Introduction
In the United States, the incidence of gastric
cancer is not particularly high compared with that observed in
other countries; however, ~21,600 patients were diagnosed with
gastric cancer in 2013, and the 5-year survival rate for all stages
of the disease was only 27% (1). The
disease is also a leading cause of cancer-associated mortality in
males and females in Japan (2). The
prognosis for patients with gastric cancer has gradually improved
over the last 10 years; however, the 5-year survival rate of
metastasized gastric cancer remains at ~40% (3). Therefore, the identification and
development of therapeutics is required to improve the prognosis of
patients with advanced gastric cancer.
Integrin associated protein (CD47) is a glycoprotein
that is ubiquitously expressed on the plasma membrane of all
hematopoietic cells and the majority of other cell types (4,5). Oldenborg
et al (6) revealed that CD47
is a marker of self on murine red blood cells (RBCs), and that
CD47-negative RBCs are rapidly eliminated from the circulation by
macrophage phagocytosis. Furthermore, tumor cells expressing CD47
evade elimination by macrophages (7).
The clinical significance of CD47 expression in blood cancer has
been extensively studied (6–8). In solid tumors, including bladder
cancer, cells expressing CD47 were identified to be of a
tumor-initiating cell population (9).
In addition, the prognosis of patients with breast cancer with high
levels of CD47 expression was significantly poorer compared with
patients with low expression of CD47 (10). In 2015, it was revealed that in
addition to being associated with macrophage phagocytosis, a CD47
blockade drives the T-cell mediated elimination of immunogenic
tumors (11). Therefore, CD47 is
emerging as a focus of the field of immunotherapy.
Targeting CD47 via anti-CD47 antibody therapy was
recently proposed (12). A variety of
cancer types may be targeted with this therapy, and therefore, the
status of CD47 expression requires investigation in various
malignant tumors. However, little is known about the status of CD47
expression in patients with gastric cancer. Thus, the present study
aimed to determine the level of CD47 expression in the primary
tumor, peripheral blood (PB) and bone marrow (BM) of patients with
gastric cancer, and to explore the potential of anti-CD47 antibody
therapy in gastric cancer.
Materials and methods
Patients
A total of 168 patients (108 male, 60 female; mean
age, 69±11.8 years) who were diagnosed with gastric cancer and
treated with gastrectomy, including lymphadenectomy, at Kyushu
University Beppu Hospital (Oita, Japan) between April 2000 and
April 2005 were enrolled in the present study. Written informed
consent was obtained from all patients according to the guidelines
provided by the Institutional Research Board of Kyushu University
Beppu Hospital, which approved the present study. None of the
patients received pre-surgical chemotherapy or radiotherapy.
Pathological diagnosis and disease staging were performed according
to the criteria of the Japanese Classification of Gastric Carcinoma
(13). All patients were monitored
closely for the recurrence of cancer following surgery every 3
months for 3–5 years.
Sample collection
PB samples were obtained from 6 healthy volunteers
and the 168 patients with gastric cancer prior to surgery. PB
samples (10 ml) were taken and mixed with 4 ml ISOGEN-LS reagent
(Nippon Gene Co., Ltd., Toyama, Japan) per 1 ml whole blood. The
sample was stored for 5 min at room temperature, then snap-frozen
immediately in liquid nitrogen and stored at −80°C until required
for RNA extraction.
BM samples were obtained from 160 patients with
gastric cancer under general anesthesia prior to surgery. BM
samples were taken from the sternum using a 15-gauge needle. Since
there was a possibility of aspirating skin with the BM samples, the
first 1 ml of the sample was discarded, and another syringe was
used to aspirate 20–30 ml of BM. ISOGEN-LS reagent was then added
as previous, and the BM samples were subsequently processed in the
same manner as the PB samples.
A total of 10 tumor and corresponding normal 4-µm
thick formalin-fixed paraffin-embedded tissue section samples from
the patients who underwent surgery were also obtained and used for
immunohistochemical analysis. A total of 6 autopsy bone marrow
samples were collected, 3 from patients who succumbed to advanced
gastric cancer, and 3 from pneumonia. Formalin-fixed
paraffin-embedded 4-µm tissue sections were made from these bone
marrow samples.
RNA preparation and reverse
transcription (RT)
Total RNA was extracted from PB and BM samples using
the ISOGEN-directed chloroform extraction and isopropanol
precipitation protocol, as described previously (14). cDNA was synthesized using RT as
described previously (15).
Cell lines
The human gastric cancer cell lines KATO-III, MKN-1,
MKN-45 and MKN-74 were provided by the Cell Resource Center for
Biomedical Research (Institute of Development, Aging and Cancer,
Tohoku University, Sendai, Japan). The NUGC-4 (JCRBO834) cell line
was provided by the Japanese Collection of Research Bioresources
Cell Bank (National Institutes of Biomedical Innovation, Health and
Nutrition, Osaka, Japan). KATO-III cells were maintained in McCoy5A
medium supplemented with 10% fetal bovine serum and 1% penicillin
and streptomycin antibiotics (all from Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The other cell lines were maintained in an
RPMI-1640 medium supplemented with 10% fetal calf serum and 1%
penicillin and streptomycin (all from Thermo Fisher Scientific,
Inc.). All cell lines were cultured in 10 cm culture dishes with 5%
CO2 at 37°C. Cultured cells from each cell line were
dissolved in 350 µl of Buffer RLT containing 1% β-mercaptoethanol,
and total RNA was extracted and purified using the RNeasy Mini kit
(Qiagen GmbH, Hilden, Germany) in accordance with the
manufacturer's protocol.
Quantitative polymerase chain reaction
(qPCR) evaluation of CD47 expression in clinical samples
The following primers were used to amplify CD47 from
the RNA samples from the cells, PB and BM: Sense,
5′-GGCAATGACGAAGGAGGTTA-3′ and antisense,
5′-ATCCGGTGGTATGGATGAGA-3′. GAPDH, used as an internal control, was
amplified using the following primers: Sense,
5′-TGAACGGGAAGCTCACTGG-3′ and antisense,
5′-TCCACCACCCTGTTGCTGTA-3′. qPCR was performed using the
LightCycler™ system and SYBR-Green I dye (both from
Roche Diagnostics, Indianapolis, IN, USA) according to the
manufacturer's protocol. Briefly, each reaction contained 80 ng
cDNA, 2 µl DNA Master SYBR-Green I mix, 50 ng primers and 2.4 µl 25
mM MgCl2. The final volume was adjusted to 20 µl with
water. qPCR was performed with the following thermocycling
conditions: Initial denaturation at 95°C for 10 min; followed by 40
cycles of 95°C for 10 sec; annealing at 60°C for 10 sec; and
extension at 72°C for 10 sec. The product was confirmed by gel
electrophoresis. Following amplification, the products were
subjected to a temperature gradient from 68–95°C at a rate of
0.2°C/sec, under continuous fluorescence monitoring, in order to
produce a melt curve for each product. All concentrations were
calculated relative to the concentration of the positive control,
cDNA produced from Human Universal Reference Total RNA (Clontech
Laboratories, Inc., Mountain View, CA, USA), using a previously
described method (16). The negative
control was water. Normalized expression values were obtained by
dividing the mRNA concentration of CD47 by that of the internal
control.
Immunohistochemistry
Formalin-fixed and paraffin-embedded tissue sections
corresponding to the samples used for mRNA expression analysis were
analyzed. Tissue sections were de-paraffinized, soaked in 0.01 M
sodium citrate buffer and boiled in a microwave oven for 15 min at
500 W to retrieve cell antigens. In the case of bone marrow
samples, decalcification was performed according to the
manufacturer's protocol (Super Decalcifier I; Polyscience Inc.,
Warminster, PA, USA). The tissue sections were stained
immunohistochemically using the streptavidin-biotin peroxidase
method (Universal Dako Cytomation LSAB® kit; Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA) according to the
manufacturer's protocol, with primary antibodies against CD47
(mouse monoclonal antibody; dilution, 1:200; cat. no. sc-12730;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Briefly, the
tissue sections were blocked with 3% H2O2 for
5 min and incubated overnight with the primary antibody at 4°C. The
samples were then washed with TBS buffer and subsequently incubated
with the secondary antibody from the LSAB® kit for 30
min at room temperature.
Cell sorting
Immunomagnetic cell sorting was performed on 30 ml
of PB collected from 6 healthy donors and 30 ml BM collected from 6
patients with gastric cancer. Fractionation was performed using the
autoMACS® Pro Separator (Miltenyi Biotec GmbH, Bergisch
Gladbach, Germany) according to the manufacturer's protocol. Cells
were prepared as follows: The PB or BM sample was loaded into 10 ml
of Ficoll density medium (GE Healthcare Life Sciences, Chalfont,
UK) prepared in a 50 ml polypropylene tube. Gradient centrifugation
was subsequently performed at room temperature for 30 min at 450 ×
g. Sample interfaces were collected subsequent to discarding the
supernatants. The interface containing the mononuclear fractionated
cells was washed twice with 20 ml PBS and centrifuged at 150 × g
for 5 min at 4°C.
Human CD326 [epithelial cell adhesion molecule
(EpCAM)]-phycoerythrin MicroBeads (Miltenyi Biotec GmbH) were used
for positive selection of viable epithelial tumor cells from the
samples, CD45-MicroBeads (Miltenyi Biotec GmbH) were used to detect
leukocytes and CD14-MicroBeads (Miltenyi Biotec GmbH) were applied
for the detection of monocytes and macrophages. The MicroBeads were
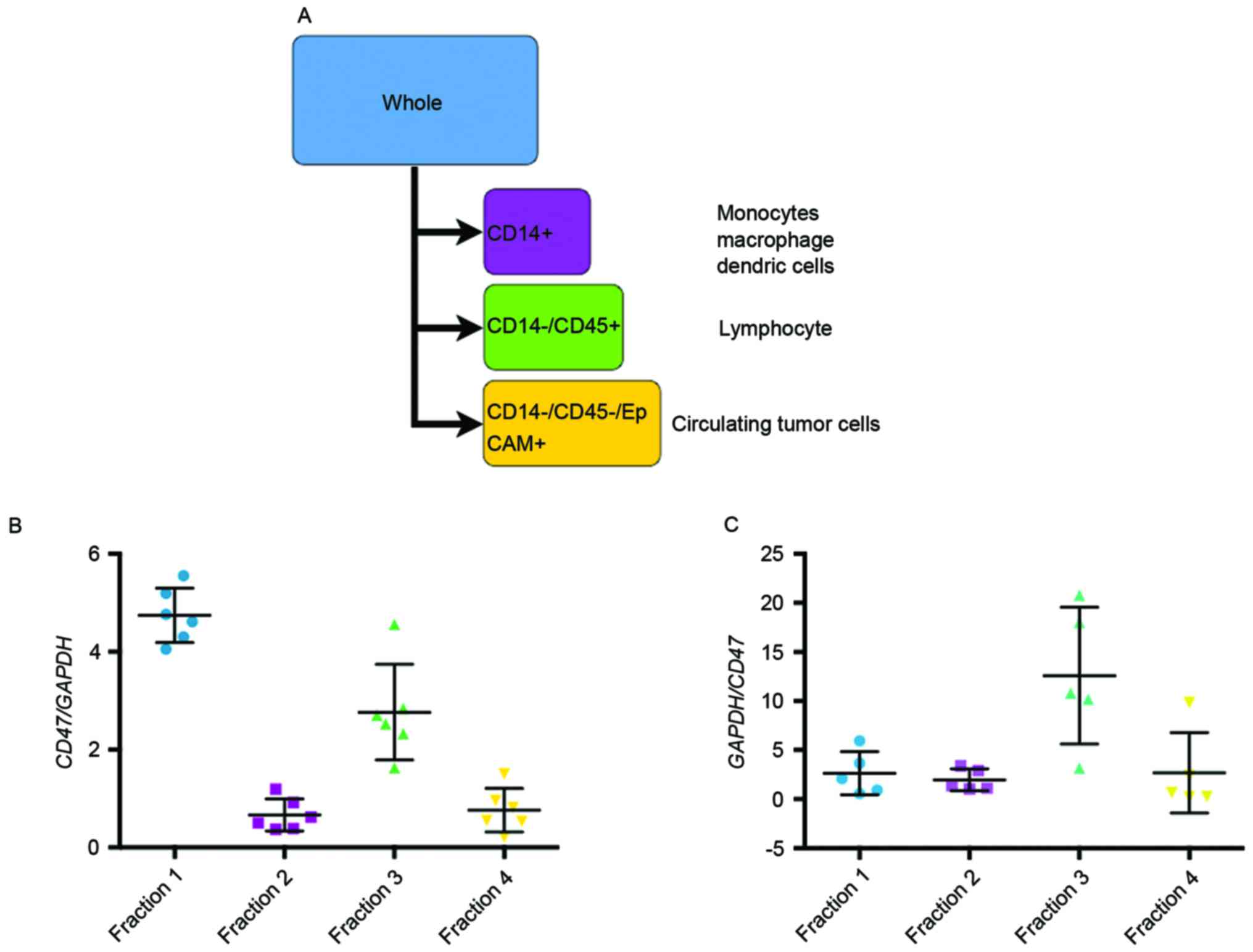
used according to the manufacturer's protocol. In brief, the CD14+
fraction was sorted, then the CD14-/CD45+ fraction and finally the
EpCAM-positive fraction (Fig. 1A).
The expression of CD47 in each fraction was measured using RT-qPCR
as described above.
Statistical analysis
The association between CD47 mRNA expression and
clinicopathological factors was evaluated using the χ2
test. For the evaluation of primary tumor tissue samples, the
median CD47 mRNA expression value was used as the cut-off to
divided patients into high CD47 expression and low CD47 expression
groups. For PB and BM samples, the median value was calculated
using all samples. Overall survival was calculated by the
Kaplan-Meier method and the differences in survival between the
groups were compared using the log-rank test. All tests were
performed using JMP software version 12.2.0 (SAS Institute, Inc.,
Cary, NC, USA) and GraphPad Prism software version 6.0 h (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of CD47 in primary gastric
tumors
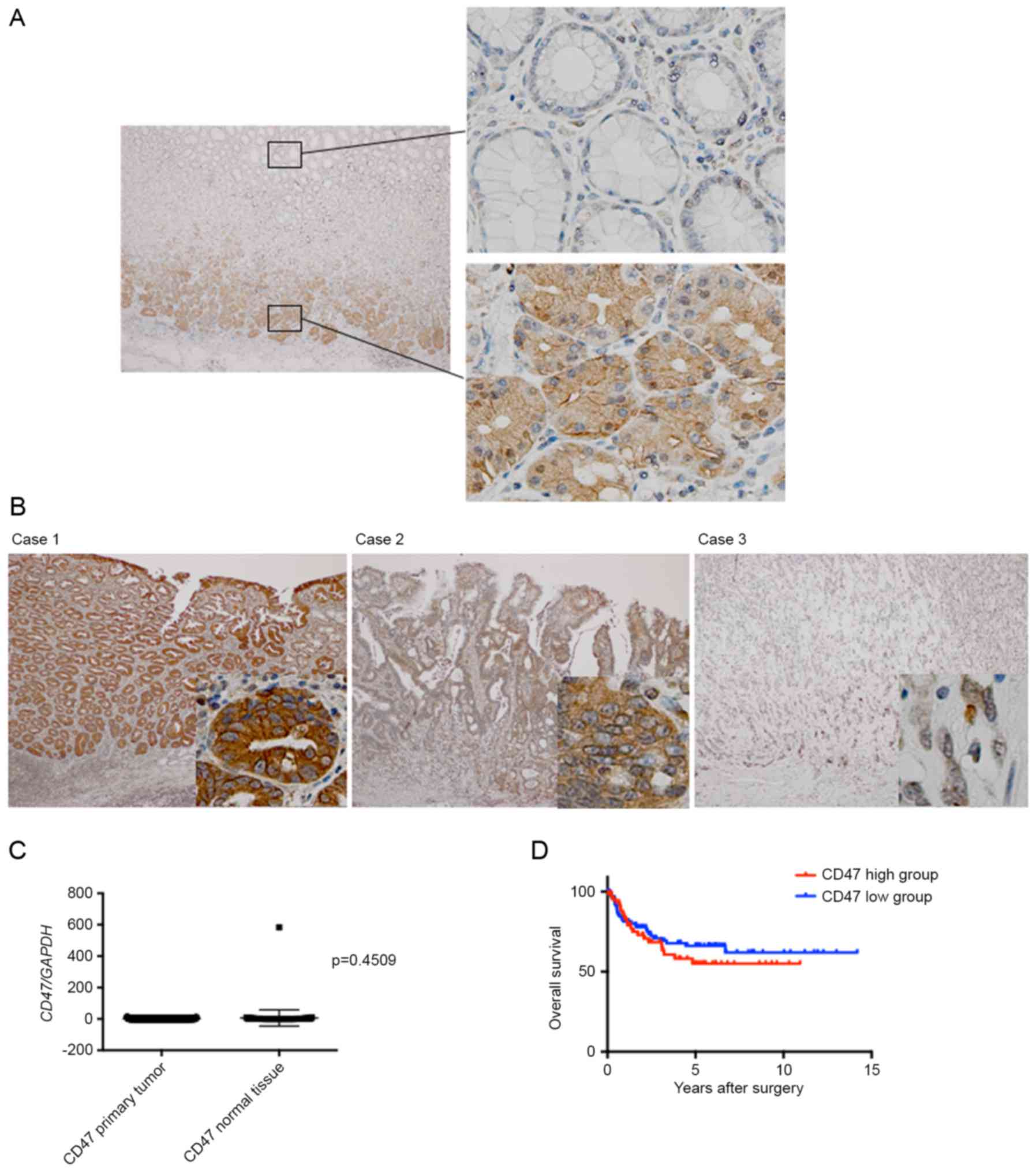
Immunohis-tochemical analysis was performed to
investigate the protein expression level of CD47 in the tissues of
gastric cancer and adjacent normal mucosa. In normal gastric
mucosa, intracellular expression of CD47 protein was observed in
the bottom layer of the gastric gland, which almost disappeared in
the upper layer (Fig. 2A). In gastric
tumor tissues, a varying pattern of CD47 protein expression was
observed. In one case, CD47 was highly expressed throughout the
tissue, and this case was diagnosed as well-differentiated
adenocarcinoma (Fig. 2B, case 1). In
another case, the expression of CD47 was relatively high, and the
expression pattern was not consistent throughout the tissue; this
case was diagnosed as moderately-differentiated adenocarcinoma
(Fig. 2B, case 2). The expression of
CD47 nearly disappeared in another case, which was diagnosed as
poorly-differentiated adenocarcinoma (Fig. 2B, case 3).
As demonstrated by immunohistochemical staining,
CD47 protein levels appeared to be associated with the histological
grade, and therefore, the CD47 mRNA expression level was compared
with the histological grade; however, no significant difference was
observed between CD47 expression and different histological grades
(data not shown). The expression of CD47 was compared between
gastric tumor tissue and corresponding normal tissue using RT-qPCR.
However, as demonstrated in Fig. 2C,
no significant difference was observed in CD47 mRNA expression
between primary tumor tissue and normal tissue.
To determine the association between CD47 mRNA
expression and the clinicopathological characteristics of patients
with gastric cancer, patients were divided into two groups: High
CD47 expression and low CD47 expression, however, no significant
association was observed between CD47 expression and any of the
clinicopathological factors tested (Table
I). In addition, the overall survival rate between the high and
low CD47 expression groups was compared, and no significant
difference was observed between the two groups (Fig. 2D).
 | Table I.Association between CD47 mRNA
expression in tumor tissue and the clinicopathological
characteristics of patients with gastric cancer. |
Table I.
Association between CD47 mRNA
expression in tumor tissue and the clinicopathological
characteristics of patients with gastric cancer.
|
| CD47 expression
group |
|
|---|
|
|
|
|
|---|
| Clinicopathological
characteristic | High | Low | P-value |
|---|
| Total, n | 65 | 68 | – |
| Age,
years, mean ± SD | 64.74±1.37 | 68.47±1.34 | 0.0528 |
| Age
distribution, n ≥70/<70 years (% ≥70) | 27/38 (41.6) | 29/39 (42.6) | 0.897 |
| Gender,
male/female (% male) | 42/23 (64.6) | 42/26 (61.7) | 0.733 |
| Tumor factors |
|
|
|
| Tumor
size, mm, mean ± SD | 49.85±26.03 | 53.79±34.19 | 0.458 |
| Tumor
size distribution, n ≥50/<50 mm (% ≥50) | 31/34 (47.6) | 39/29 (57.3) | 0.0497a |
| Depth
of tumor, n T1-2/T3-4 (% T1-2) | 43/22 (66.2) | 46/22 (67.7) | 0.855 |
| Tumor
type, n type 0–1/2-5 (% 0–1) | 13/52 (20.0) | 21/47 (30.9) | 0.149 |
|
Lymphatic invasion, n +/− (%
+) | 43/22 (61.2) | 42/26 (61.8) | 0.598 |
|
Vascular invasion, n +/− (%
+) | 19/46 (29.2) | 14/54 (20.6) | 0.248 |
| Metastatic
factors |
|
|
|
| Lymph
node metastasis, n +/− (% +) | 41/24 (63.1) | 44/24 (64.7) | 0.845 |
| Liver
metastasis, n +/− (% +) | 2/63 (3.1) | 6/62 (8.8) | 0.154 |
|
Peritoneal (micro) metastasis,
n +/− (% +) | 9/56
(13.9) | 9/59
(13.2) | 0.918 |
|
Peritoneal (macro) metastasis,
n +/− (% +) | 9/56
(13.9) | 9/59
(13.2) | 0.918 |
| Distant
metastasis, n +/− (% +) | 1/64 (1.5) | 4/64 (5.9) | 0.173 |
| Clinical stage, n
I/II–IV (% I) | 21/44 (32.3) | 27/41 (39.7) | 0.374 |
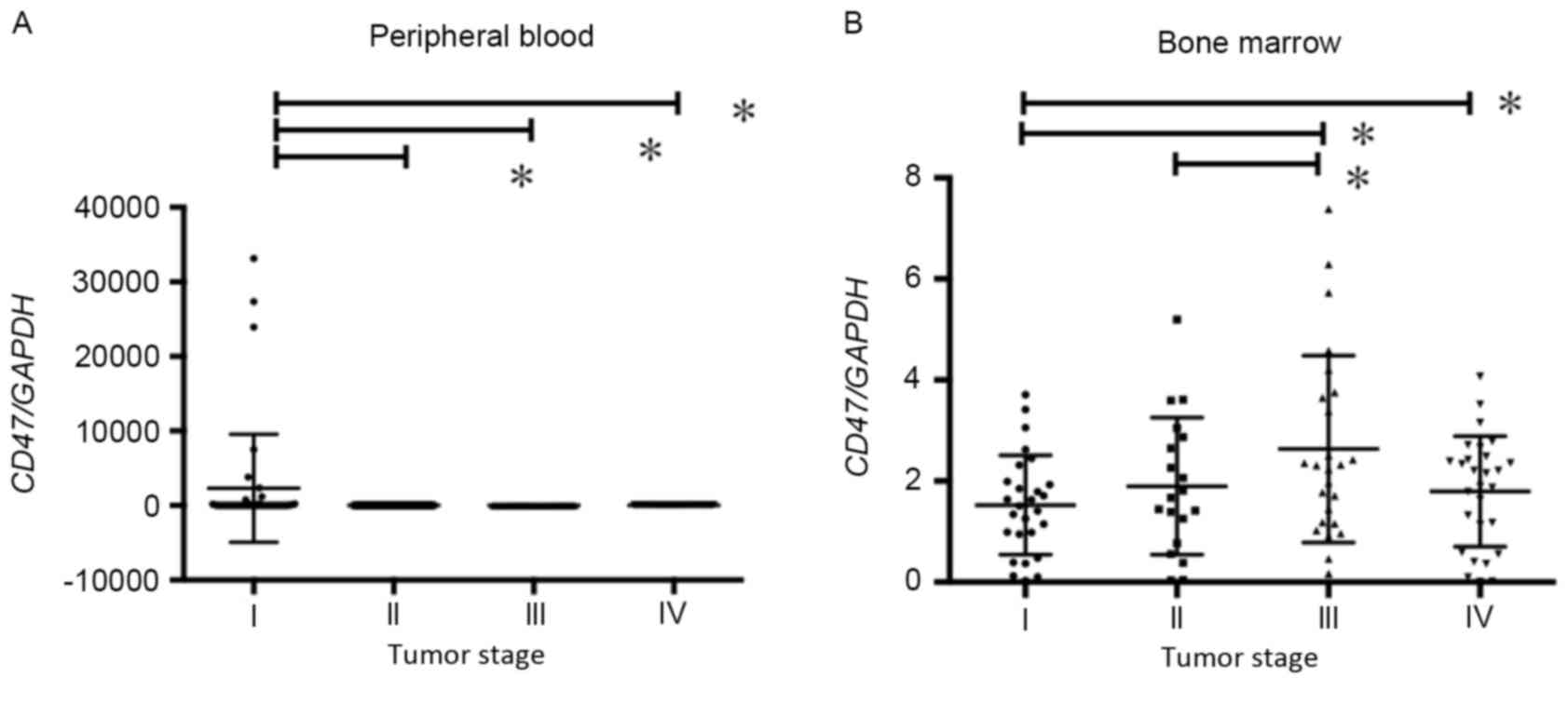
Expression of CD47 in the PB
CD47 expression in PB was analyzed, and its
association with the clinicopathological characteristics of
patients with gastric cancer was investigated (Table II). Tumor size and depth were
significantly increased in patients with low compared with high
CD47 expression. The frequency of lymphatic invasion was also
significantly higher in the low compared with high CD47 expression
group. The percentage of tumor type 2–5 was significantly higher at
73% in the CD47 low expression group compared with 44% in the CD47
high expression group. The frequency of lymph node metastasis and
lymphatic invasion in the low CD47 expression group was
significantly increased compared with that in the high CD47
expression group. As a result, the clinical tumor stage of the low
CD47 expression group was significantly increased compared with
that of the high CD47 expression group. The expression level of
CD47 mRNA was compared with the clinical tumor stage in the PB
sample (Fig. 3A). CD47 expression was
demonstrated to be highest in tumor stage I in PB samples (Fig. 3A).
 | Table II.Association between CD47 mRNA
expression in peripheral blood and the clinicopathological
characteristics of patients with gastric cancer. |
Table II.
Association between CD47 mRNA
expression in peripheral blood and the clinicopathological
characteristics of patients with gastric cancer.
|
| CD47 expression
group |
|
|---|
|
|
|
|
|---|
| Clinicopathological
characteristic | High | Low | P-value |
|---|
| Total, n | 86 | 88 | – |
| Age,
years, mean ± SD | 59.45±1.32 | 61.76±1.26 | 0.207 |
| Age
distribution, n ≥70/<70 years (% ≥70) | 18/68 (20.9) | 26/62 (29.5) | 0.460 |
| Gender,
male/female (% male) | 59/27 (68.6) | 55/33 (62.5) | 0.397 |
| Tumor factors |
|
|
|
| Tumor
size, mm, mean ± SD | 63.29±44.71 | 82.58±44.45 | 0.0025a |
| Tumor
size distribution, n ≥50/<50 mm (% ≥50) | 38/48 (44.1) | 61/27 (69.3) | 0.0052a |
| Depth
of tumor, n T1-2/T3-4 (% T1-2) | 37/49 (43.0) | 7/81 (7.9) |
<0.0001a |
| Tumor
type, n type 0–1/2-5 (% 0–1) | 48/38 (55.8) | 23/65 (26.1) | 0.0001a |
|
Lymphatic invasion, n +/− (%
+) | 47/39 (54.6) | 63/25 (71.6) |
0.00316a |
|
Vascular invasion, n +/− (%
+) | 20/66 (23.2) | 63/25 (71.6) | 0.278 |
| Metastatic
factors |
|
|
|
| Lymph
node metastasis, n +/− (% +) | 45/41 (52.3) | 71/17 (80.6) | 0.0002a |
| Liver
metastasis, n +/− (% +) | 1/85 (1.1) | 3/85 (3.4) | 0.324 |
|
Peritoneal (micro) metastasis,
n +/− (% +) | 11/75 (14.7) | 20/68 (22.7) | 0.0868 |
|
Peritoneal (macro) metastasis,
n +/− (% +) | 7/79 (8.1) | 11/77 (12.5) | 0.340 |
| Distant
metastasis, n +/− (% +) | 2/84 (2.3) | 1/87 (1.1) | 0.527 |
| Clinical stage, n
I/II–IV (% I) | 45/41 (52.1) | 9/79
(10.2) |
<0.0001a |
Expression of CD47 in the BM
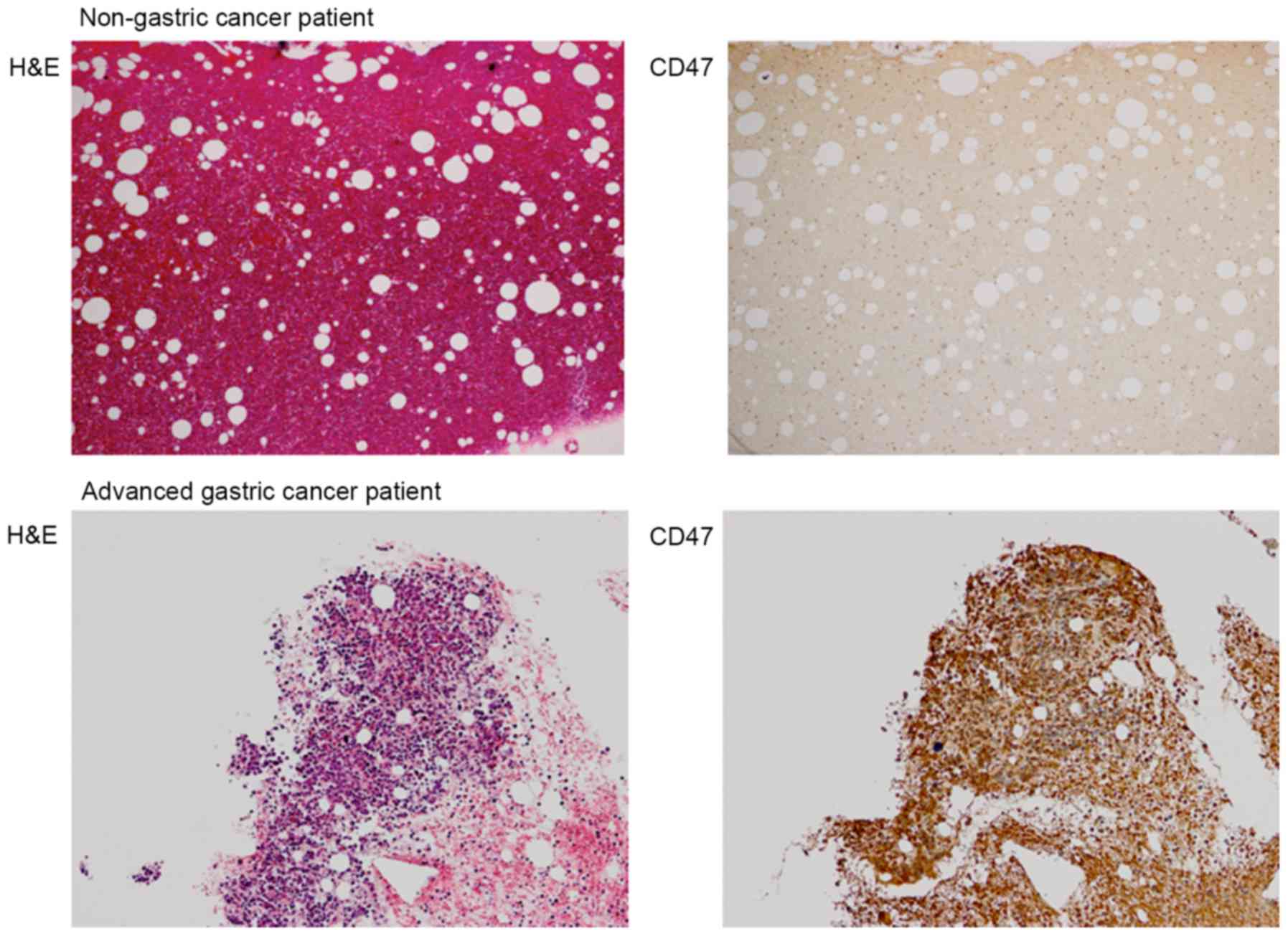
CD47 protein expression in BM samples was analyzed
using immunohistochemical staining. As demonstrated in Fig. 4, CD47 expression was markedly
increased in the BM of patients with stage IV gastric cancer. To
assess the importance of CD47 expression in the BM, the
clinicopathological characteristics of patients with gastric cancer
who exhibited high or low CD47 mRNA expression were compared. As
indicated in Table III, the
percentages of lymphatic invasion and lymph node metastasis were
significantly higher in the high CD47 expression group compared
with the low CD47 expression group. By contrast, the frequency of
distant metastasis was significantly higher in the low CD47
expression group compared with the high CD47 expression group. In
addition, the clinical tumor stage was significantly increased in
the high compared low CD47 expression group. The BM expression of
CD47 mRNA was significantly higher in stage III/IV patients than in
stage I/II patients (Fig. 3B).
 | Table III.Association between CD47 mRNA
expression in bone marrow and the clinicopathological
characteristics of patients with gastric cancer. |
Table III.
Association between CD47 mRNA
expression in bone marrow and the clinicopathological
characteristics of patients with gastric cancer.
|
| CD47 expression
group |
|
|---|
|
|
|
|
|---|
| Clinicopathological
characteristic | High | Low | P-value |
|---|
| Total, n | 97 | 98 | – |
| Age,
years, mean ± SD | 61.2±1.21 | 62.5±1.21 | 0.445 |
| Age
distribution, n ≥70/<70 years (% ≥70) |
|
|
|
| Gender,
male/female (% male) | 59/38 (60.8) | 73/25 (74.5) | 0.0408a |
| Tumor factors |
|
|
|
| Tumor
size, mm, mean ± SD | 77.23±48.11 | 65.87±47.63 | 0.101 |
| Tumor
size distribution, n ≥50/<50 mm (% ≥50) | 60/37 (61.9) | 53/45 (54.1) | 0.31 |
| Depth
of tumor, n T1-2/T3-4 (% T1-2) | 28/69 (28.7) | 46/52 (47.4) | 0.0076a |
| Tumor
type, n type 0–1/2-5 (% 0–1) | 60/37 (61.9) | 49/49 (50.0) | 0.220 |
|
Lymphatic invasion, n +/− (%
+) | 63/34 (65.2) | 44/54 (45.2) | 0.0071a |
|
Vascular invasion, n +/− (%
+) | 20/77 (20.9) | 24/74 (24.7) | 0.545 |
| Metastatic
factors |
|
|
|
| Lymph
node metastasis, n +/− (% +) | 74/23 (76.4) | 53/45 (54.3) | 0.0015a |
| Liver
metastasis, n +/− (% +) | 1/96 (1.0) | 2/96 (2.0) | 0.563 |
|
Peritoneal (micro) metastasis,
n +/− (% +) | 17/80 (17.5) | 16/82 (16.5) | 0.848 |
|
Peritoneal (macro) metastasis,
n +/− (% +) | 10/87 (10.3) | 5/93 (5.1) | 0.169 |
| Distant
metastasis, n +/− (% +) | 0/97 (0.0) | 5/93 (5.1) | 0.0081a |
| Clinical stage, n
I/II–IV (% I) | 14/83 (14.9) | 33/65 (34.0) | 0.0019a |
Origin of CD47 in the PB and BM
The results of the analysis of the association
between CD47 mRNA expression and the clinicopathological
characteristics of patients with gastric cancer were different in
primary tumors, PB and BM. To detect the original source of CD47
expression in PB and BM, cell sorting was performed. PB and BM
samples were sorted into the following four fractions (Fig. 1A): Fraction 1, whole blood cells;
fraction 2 [myeloid cell-specific leucine-rich glycoprotein
(CD14)+ cells], macrophages or monocytes; fraction 3
(CD14−/CD45+ cells), lymphocytes; and
fraction 4 (CD14−/CD45−/EpCAM+
cells), circulating tumor cells. Prior to the analysis, CD47
expression was confirmed in 4 gastric cancer cell lines by RT-qPCR
(data not shown). The expression of CD47 mRNA in each fraction is
demonstrated in Fig. 1B and C. CD47
mRNA expression was expected to be highest in the circulating tumor
cell fraction; however, the fraction expressing CD47 mRNA was
larger in the PB (Fig. 1B) and BM
(Fig. 1C) samples than the lymphocyte
fraction.
Discussion
In the present study, the localization of CD47
protein was confirmed in normal tissue and primary gastric tumor
tissue using immunohistochemical staining. Despite initial
expectations, no statistically significant association was observed
between primary tumor CD47 expression and clinicopathological
factors, which has previously been detected in hematological
malignancies (7,17,18) and
breast cancer (10). The Kaplan-Meier
overall survival curve revealed that the survival rate of the high
CD47 expression group was decreased compared with the low CD47
expression group from 3 years following surgery. This indicates
that high CD47 expression is associated with late phase metastasis
or recurrence of gastric cancer; however, the difference in
survival between the two groups did not reach a statistically
significant level. By contrast, low expression of CD47 in PB
samples was significantly associated with increased tumor depth,
lymphatic invasion, lymph node metastasis and clinical tumor stage.
Conversely, high expression of CD47 in BM samples was associated
with increased tumor depth, lymphatic invasion, lymph node
metastasis and clinical stage. It was also revealed that CD47 mRNA
expression was highest in the lymphocyte fraction in PB and BM
samples. The contradiction of the CD47 expression pattern between
the primary tumor, PB and BM must be explained.
In normal gastric tissue, CD47 expression was
limited to the lower layer, termed the fundic glands, in the
current study. The expression of CD47 is particularly observed from
the portion of isthmus to the base of the glands. The fundic glands
contain gastric mucosal stem cells and progenitor cells (19–21). By
expressing CD47, the stem cells evade macrophage phagocytosis and
elimination (6), and therefore the
CD47 expression observed in normal mucosa is consistent with
previous reports.
In primary gastric tumors, there was no indication
of any statistical association between CD47 mRNA expression and
clinicopathological factors. In the immunohistochemical staining
analysis, a varied pattern of staining was observed, but there was
no definitive pattern indicating that CD47 protein expression
contributes to macrophage phagocytosis evasion. In addition, it was
difficult to objectively evaluate which staining patterns were
CD47-dominant or -negative; thus, it may not be appropriate to
evaluate CD47 expression using immunohistochemical staining
analysis in cancer. To make objective evaluations, the mRNA
expression level of CD47 was calculated; however, bulky samples
were used for mRNA collection in the present study, such that the
heterogeneity of the tumor as a whole may have been erased.
In PB, upregulation of CD47 mRNA expression was
associated with improved clinicopathological factors and early
stage disease. By contrast, the association between CD47 expression
and clinicopathological factors was the opposite in the BM. CD47
mRNA was expressed in the lymphocyte fraction in PB and BM samples,
and this expression was low in the circulating tumor cell fraction,
indicating that CD47 mRNA expression reflects lymphocytes but not
cancer cells. Downregulation of CD47 is presumed to be caused by a
reduction in lymphocytes. Several studies have indicated that the
neutrophil:lymphocyte ratio (NLR) is a prognostic marker for
gastric cancer (22–27). In early gastric cancer,
cancer-associated mortality has been reported to be significantly
more frequent among patients with an elevated NLR (>2) (28). In advanced gastric cancer,
upregulation of the NLR to >2.5 is associated with a poor
prognosis (29). The cause of the
elevated NLR in this study was due to a surge in neutrophils and
decline in lymphocytes, which is consistent with the decline in
CD47 expression observed in the present study.
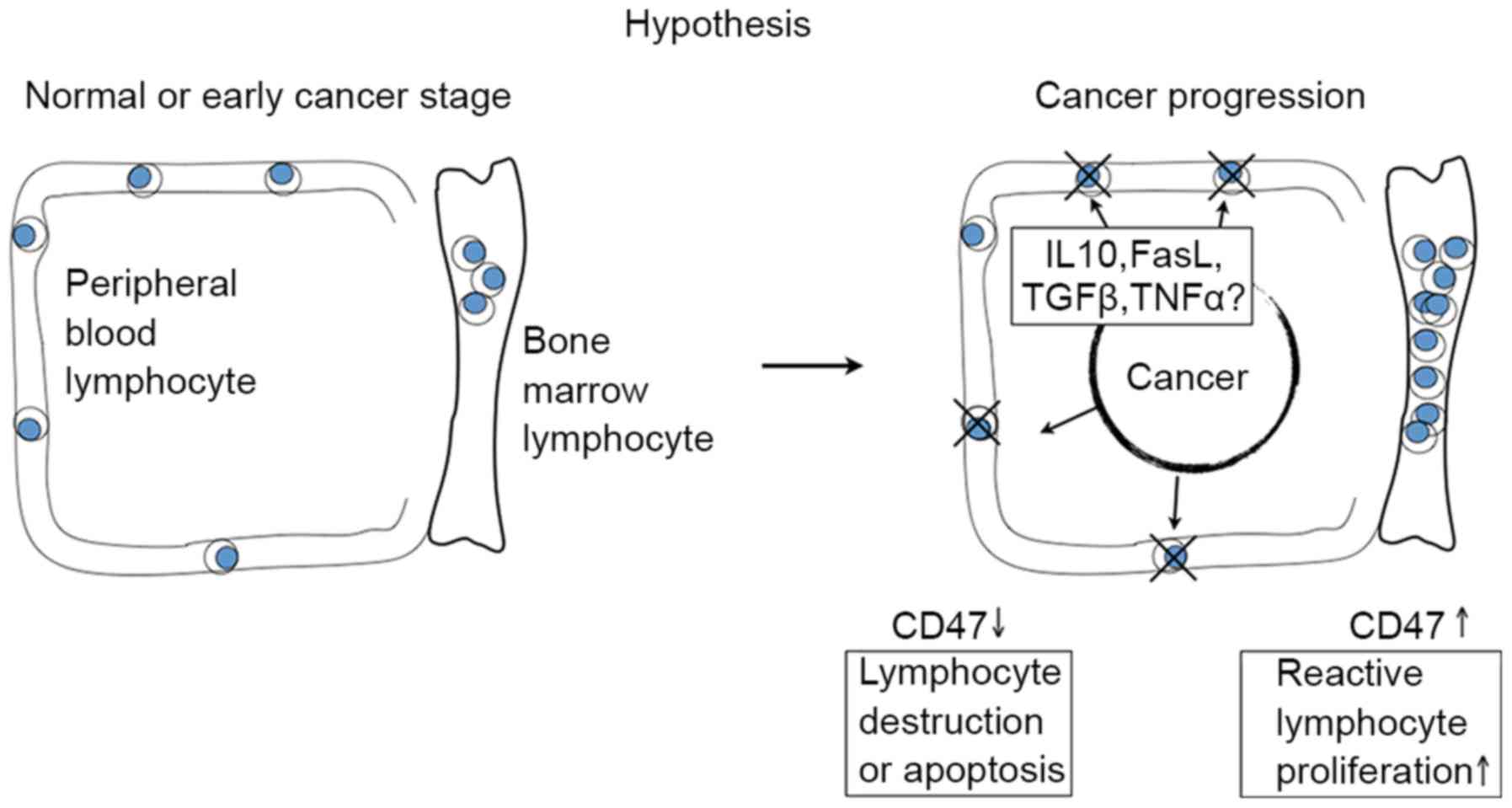
Lymphocyte reduction may be explained partially by
the effect of an elevated release of several biological factors and
cytokines into the PB from cancer tissues. Saito et al
(30) demonstrated that a decline in
natural killer (NK) cells, a type of lymphocyte, was caused by
elevated tumor necrosis factor ligand superfamily member 6
expression, which shortened the lifespan of circulating NK cells by
inducing their apoptosis. By contrast, the elevation of lymphocytes
in BM is potentially the result of reactive proliferation in
response to decreased lymphocytes in the PB (Fig. 5).
The reason for the discrepancy between the findings
for CD47 expression in gastric cancer compared with blood and
breast cancer requires further analysis. In the present study, the
level of CD47 expression was investigated using PB samples, so that
the expression level of CD47 would be representative of all cells
in the blood, including lymphocyte and RBCs expressing CD47. Future
studies should also collect the circulating tumor cells for
analysis of CD47 expression levels.
Recently, Yoshida et al (31) reported that the survival rate of
patients with CD47-expressing gastric cancer was significantly
worse compared with that of patients with CD47-negative gastric
cancer, using immunohistochemical staining analysis. In the present
study, increased CD47 mRNA expression in the primary gastric tumor
was not an adverse prognostic factor. This suggests that there may
be post-transcriptional differences that cause CD47 protein
expression to be higher, such as disruption of the protein
degradation or transportation.
To the best of our knowledge, this is the first
study to analyze CD47 expression in primary tumors, PB and BM
simultaneously in patients with gastric cancer. The results
indicate that CD47 expression in the PB and BM may serve as a
marker to analyze the immunological function of patients with
gastric cancer; however, to elucidate the significance of CD47 in
gastric cancer is a complicated task. Therefore, if anti-CD47
antibody treatment is considered for gastric cancer, it may not be
appropriate to make the decision based on the measurement of CD47
for this disease.
Glossary
Abbreviations
Abbreviations:
|
CD47
|
integrin-associated protein
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
CD14
|
myeloid cell-specific leucine-rich
glycoprotein
|
|
CD45
|
protein tyrosine phosphatase receptor
type C
|
|
CD325
|
neural cadherin
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katanoda K, Hori M, Matsuda T, Shibata A,
Nishino Y, Hattori M, Soda M, Ioka A, Sobue T and Nishimoto H: An
updated report on the trends in cancer incidence and mortality in
Japan, 1958–2013. Jpn J Clin Oncol. 45:390–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsuda T, Ajiki W, Marugame T, Ioka A,
Tsukuma H and Sobue T: Research group of population-based cancer
registries of Japan: Population-based survival of cancer patients
diagnosed between 1993 and 1999 in Japan: A chronological and
international comparative study. Jpn J Clin Oncol. 41:40–51. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mawby WJ, Holmes CH, Anstee DJ, Spring FA
and Tanner MJ: Isolation and characterization of CD47 glycoprotein:
A multispanning membrane protein which is the same as
integrin-associated protein (IAP) and the ovarian tumour marker
OA3. Biochem J. 304:525–530. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reinhold MI, Lindberg FP, Plas D, Reynolds
S, Peters MG and Brown EJ: In vivo expression of alternatively
spliced forms of integrin-associated protein (CD47). J cell Sci.
108:3419–3425. 1995.PubMed/NCBI
|
|
6
|
Oldenborg PA, Zheleznyak A, Fang YF,
Lagenaur CF, Gresham HD and Lindberg FP: Role of CD47 as a marker
of self on red blood cells. Science. 288:2051–2054. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jaiswal S, Jamieson CH, Pang WW, Park CY,
Chao MP, Majeti R, Traver D, van Rooijen N and Weissman IL: CD47 is
upregulated on circulating hematopoietic stem cells and leukemia
cells to avoid phagocytosis. Cell. 138:271–285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chao MP, Weissman IL and Majeti R: The
CD47-SIRPα pathway in cancer immune evasion and potential
therapeutic implications. Curr Opin Immunol. 24:225–232. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chan KS, Espinosa I, Chao M, Wong D,
Ailles L, Diehn M, Gill H, Presti J Jr, Chang HY, van de Rijn M, et
al: Identification, molecular characterization, clinical prognosis,
and therapeutic targeting of human bladder tumor-initiating cells.
Proc Natl Acad Sci USA. 106:pp. 14016–14021. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagahara M, Mimori K, Kataoka A, Ishii H,
Tanaka F, Nakagawa T, Sato T, Ono S, Sugihara K and Mori M:
Correlated expression of CD47 and SIRPA in bone marrow and in
peripheral blood predicts recurrence in breast cancer patients.
Clin Cancer Res. 16:4625–4635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu X, Pu Y, Cron K, Deng L, Kline J,
Frazier WA, Xu H, Peng H, Fu YX and Xu MM: CD47 blockade triggers T
cell-mediated destruction of immunogenic tumors. Nat Med.
21:1209–1215. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soto-Pantoja DR, Stein EV, Rogers NM,
Sharifi-Sanjani M, Isenberg JS and Roberts DD: Therapeutic
opportunities for targeting the ubiquitous cell surface receptor
CD47. Expert Opin Ther Targets. 17:89–103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric
Cancer. 20:1–19. 2017. View Article : Google Scholar
|
|
14
|
Chomczynski P: A reagent for the
single-step simultaneous isolation of RNA, DNA and proteins from
cell and tissue samples. Bio Techniques. 15:532–534, 536-537.
1993.
|
|
15
|
Inoue H, Mori M, Honda M, Li J, Shibuta K,
Mimori K, Ueo H and Akiyoshi T: The expression of tumor-rejection
antigen ‘MAGE’ genes in human gastric carcinoma. Gastroenterology.
109:1522–1525. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Majeti R, Chao MP, Alizadeh AA, Pang WW,
Jaiswal S, Gibbs KD Jr, van Rooijen N and Weissman IL: CD47 is an
adverse prognostic factor and therapeutic antibody target on human
acute myeloid leukemia stem cells. Cell. 138:286–299. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chao MP, Alizadeh AA, Tang C, Jan M,
Weissman-Tsukamoto R, Zhao F, Park CY, Weissman IL and Majeti R:
Therapeutic antibody targeting of CD47 eliminates human acute
lymphoblastic leukemia. Cancer Res. 71:1374–1384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoffmann W: Regeneration of the gastric
mucosa and its glands from stem cells. Curr Med Chem. 15:3133–3144.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoffmann W: Stem cells, self-renewal and
cancer of the gastric epithelium. Curr Med Chem. 19:5975–5983.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee ER and Leblond CP: Dynamic histology
of the antral epithelium in the mouse stomach: II. Ultrastructure
and renewal of isthmal cells. Am J Anat. 172:205–224. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimada H, Takiguchi N, Kainuma O, Soda H,
Ikeda A, Cho A, Miyazaki A, Gunji H, Yamamoto H and Nagata M: High
preoperative neutrophil-lymphocyte ratio predicts poor survival in
patients with gastric cancer. Gastric Cancer. 13:170–176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Balta S, Unlu M, Arslan Z and Demırkol S:
Neutrophil-to- lymphocyte ratio in prognosis of gastric cancer. J
Gastric Cancer. 13:196–197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho IR, Park JC, Park CH, Jo JH, Lee HJ,
Kim S, Shim CN, Lee H, Shin SK, Lee SK and Lee YC: Pre-treatment
neutrophil to lymphocyte ratio as a prognostic marker to predict
chemotherapeutic response and survival outcomes in metastatic
advanced gastric cancer. Gastric Cancer. 17:703–710. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Petrillo A, Laterza MM, Ventriglia J,
Savastano B, Tirino G, Pompella L, Martinelli E, Morgillo F,
Orditura M, Ciardiello F and De Vita F: Prognostic implications of
baseline neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte
ratio (PLR) in metastatic gastric cancer (GC) patients. Ann Oncol.
27:(Suppl 6): 652P2016. View Article : Google Scholar
|
|
26
|
Zhang X, Zhang W and Feng LJ: Prognostic
significance of neutrophil lymphocyte ratio in patients with
gastric cancer: A meta-analysis. PLoS One. 9:e1119062014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim
KH and Kim HJ: Prognostic significance of neutrophil lymphocyte
ratio and platelet lymphocyte ratio in advanced gastric cancer
patients treated with FOLFOX chemotherapy. BMC Cancer. 13:3502013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hirashima M, Higuchi S, Sakamoto K,
Nishiyama T and Okada H: The ratio of neutrophils to lymphocytes
and the phenotypes of neutrophils in patients with early gastric
cancer. J Cancer Res Clin Oncol. 124:329–334. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamanaka T, Matsumoto S, Teramukai S,
Ishiwata R, Nagai Y and Fukushima M: The baseline ratio of
neutrophils to lymphocytes is associated with patient prognosis in
advanced gastric cancer. Oncology. 73:215–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saito H, Takaya S, Osaki T and Ikeguchi M:
Increased apoptosis and elevated Fas expression in circulating
natural killer cells in gastric cancer patients. Gastric Cancer.
16:473–479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoshida K, Tsujimoto H, Matsumura K,
Kinoshita M, Takahata R, Matsumoto Y, Hiraki S, Ono S, Seki S,
Yamamoto J and Hase K: CD47 is an adverse prognostic factor and a
therapeutic target in gastric cancer. Cancer Med. 4:1322–1333.
2015. View
Article : Google Scholar : PubMed/NCBI
|