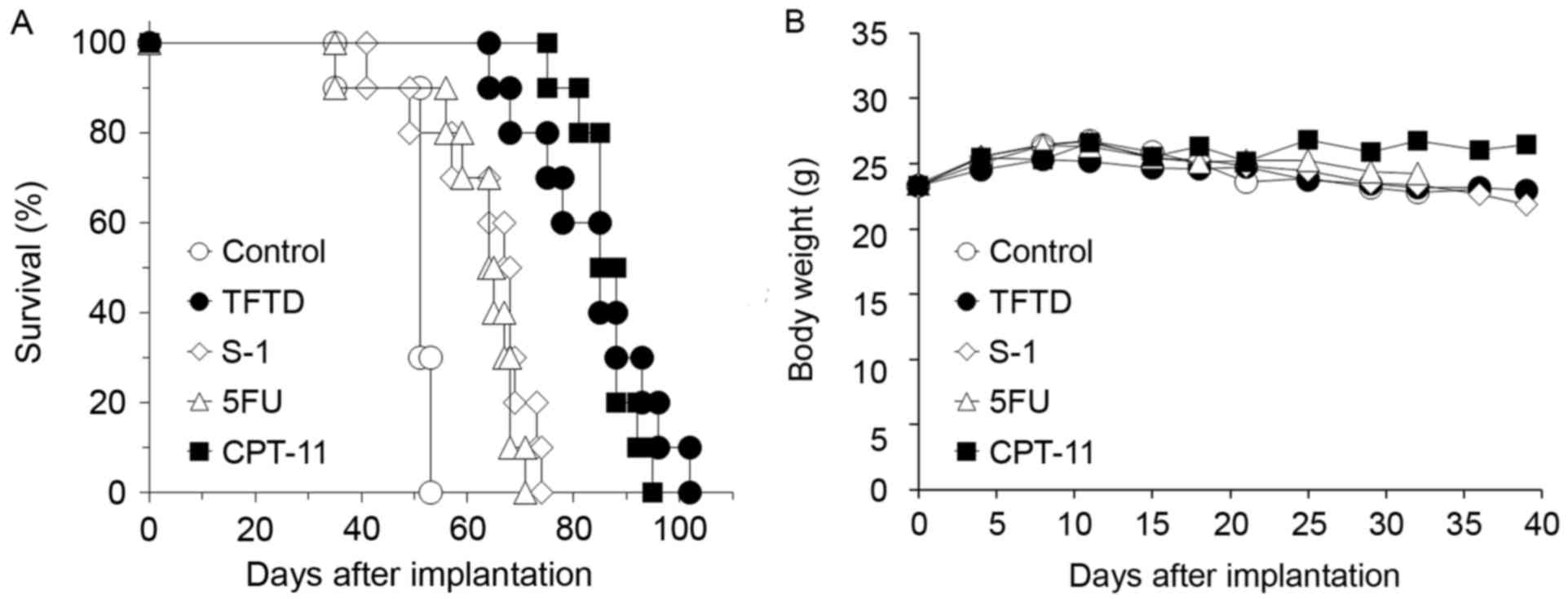 | Figure 2.Effect of TFTD (A) on the survival
intraperitoneally transplanted with human colorectal cancer DLD-1
cells and on the (B) body weight of DLD-1 tumor-bearing nude mice.
Mice were treated with (○) vehicle, (●) TFTD (200 mg/kg, orally
twice daily for 5 consecutive days followed by 2 drug-free days for
6 weeks), (◊) S-1 (10 mg/kg/day, orally once daily for 5
consecutive days followed by 2 drug-free days for 6 weeks), (∆) 5FU
(12 mg/kg, intravenous injection three times a week every 3 weeks
for 6 weeks) and (■) CPT-11 (100 mg/kg, intravenously once-weekly
during 6 weeks). Body weight was measured twice weekly. Results are
presented as means (n=10). TFTD, trifluridine/tipiracil; S-1,
tegafur, gimeracil and potassium oxonate; 5FU, 5-fluorouracil;
CPT-11, irinotecan. |
 | Table I.Antitumor activity of TFTD in the
human colorectal intraperitoneal xenograft model. |
Table I.
Antitumor activity of TFTD in the
human colorectal intraperitoneal xenograft model.
|
| Survival day, median
(ILS, %) |
|---|
|
|
|
|---|
| Cell line | Control | TFTD | S-1 | 5FU | CPT-11 |
|---|
| DLD-1 | 51 (−) | 85a–c
(67) | 68a (32) | 65a (26) | 87a–c
(70) |
| DLD-1/5FU | 64 (−) | 91a–c
(43) | 64 (0.80) | 65 (1) | 109a–c,e
(70) |
| HT-29 | 40 (−) | 83a–d
(106) | 64a,c
(60) | 58a (44) | 69a,c
(73) |
| HCT116 | 29 (−) | 58a,b
(98) | 34a (17) | n.d. | 54a (84) |
In the DLD-1/5FU intraperitoneal xenograft model, no
significant antitumor effect of 5FU or S-1 was identified compared
with the untreated control group (Fig.
3A and Table I). TFTD exhibited a
significant antitumor activity compared with that of the untreated
control group (P<0.01), but the effect of TFTD was weaker
compared with that of CPT-11 in the DLD-1/5FU model (Table I). No significant body weight loss of
drug treated groups was identified compared with those in the
untreated control group (Fig.
3B).
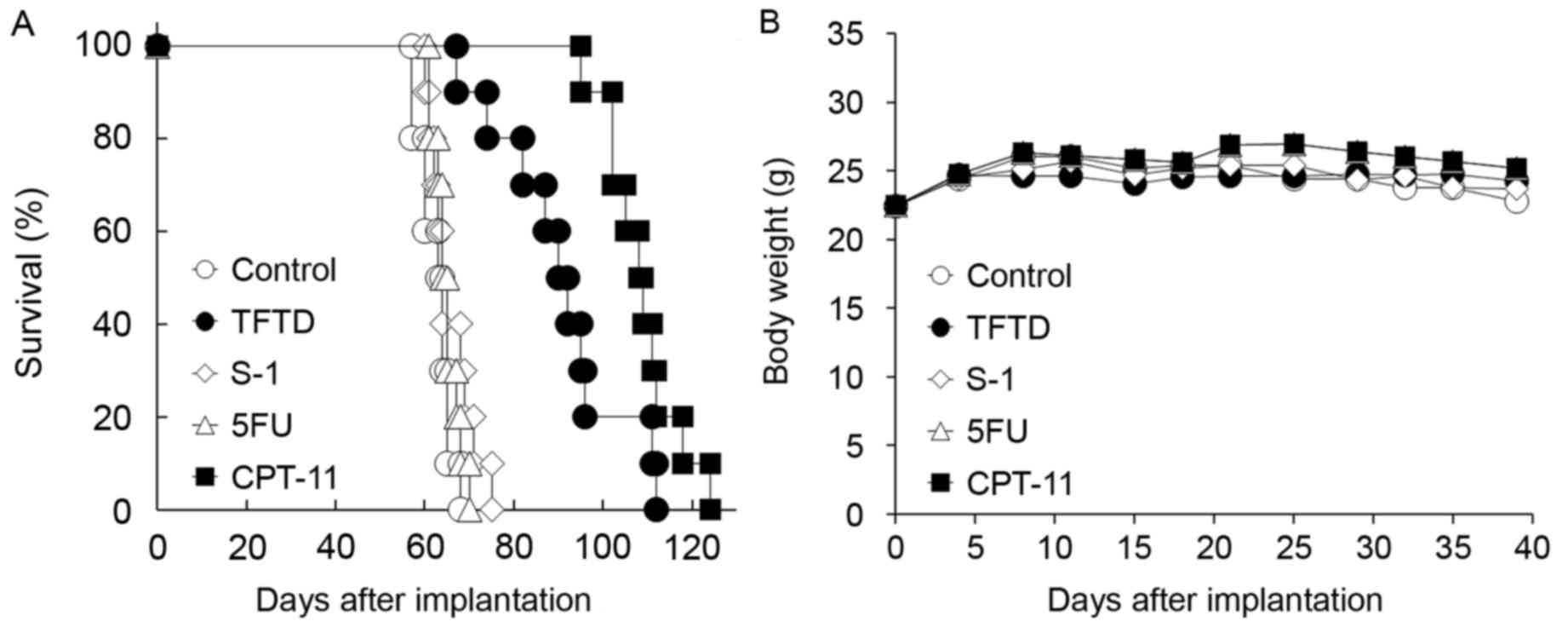 | Figure 3.Effect of TFTD (A) on the survival of
mice intraperitoneally transplanted with human colorectal cancer
DLD-1/5FU cells and (B) on the body weight of DLD-1/5FU
tumor-bearing nude mice. Mice were treated with (○) vehicle, (●)
TFTD (200 mg/kg, orally twice daily for 5 consecutive days followed
by 2 drug-free days for 6 weeks), (◊) S-1 (10 mg/kg/day, orally
once daily for 5 consecutive days followed by 2 drug-free days for
6 weeks), (∆) 5FU (12 mg/kg, intravenous injection three times a
week every three weeks for 6 weeks) and (■) CPT-11 (100 mg/kg,
intravenously once weekly for 6 weeks). Body weight was measured
twice weekly. Results are presented as means (n=10). TFTD,
trifluridine/tipiracil; S-1, tegafur, gimeracil and potassium
oxonate; 5FU, 5-fluorouracil; CPT-11, irinotecan. |
In contrast, TFTD exhibited a significant antitumor
effect when compared with CPT-11 in the HT-29 intraperitoneal
xenograft model (P<0.01; Fig. 4A
and Table I). TFTD also exhibited a
significant antitumor effect when compared with the 5FU and S-1
groups in the HT-29 xenograft model (P<0.01; Table I). In HT-29 cells, no significant body
weight loss in the drug treated groups was identified compared with
those in the untreated control group (Fig. 4B).
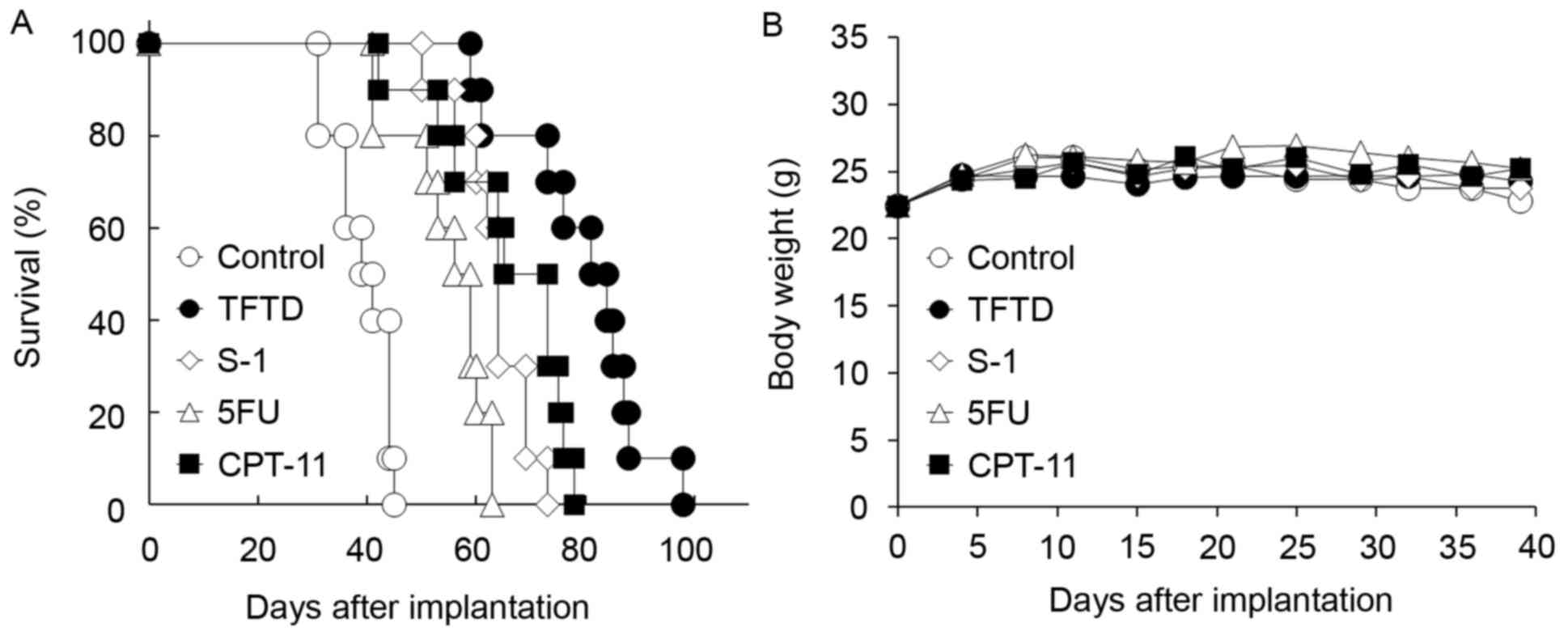 | Figure 4.Effect of TFTD (A) on the survival of
mice intraperitoneally transplanted with human colorectal cancer
HT-29 cells and (B) on body weight in HT-29 tumor-bearing nude
mice. Mice were treated with (○) vehicle, (●) TFTD (200 mg/kg,
orally twice daily for 5 consecutive days followed by 2 drug-free
days for 6 weeks), (◊) S-1 (10 mg/kg/day, orally once daily for 5
consecutive days followed by 2 drug-free days for 6 weeks), (∆) 5FU
(12 mg/kg, intravenous injection three times a week every 3 weeks
for 6 weeks) and (■) CPT-11 (100 mg/kg, intravenously once weekly
for 6 weeks). Body weight was measured twice weekly. Results are
presented as means (n=10). TFTD, trifluridine/tipiracil; 5FU,
5-fluorouracil; S-1, tegafur, gimeracil and potassium oxonate;
CPT-11, irinotecan. |
TFTD, S-1 and CPT-11 significantly increased the
survival in the HCT116 xenograft model (P<0.01; Fig. 5 and Table
I). TFTD and CPT-11 exhibited significant antitumor activities
compared with S-1 in the HCT116 xenograft model (P<0.01;
Table I). In the HCT116
intraperitoneal xenograft models, no significant body weight loss
of drug treated groups were identified compared with those in the
untreated control group (Fig.
5B).
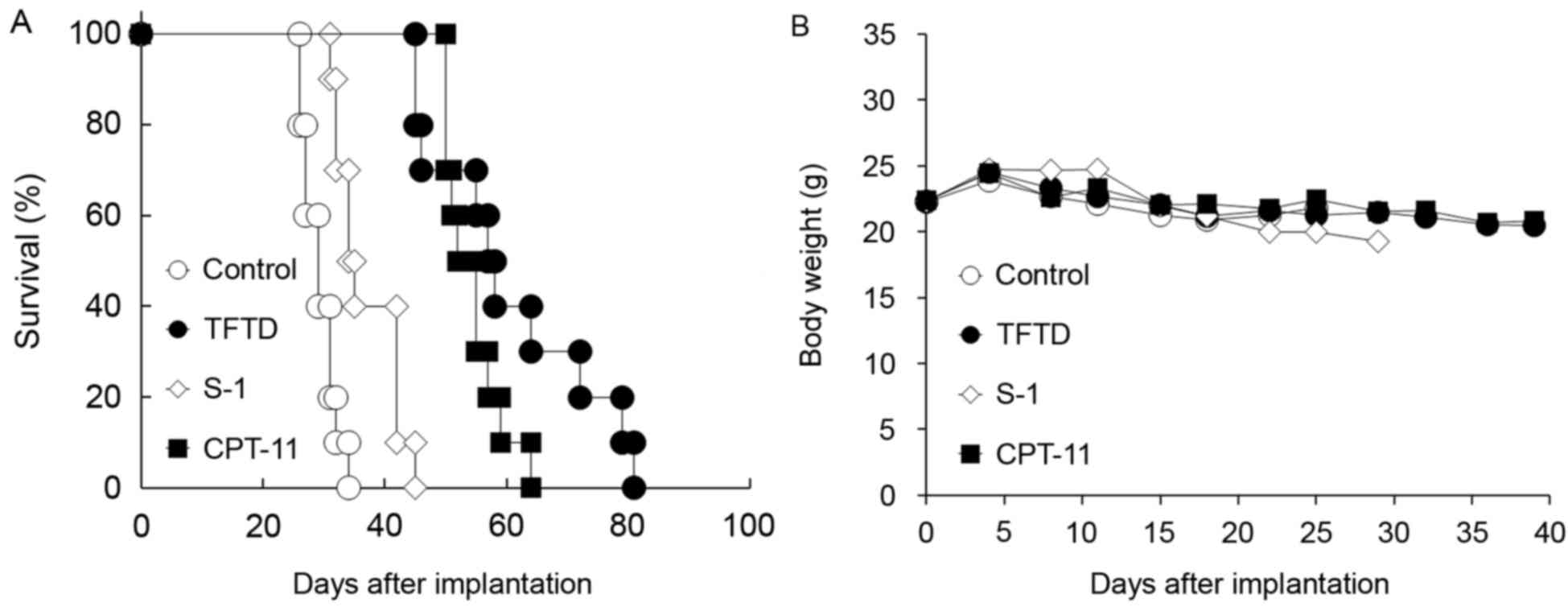 | Figure 5.Effect of TFTD (A) on the survival of
mice intraperitoneally transplanted with human colorectal cancer
HCT116 cells and (B) on the body weight of HCT116 tumor-bearing
nude mice. Mice were treated with (○) vehicle, (●) TFTD (200 mg/kg,
orally twice daily for 5 consecutive days followed by 2 drug-free
days for 6 weeks), (◊) S-1 (10 mg/kg/day, orally once daily for 5
consecutive days followed by 2 drug-free days for 6 weeks), (∆) 5FU
(12 mg/kg, intravenous injection three times a week every 3 weeks
for 6 weeks) and (■) CPT-11 (100 mg/kg, intravenously once weekly
for 6 weeks). Body weight was measured twice weekly. Results are
presented as means (n=10). TFTD, trifluridine/tipiracil; S-1,
tegafur, gimeracil and potassium oxonate; 5FU, 5-fluorouracil;
CPT-11, irinotecan. |
Antitumor activity of TFTD in the
human gastric MKN45 intraperitoneal xenograft model
Fig. 6A and B present
the survival curve and body weight of nude mice inoculated with the
human gastric MKN45 cells, respectively. Untreated control mice
succumbed to peritoneal dissemination after ~4 weeks. TFTD
exhibited a significant antitumor effect compared with S-1, 5FU and
CDDP in the MKN45 intraperitoneal xenograft model (P<0.01;
Table II). The body weight in the
control group gradually decreased during the dosing period. No
significant difference in weight loss of the drug-treated mice
(TFTD, S-1 and CDDP) was identified compared with that of the
control mice (Fig. 6B). No
drug-related toxicity was detected during the present study.
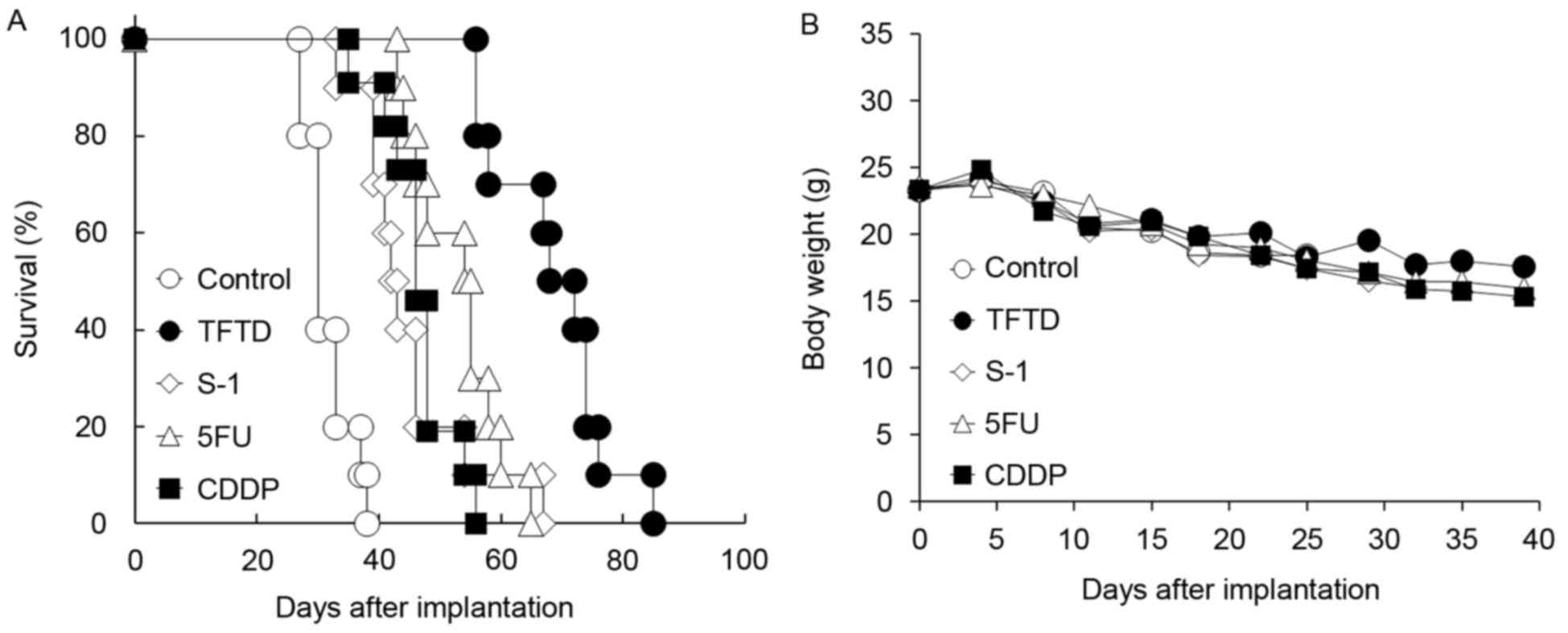 | Figure 6.Effect of TFTD (A) on the survival of
mice intraperitoneally transplanted with human gastric cancer MKN45
cells and (B) on the body weight of MKN45 tumor-bearing nude mice.
Mice were treated with (○) vehicle, (●) TFTD (200 mg/kg, orally
twice daily for 5 consecutive days followed by 2 drug-free days for
6 weeks), (◊) S-1 (10 mg/kg/day, orally once daily for 5
consecutive days followed by 2 drug-free days for 6 weeks), (∆) 5FU
(12 mg/kg, intravenous injection three times a week every three
weeks for 6 weeks) and (■) CDDP (7 mg/kg, intravenously once every
three weeks for 6 weeks). Body weight was measured twice weekly.
Results are presented as means (n=10). TFTD,
trifluridine/tipiracil; S-1, tegafur, gimeracil and potassium
oxonate; 5FU, 5-fluorouracil; CDDP, cisplatin. |
 | Table II.Antitumor activity of TFTD in the
human gastric MKN45 intraperitoneal xenograft model. |
Table II.
Antitumor activity of TFTD in the
human gastric MKN45 intraperitoneal xenograft model.
|
| Survival day,
median (ILS, %) |
|---|
|
|
|
|---|
| Cell line | Control | TFTD | S-1 | 5FU | CDDP |
|---|
| MKN45 | 30 (−) | 70a–c
(133) | 43a (42) | 46a (53) | 56a (85) |
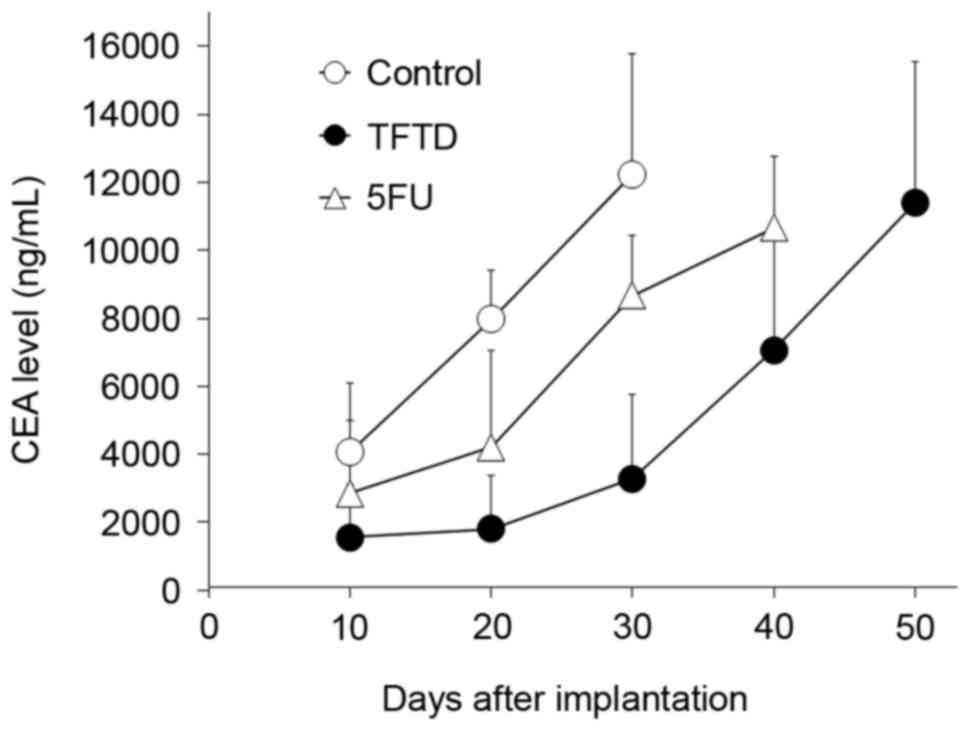
Peritoneal fluid CEA levels in mice
treated with TFTD and 5FU in the human gastric MKN45
intraperitoneal xenograft model
On day 10 following intraperitoneal inoculation of
MKN45 tumor cells, the mean CEA level in the peritoneal fluid of
untreated control mice was 4,061 ng/ml. The CEA level in control
mice increased to 12,234 ng/ml on day 30 (Fig. 7). The CEA level in mice treated with
5FU increased from 2,855 ng/ml (70.3% of control) on day 10 to
4,210 ng/ml (52.8% of control) on day 20, 8,665 ng/ml (70.8% of
control) on day 30 and 10,658 ng/ml on day 40. In contrast, the CEA
level in mice treated with TFTD was 1,547 ng/ml (38.1% of control)
on day 10, 1,795 ng/ml (22.5% of control) on day 20, and 3,284
ng/ml (26.8% of control) on day 30. Almost all mice succumbed to
peritoneal dissemination when the CEA level in the peritoneal fluid
was >10,000 ng/ml.
Discussion
In the present study, a reliable and feasible nude
mouse survival model of peritoneal dissemination of colorectal and
gastric cancers was developed. Using these models, it was
determined that i) TFTD treatment significantly prolonged survival
in nude mice bearing intraperitoneal human colorectal tumors and
human gastric tumors, and ii) the antitumor effects of TFTD were
similar to those of CPT-11 and were significantly increased
compared with those of 5FU, S-1 and CDDP without body weight
loss.
5FU and its derivatives are uracil-based nucleic
acid analogs that inhibit thymidylate synthase, which is a key
enzyme in DNA synthesis, and are incorporated into nucleic acids,
causing RNA damage. Unlike 5FU and its derivatives, the primary
cytotoxic mechanism of trifluridine with twice-daily oral dosing is
hypothesized to be DNA incorporation, causing DNA dysfunction
(19). TFTD has a unique mechanism of
action and is used for the treatment of patients with unresectable
advanced or recurrent colorectal cancer that is refractory to
standard therapies. In preclinical studies, when 5FU-resistant
human tumors are transplanted subcutaneously into the dorsal region
of each animal, TFTD exhibited a significant antitumor effect
compared with that of 5FU and its derivatives (18). The mechanism of tumor cell resistance
to 5FU of DLD-1/5FU is hypothesized to involve decreased
incorporation of 5FU into RNA. The effect of TFTD on the DLD-1/5FU
intraperitoneal xenograft is consistent with previous results
(18).
In the present study, four colorectal cancer cell
lines and one gastric cancer cell line were used. HT-29 and MKN45
are KRAS wild-type, whereas DLD-1 and HCT116 carry a KRAS mutation.
In the present study, TFTD exhibited similar anticancer activities
on these colorectal or gastric cancer cell lines regardless of the
KRAS status (Tables I and II). Consistent with these results, TFTD has
been shown to improve overall survival in a clinical setting
regardless of the KRAS status of tumors (20). Furthermore, the BRAF mutation status
of DLD-1, HCT116 and MKN45 is wild-type and that of HT-29 is
mutant. The p53 mutation status of DLD-1, HT-29 and MKN45 is
wild-type and that of HCT116 is mutant. In the present study, TFTD
also exhibited similar anticancer activities regardless of the BRAF
or p53 status on these cancer cell lines. Therefore, TFTD may be
beneficial to patients with mutated and wild-type KRAS tumors, and
to patients with mutated and wild-type BRAF or p53 tumors.
In the present study, the effects of TFTD were
identified to be significantly increased compared with those of 5FU
and S-1 in nude mice bearing intraperitoneal human colorectal
tumors and human gastric tumors. These data are consistent with
results obtained using KM20C colon cancer cells (29). Trifluridine is incorporated in place
of thymidine bases in DNA (30). DNA
glycosylases involved in the excision of uracil and 5-FU from DNA
include uracil N-glycosylase (UNG), single-strand-selective
monofunctional uracil-DNA glycosylase 1 (SMUG1), thymine DNA
glycosylase (TDG), and methyl-CpG-binding domain 4 (MBD4) (31). Trifluridine inserted at T-sites
(paired to A) is not excised by UNG, SMUG1, TDG or MBD4 (30). The incorporated trifluridine into DNA
is sustained in the DNA for a marked period, and its incorporation
into DNA induces instability of the DNA (32). This persistent antitumor activity is
likely to account for the ability of TFTD to prolong the survival
of mice intraperitoneally transplanted with human tumor cells. The
preclinical data of the present study may be useful to predict the
potential clinical benefits of an anticancer agent.
The CEA levels of 5FU-treated mice between 10 and 30
days ranged between 50 and 70%, whereas that of the TFTD-treated
mice ranged between 20 and 40%. On early days following
intraperitoneal inoculation of MKN45 tumor cells, the increase in
CEA levels was inhibited by TFTD. These TFTD inhibitory effects may
be related to the effects of TFTD on the survival of mice
transplanted with tumor cells when compared with those of other
drugs. In clinical settings, TFTD may inhibit tumor relapse
following surgery at an early stage of colorectal or gastric cancer
in patients.
Certain anticancer agents failed to show any
benefits in clinical trials in spite of being highly effective in a
mouse xenograft model (33–35). The majority of clinical studies enroll
advanced and late-stage patients. Conversely, almost all mouse
studies do not measure therapeutic effects on advanced metastatic
disease (33). The experimental model
of the present study mimicked clinical studies. However, the
effects of TFTD were only evaluated at a single dose (200 mg/kg) by
using a single schedule (twice daily for 5 consecutive days
followed by 2 drug-free days for 6 weeks) in the present study.
Further investigation of the optimal dosing schedule for TFTD is
required to predict the clinical response to TFTD.
Finally, in the present study, TFTD exhibited
superior antitumor efficacy to that of the fluoropyrimidines and
CDDP in a peritoneal dissemination mouse model using human
colorectal and gastric cancer cells. The activity was also
confirmed by measuring CEA levels in MKN45 tumors. A clinical
randomized double-blind Phase III study evaluating TAS-102 plus BSC
compared with placebo plus BSC in patients with metastatic gastric
cancer refractory to standard treatments is currently ongoing
(22), and its outcome is expected to
be informative.
Glossary
Abbreviations
Abbreviations:
|
5FU
|
5-fluorouracil
|
|
BSC
|
best supportive care
|
|
CEA
|
carcinoembryonic antigen
|
|
CPT-11
|
irinotecan
|
|
ILS
|
increase in lifespan
|
|
OS
|
overall survival
|
|
TFTD
|
trifluridine and tipiracil
|
|
TPI
|
tipiracil
|
References
|
1
|
Spolverato G, Ejaz A, Azad N and Pawlik
TM: Surgery for colorectal liver metastases: The evolution of
determining prognosis. World J Gastrointest Oncol. 5:207–221. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lemmens VE, Klaver YL, Verwaal VJ, Rutten
HJ, Coebergh JW and de Hingh IH: Predictors and survival of
synchronous peritoneal carcinomatosis of colorectal origin: A
population-based study. Int J Cancer. 128:2717–2725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sobrero AF, Maurel J, Fehrenbacher L,
Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C,
Steinhauer EU, Prausova J, et al: EPIC: Phase III trial of
cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin
failure in patients with metastatic colorectal cancer. J Clin
Oncol. 26:2311–2319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldberg RM, Sargent DJ, Morton RF, Fuchs
CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC and Alberts
S: Randomized controlled trial of reduced-dose bolus fluorouracil
plus leucovorin and irinotecan or infused fluorouracil plus
leucovorin and oxaliplatin in patients with previously untreated
metastatic colorectal cancer: A North American Intergroup Trial. J
Clin Oncol. 24:3347–3353. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
ClinicalTrials.gov ClinicalTrials.gov
Identifier: NCT00003594: Combination Chemotherapy in Treating
Patients With Advanced Colorectal Cancer. http://clinicaltrials.gov/show/NCT00003594March
10–2017
|
|
8
|
ClinicalTrials.gov ClinicalTrials.gov
Identifier: NCT00005036: Irinotecan Compared With Combination
Chemotherapy in Treating Patients With Advanced Colorectal Cancer.
http://clinicaltrials.gov/show/NCT00005036March
10–2017
|
|
9
|
Franko J, Shi Q, Goldman CD, Pockaj BA,
Nelson GD, Goldberg RM, Pitot HC, Grothey A, Alberts SR and Sargent
DJ: Treatment of colorectal peritoneal carcinomatosis with systemic
chemotherapy: A pooled analysis of north central cancer treatment
group phase III trials N9741 and N9841. J Clin Oncol. 30:263–267.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maehara Y, Hasuda S, Koga T, Tokunaga E,
Kakeji Y and Sugimachi K: Postoperative outcome and sites of
recurrence in patients following curative resection of gastric
cancer. Br J Surg. 87:353–357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sadeghi B, Arvieux C, Glehen O, Beaujard
AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL,
Faure JL, et al: Peritoneal carcinomatosis from non-gynecologic
malignancies: Results of the EVOCAPE 1 multicentric prospective
study. Cancer. 88:358–363. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamaguchi H, Kitayama J, Ishigami H, Emoto
S, Yamashita H and Watanabe T: A phase 2 trial of intravenous and
intraperitoneal paclitaxel combined with S-1 for treatment of
gastric cancer with macroscopic peritoneal metastasis. Cancer.
119:3354–3358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heidelberger C, Birnie GD, Boohar J and
Wentland D: Fluorinated pyrimidines. XX. Inhibition of the
nucleoside phosphorylase cleavage of 5-fluoro-2′-deoxyuridine by
5-trifluoromethyl-2′-deoxyuridine. Biochim Biophys Acta.
76:315–318. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gottschling H and Heidelberger C:
Fluorinated pyrimidines: XIX some biological effects of
5-trifluoromthyluracil and 5-trifluoromethyl-2′-deoxyuridine of
Escherichia coli and bacteriophage T4 G. J Mol Biol. 7:541–560.
1963. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reyes P and Heidelberger C: Fluorinated
pyrimidines. XXVI. Mammalian thymidylate synthetase: Its mechanism
of action and inhibition by fluorinated nucleotides. Mol Pharmacol.
1:14–30. 1965.PubMed/NCBI
|
|
16
|
Fujiwara Y, Oki T and Heidelberger C:
Fluorinated pyrimidines. XXXVII. Effects of
5-trifluoromethyl-2′-deoxyuridine on the synthesis of
deoxyribonucleic acid of mammalian cells in culture. Mol Pharmacol.
6:273–280. 1970.PubMed/NCBI
|
|
17
|
Fukushima M, Suzuki N, Emura T, Yano S,
Kazuno H, Tada Y, Yamada Y and Asao T: Structure and activity of
specific inhibitors of thymidine phosphorylase to potentiate the
function of antitumor 2′-deoxyribonucleosides. Biochem Pharmacol.
59:1227–1236. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Emura T, Suzuki N, Yamaguchi M, Ohshimo H
and Fukushima M: A novel combination antimetabolite, TAS-102,
exhibits antitumor activity in FU-resistant human cancer cells
through a mechanism involving FTD incorporation in DNA. Int J
Oncol. 25:571–578. 2004.PubMed/NCBI
|
|
19
|
Lenz HJ, Stintzing S and Loupakis F:
TAS-102, a novel antitumor agent: A review of the mechanism of
action. Cancer Treat Rev. 41:777–783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mayer RJ, van Cutsem E, Falcone A, Yoshino
T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero
J, Komatsu Y, et al: Randomized trial of TAS-102 for refractory
metastatic colorectal cancer. N Engl J Med. 372:1909–1919. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bando H, Doi T, Muro K, Yasui H, Nishina
T, Yamaguchi K, Takahashi S, Nomura S, Kuno H, Shitara K, et al: A
multicenter phase II study of TAS-102 monotherapy in patients with
pre-treated advanced gastric cancer (EPOC1201). Eur J Cancer.
62:46–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
ClinicalTrials.gov ClinicalTrials.gov
Identifier: NCT02500043: Study of TAS-102 or Placebo Plus BSC in
Patients With Metastatic Gastric Cancer. http://clinicaltrials.gov/show/NCT02500043March
10–2017
|
|
23
|
Murakami Y, Kazuno H, Emura T, Tsujimoto
H, Suzuki N and Fukushima M: Different mechanisms of acquired
resistance to fluorinated pyrimidines in human colorectal cancer
cells. Int J Oncol. 17:277–283. 2000.PubMed/NCBI
|
|
24
|
Motoyama T, Hojo H and Watanabe H:
Comparison of seven cell lines derived from human gastric
carcinomas. Acta Pathol Jpn. 36:65–83. 1986.PubMed/NCBI
|
|
25
|
Kasuya K, Nagakawa Y, Suzuki M, Suzuki Y,
Kyo B, Suzuki S, Matsudo T, Itoi T, Tsuchida A and Aoki T:
Combination therapy of gemcitabine or oral S-1 with the anti-VEGF
monoclonal antibody bevacizumab for pancreatic neuroendocrine
carcinoma. Exp Ther Med. 3:599–602. 2012.PubMed/NCBI
|
|
26
|
Guo XF, Yang ZR, Wang J, Lei XF, Lv XG and
Dong WG: Synergistic antitumor effect of puerarin combined with
5-fluorouracil on gastric carcinoma. Mol Med Rep. 11:2562–2568.
2015.PubMed/NCBI
|
|
27
|
Kawato Y, Furuta T, Aonuma M, Yasuoka M,
Yokokura T and Matsumoto K: Antitumor activity of a camptothecin
derivative, CPT-11, against human tumor xenografts in nude mice.
Cancer Chemother Pharmacol. 28:192–198. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arjumand W and Sultana S: Glycyrrhizic
acid: A phytochemical with a protective role against
cisplatin-induced genotoxicity and nephrotoxicity. Life Sci.
89:422–429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tanaka N, Sakamoto K, Okabe H, Fujioka A,
Yamamura K, Nakagawa F, Nagase H, Yokogawa T, Oguchi K, Ishida K,
et al: Repeated oral dosing of TAS-102 confers high trifluridine
incorporation into DNA and sustained antitumor activity in mouse
models. Oncol Rep. 32:2319–2326. 2014.PubMed/NCBI
|
|
30
|
Suzuki N, Emura T and Fukushima M: Mode of
action of trifluorothymidine (TFT) against DNA replication and
repair enzymes. Int J Oncol. 39:263–270. 2011.PubMed/NCBI
|
|
31
|
Grogan BC, Parker JB, Guminski AF and
Stivers JT: Effect of the thymidylate synthase inhibitors on dUTP
and TTP pool levels and the activities of DNA repair glycosylases
on uracil and 5-fluorouracil in DNA. Biochemistry. 50:618–627.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Markley JC, Chirakul P, Sologub D and
Sigurdsson ST: Incorporation of 2′-deoxy-5-(trifluoromethyl)uridine
and 5-cyano-2′-deoxyuridine into DNA. Bioorg Med Chem Lett.
11:2453–2455. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kerbel RS: Human tumor xenografts as
predictive preclinical models for anticancer drug activity in
humans: Better than commonly perceived-but they can be improved.
Cancer Biol Ther. 2 4 Suppl 1:S134–S139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang C, Awasthi N, Schwarz MA and Schwarz
RE: Establishing a peritoneal dissemination xenograft mouse model
for survival outcome assessment of experimental gastric cancer. J
Surg Res. 182:227–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gould SE, Junttila MR and de Sauvage FJ:
Translational value of mouse models in oncology drug development.
Nat Med. 21:431–439. 2015. View
Article : Google Scholar : PubMed/NCBI
|