Introduction
Although a number of previous studies have attempted
to detect circulating tumor cells (CTCs), these have been unable to
identify a reliable target so far. The majority of these studies
have focused on surface antigens, including the epithelial cell
adhesion molecule (1) and cluster of
differentiation 45 (CD45) (2). The
majority of these methods have involved immunocytochemical
detection, but the main drawback of this is the inability to detect
cells that do not express these epithelial antigens (3). Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) protocols have been developed
to enhance sensitivity, but have demonstrated limited specificity
(4). If it were possible to target a
tumor cell-specific gene, a gene-specific PCR method may prove to
be the most sensitive and specific approach to detect CTCs.
The melanoma antigen-encoding gene (MAGE) family are
known to be cancer-specific genes, but are poorly expressed in
cancer cells (5,6). Common MAGE primers that can detect MAGE
family member A1 to MAGE family member A6 (MAGE A1-6) genes
simultaneously were developed and have been used in various types
of cancer (7–9). The present study investigated the
potential of using MAGE A1-6 primers to detect CTCs. The human
telomerase reverse transcriptase (hTERT) gene has also been studied
in multiple types of cancer cell (10–12).
However, because the hTERT gene is expressed in activated
lymphocytes (13), it was believed to
be of limited use in CTC detection.
The present study used the MAGE A1-6 and hTERT genes
individually and in combination to detect CTCs in blood, which had
mononuclear cells (MNCs) removed by CD45 antibody capture prior to
detection. The removal of MNCs should enhance CTC detection rates
(14) and reduce hTERT expression
levels in the blood samples. In addition, a number of RNA
extraction methods were compared in order to achieve amplification
of rare cancer cells in the blood. Finally, the clinical
sensitivity and specificity of this CTC detection system was
evaluated.
Materials and methods
Cancer cell lines
To determine the expression levels of MAGE and hTERT
genes in various cancer cells, 14 cancer cell lines were selected
as follows: Five gastric (NCI-N87, SNU1, SNU216, SNU484 and
SNU688), four colorectal (CRC1306, SNU1197, SNU1411 and SNU175),
one cervical (CaSki), one liver (SK-Hep1), one lung (A549), one
breast (MDA-MB-361) and one renal (Caki-1) cancer cell line. All
cells were provided by the Korean Cell Line Bank (Seoul,
Korea).
Comparison of RNA extraction methods
using SNU1 cells
To detect cancer cells that circulate in small
numbers in the blood, four commercially available RNA extraction
methods were compared: The NucleoSpin kit (Macherey-Nagel GmbH,
Düren, Germany), the RNeasy Mini kit (Qiagen AB, Sollentuna,
Sweden) and the TRIzol and TRIzol Plus kits (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). SNU1 cancer cells were
counted and serially diluted to produce various cell concentrations
(5,000, 500, 50 and 5 cells); these solutions were then lysed with
the specific cell lysis buffers included with each RNA extraction
kit and mixed with 50 ng duck RNA (kindly donated by the Department
of Immunology, School of Medicine, Keimyung University, Daegu,
Korea), which was used as a carrier RNA to enhance RNA extraction
efficiency. The prepared lysis solutions were then subjected to the
various RNA extraction methods, according to the manufacturer's
protocols, and the MAGE A1-6 and hTERT genes were amplified as
subsequently described to compare the RNA extraction efficiency of
each reagent.
Patients and blood sample
collection
To determine the expression levels of MAGE and hTERT
genes in the blood, 76 blood samples (3 ml) from patients without
malignant disease were used (age range, 57.8±15.4; male:female
ratio, 0.9:1), including 30 samples from healthy people obtained
via medical examination, 10 from patients with local inflammation,
9 with trauma, 5 with end-stage renal disease, 5 with central
nervous system (CNS) infarction, 4 with heart disease, 3 with CNS
hemorrhage, 3 with diabetes, 2 with hepatitis, 2 with
pyelonephritis, 2 with gastrointestinal disease and 1 with portal
vein thrombosis. Diagnoses of patients with non-malignant diseases
were confirmed after physical and medical examinations at the Daegu
Catholic University Hospital (Daegu, Republic of Korea) between
July 1, 2013 and June 30, 2014.
To detect CTCs, 107 blood samples (3 ml each) were
additionally collected from patients with malignant disease (age
range, 65.6±12.1; male:female ratio, 1.36:1) between July 1, 2013
and December 30, 2014, including 70 patients with colorectal
cancer, 11 with breast cancer, 10 with gastric cancer, 8 with liver
cancer, 4 with bile duct cancer, 2 with lung cancer and 2 with
pancreatic cancer at the Daegu Catholic University Hospital.
Diagnoses of malignant diseases were confirmed following a review
of patient medical records and pathology reports. The cancer stage
was classified according to the ‘T-stage’ system by the American
Joint Committee on Cancer, 7th edition (15).
Written informed consent was obtained from all
patients prior to enrollment in the present study. The study
protocols were approved by the Institutional Review Board of the
School of Medicine, Catholic University of Daegu.
All blood samples were immediately stored at 4°C and
RNA extraction was performed on the day of sample collection. The
blood samples were treated using the CD45 antibody capture system
as subsequently described and RNA was extracted from the eluted
cells using the RNeasy Mini method, according to the manufacturer's
protocol.
Mixing of SNU1 cells with blood and
CD45 antibody capture
Blood left over from samples that had been drawn for
routine clinical testing from patients with non-malignant disease
was used. These remnant blood samples were mixed into one tube, and
then divided into 2 ml aliquots. SNU1 cancer cells were counted and
serially diluted to produce various cell concentrations (5,000,
500, 50 and 5 cells), and these solutions were then added to the 2
ml aliquots of blood.
To remove the CD45-positive blood cells from the
blood samples, the red blood cells were lysed with red blood cell
lysis buffer (Roche Diagnostics GmbH, Mannheim, Germany). The MNCs
were suspended in 80 µl of CD45 binding buffer and reacted with 20
µl of microbeads conjugated to monoclonal antihuman CD45 antibodies
(cat. no., 130-045-801; Miltenyi Biotec GmbH, Bergisch Gladbach,
Germany) for 15 min at 4°C. The CD45+ cells were
captured using a magnetic separator, and CD45− cells
were eluted and collected. From the eluted cells, RNA was extracted
using the RNeasy Mini kit and TRIzol reagent, and the MAGE A1-6 and
hTERT genes were amplified as subsequently described to detect the
presence of SNU1 cells in the blood.
Gene amplification of MAGE A1-6, hTERT
and GAPDH via RT-qPCR
Extracted RNA from cancer cell lines and blood
samples was reverse transcribed using the ImProm-II Reverse
Transcription System (Promega Corporation, Madison, WI, USA)
according to the manufacturer's protocol. For reverse
transcription, the reaction mixture was incubated at 25°C for 10
min, 42°C for 60 min, at 70°C for 15 min and at 5°C for 5 min, then
stored at −80°C. The MAGE A1-6, hTERT and GAPDH genes were
amplified using the LightCycler FastStart DNA Master System (Roche
Diagnostics GmbH) according to the manufacturer's protocol, then
detected with a LightCycler 2.0 (Roche Diagnostics GmbH). The MAGE
A1-6 gene was amplified with nested PCR. The GAPDH gene was used as
a ‘housekeeping’ gene. Table I
contains a list of the primer sequences and thermocycler conditions
used.
 | Table I.Gene-specific primers used for the
polymerase chain reaction. |
Table I.
Gene-specific primers used for the
polymerase chain reaction.
| Gene | Sequences | Products (bp) | PCR steps |
|---|
| MAGE A1-6 | OF 5′-CTG AAG GAG AAG
ATC TGC C-3′ | 465 | 95°C 5 sec, 60°C 5
sec, |
|
| OR 5′-CCA GCA TTT CTG
CCT TTG TGA-3′ |
| 72°C 25 sec |
|
| IF 5′-AAG GAG AAG ATC
TGC CAG TG-3′ | 262 | 95°C 5 sec, 62°C 5
sec, |
|
| IR 5′-GAG GCT CCC TGA
GGA CTC T-3′ |
| 72°C 12 sec |
| hTERT | OF 5-CGG GCT GCT CCT
GCG TTT GGT G-3′ | 311 | 95°C 5 sec, 68°C 5
sec, |
|
| OR 5′-AGC CGC GGT TGA
AGG TGA GAC TGG-3′ |
| 72°C 16 sec |
| GAPDH | OF 5′-TCG GAG TCA ACG
GAT TTG GTC GTA-3′ | 320 | 95°C 5 sec, 59°C 5
sec, |
|
| OR 5′-CAA ATG AGC CCC
AGC CTT CTC CA-3′ |
| 72°C 18 sec |
Statistical analysis
The χ2 test was used to compare the
sensitivity of the four RNA extraction methods and the MAGE A1-6
and hTERT positive expression rates among the patient groups. A
paired t-test was used to compare MAGE A1-6 and hTERT expression
between disease stage T1/2 and T3/4. Statistical analyses were
performed using SPSS version 23.0 software (IBM SPSS, Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
MAGE A1-6, hTERT and GAPDH gene
expression levels in cancer cell lines
Analysis of 14 cancer cell lines revealed that the
levels of expression of the MAGE A1-6, hTERT and GAPDH genes were
85.7, 100 and 100%, respectively (Table
II). The MAGE A1-6 gene was not expressed in 2 of the 5 gastric
cancer cell lines (NCI-N87 and SNU688). The average cycle
thresholds of MAGE A1-6, hTERT and GAPDH were 30.1, 25.2 and 12.5,
respectively (Table II). In all cell
lines, the levels of hTERT expression were higher than MAGE A1-6
expression (Paired t-test, P<0.001).
 | Table II.Expression levels of MAGE A1-6, hTERT
and GAPDH mRNA in the 14 cancer cell lines. |
Table II.
Expression levels of MAGE A1-6, hTERT
and GAPDH mRNA in the 14 cancer cell lines.
| Parameter | MAGE A1-6 | hTERT | GAPDH | MAGE Cq | hTERT Cq | GAPDH Cq |
|---|
| Cell line
(origin) |
|
|
|
|
|
|
| NCI-N87
(gastric) | Neg | Pos | Pos | N/A | 26.7 | 11.0 |
| SNU1
(gastric) | Pos | Pos | Pos | 28.8 | 22.8 | 19.7 |
| SNU216
(gastric) | Pos | Pos | Pos | 28.7 | 24.5 | 11.7 |
| SNU484
(gastric) | Pos | Pos | Pos | 24.9 | 21.5 | 11.8 |
| SNU688
(gastric) | Neg | Pos | Pos | N/A | 25.1 | 11.5 |
| CRC1306
(colorectal) | Pos | Pos | Pos | 32.2 | 26.7 | 12.6 |
| SNU1197
(colorectal) | Pos | Pos | Pos | 32.8 | 29.6 | 12.7 |
| SNU1411
(colorectal) | Pos | Pos | Pos | 35.1 | 23.8 | 12.3 |
| SNU175
(colorectal) | Pos | Pos | Pos | 24.7 | 22 | 12.1 |
| CaSki
(cervical) | Pos | Pos | Pos | 31.2 | 25.7 | 10.9 |
| SK-Hep1
(liver) | Pos | Pos | Pos | 29.6 | 24.5 | 16.7 |
| A549
(lung) | Pos | Pos | Pos | 29.6 | 24.5 | 10.4 |
|
MDA-MB-361 (breast) | Pos | Pos | Pos | 32.8 | 29.9 | 11.0 |
| Caki
(renal) | Pos | Pos | Pos | 30.9 | 25.7 | 10.7 |
| % Positive | 85.7 | 100.0 | 100.0 |
|
|
|
| Mean Cq |
|
|
| 30.1 | 25.2 | 12.5 |
Sensitivity of the four RNA extraction
methods for the amplification of MAGE A1-6 and hTERT in SNU1
cells
The positive expression levels of MAGE A1-6 and
hTERT in SNU1 cells are summarized in Table III. In the samples containing 5 SNU1
cells, the results obtained using the TRIzol method were deemed the
most sensitive, with positive expression levels of 40% for MAGE
A1-6 and hTERT genes, although the difference between methods was
statistically insignificant. In the samples containing 50 SNU1
cells, the TRIzol and RNeasy Mini methods were the most sensitive
tests, with positive expression levels of 60%. On this basis, the
TRIzol and RNeasy Mini methods were selected as the RNA extraction
methods for the mixed blood samples.
 | Table III.SNU1 cancer cell detection rates
according to RNA extraction method (n=5). |
Table III.
SNU1 cancer cell detection rates
according to RNA extraction method (n=5).
|
| MAGE A1-6 (%) |
| hTERT (%) |
|
|---|
|
|
|
|
|
|
|---|
| No. SNU1 cells | Nucleo Spin | RNeasy Mini | TRIzol | TRIzol Plus | P-value | Nucleo Spin | RNeasy Mini | TRIzol | TRIzol Plus | P-value |
|---|
| 5,000 | 80 | 100 | 100 | 100 | 0.368 | 100 | 100 | 100 | 100 | 1.000 |
| 500 | 60 | 80 | 100 | 100 | 0.230 | 60 | 80 | 100 | 100 | 0.230 |
| 50 | 20 | 60 | 60 | 40 | 0.528 | 20 | 60 | 80 | 60 | 0.279 |
| 5 | 0 | 0 | 40 | 20 | 0.230 | 0 | 0 | 40 | 20 | 0.230 |
Sensitivity of the four RNA extraction
methods for the amplification of MAGE A1-6 and hTERT in blood mixed
with SNU1 cells
The positive expression levels of MAGE A1-6 and
hTERT in the blood mixed with SNU1 cells are summarized in Table IV. In the samples containing 5 SNU1
cells, the TRIzol and RNeasy Mini methods revealed similar positive
expression levels for both genes; although the differences were not
statistically significant, the results obtained using the RNeasy
Mini method exhibited consistent positive rates with zero standard
deviation. In the samples containing 50 SNU1 cells, the results of
the TRIzol and RNeasy Mini methods demonstrated the same positive
expression levels. On this basis, the RNeasy Mini method was
selected as the final RNA extraction method for CTC detection.
 | Table IV.Cancer cell detection rates in blood
mixed with SNU1 cells following cluster of differentiation 45
antibody capturing according to RNA extraction method (n=6). |
Table IV.
Cancer cell detection rates in blood
mixed with SNU1 cells following cluster of differentiation 45
antibody capturing according to RNA extraction method (n=6).
|
| MAGE A1-6 (%) |
| hTERT (%) |
|
|---|
|
|
|
|
|
|
|---|
| No. SNU1 cells | RNeasy Mini | TRIzol | P-value | RNeasy Mini | TRIzol | P-value |
|---|
| 5,000 | 100.0 | 100.0 | 1.000 | 100.0 | 100.0 | 1.000 |
| 500 | 66.7 | 66.7 | 1.000 | 83.3 | 83.3 | 1.000 |
| 50 | 50.0 | 50.0 | 1.000 | 50.0 | 50.0 | 1.000 |
| 5 | 33.3 | 16.7 | 0.605 | 33.3 | 50.0 | 0.334 |
Amplification of MAGE A1-6, hTERT, and
GAPDH genes in patients with non-malignant vs. malignant
diseases
As presented in Tables
V and VI, the positive
expression rates of MAGE A1-6, hTERT and MAGE A1-6+hTERT were 5.3,
6.6 and 11.8%, respectively, in the patients with non-malignant
diseases and 41.1, 41.1, and 58.9%, respectively, in the patients
with malignant diseases. GADPH was amplified in all cases. The
hTERT positive expression rates for bile duct, lung and pancreatic
cancer were 100%.
 | Table V.Positive expression levels of GAPDH,
MAGE and hTERT in the blood of patients with non-malignant
diseases. |
Table V.
Positive expression levels of GAPDH,
MAGE and hTERT in the blood of patients with non-malignant
diseases.
|
| No. positive
samples |
|---|
|
|
|
|---|
| Parameter | GAPDH | MAGE A1-6 | hTERT | MAGE + hTERT |
|---|
| Diagnosis (n) |
|
|
|
|
| Healthy
individuals (30) | 30 | 0 | 0 | 0 |
| Local
inflammation (10) | 10 | 1 | 0 | 1 |
| Trauma
(9) | 9 | 0 | 2 | 2 |
| End
stage renal disease (5) | 5 | 1 | 0 | 1 |
| CNS
infarction (5) | 5 | 1 | 0 | 1 |
| Heart
disease (4) | 4 | 1 | 0 | 1 |
| CNS
hemorrhage (3) | 3 | 0 | 0 | 0 |
|
Diabetes (3) | 3 | 0 | 1 | 1 |
|
Hepatitis (2) | 2 | 0 | 0 | 0 |
|
Pyelonephritis (2) | 2 | 0 | 1 | 1 |
|
Gastrointestinal diseases
(2) | 2 | 0 | 0 | 0 |
| Portal
vein thrombosis (1) | 1 | 0 | 1 | 1 |
| Total number | 76 | 4 | 5 | 9 |
| Positive rate
(%) |
100.0 |
5.3 |
6.6 |
11.8 |
 | Table VI.Positive expression levels of GAPDH,
MAGE and hTERT in the blood of patients with malignant
diseases. |
Table VI.
Positive expression levels of GAPDH,
MAGE and hTERT in the blood of patients with malignant
diseases.
|
|
|
| No. patients with
positive expression |
|---|
|
|
|
|
|
|---|
| Parameter | T Stage (n) | No. patients | GAPDH | MAGE | hTERT | MAGE + hTERT | Positive rate
(%) |
|---|
| Diagnosis |
|
|
|
|
|
|
|
| Colorectal
cancer | T1(8), T2(13),
T3(35), T4(14) | 70 | 70 | 23 | 21 | 36 | 51.4 |
| Breast
cancer | T1(6), T2(5) | 11 | 11 | 7 | 7 | 8 | 72.7 |
| Gastric
cancer | T1(2), T2(2),
T4(6) | 10 | 10 | 4 | 4 | 6 | 60.0 |
| Liver
cancer | T1(2), T2(4),
T3(2) | 8 | 8 | 5 | 4 | 5 | 62.5 |
| Bile
duct cancer | T2(1), T3(3) | 4 | 4 | 1 | 4 | 4 | 100.0 |
| Lung
cancer | T3(2) | 2 | 2 | 2 | 2 | 2 | 100.0 |
|
Pancreatic cancer | T3(1), T4(1) | 2 | 2 | 2 | 2 | 2 | 100.0 |
| Total number |
| 107 | 107 | 44 | 44 | 63 | 58.9 |
| Positive rate
(%) |
|
|
100.0 |
41.1 |
41.1 |
58.9 |
|
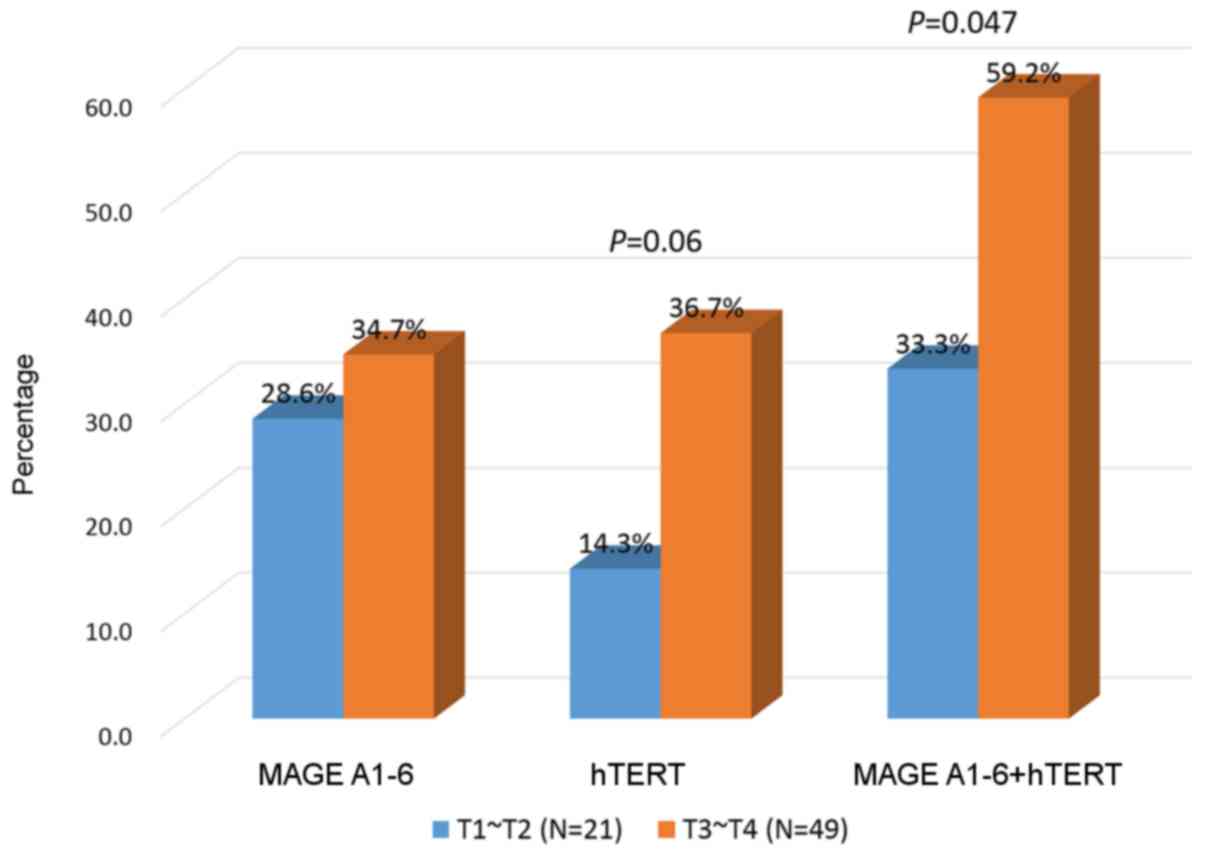
MAGE A1-6 or hTERT expression levels
are increased in the blood of patients with stage T3 or T4
colorectal cancer
The 70 patients with colorectal cancer were
classified by T stage. Though the rates of MAGE A1-6 combined with
hTERT expression were not significantly different, the positive
expression levels of MAGE A1-6 or hTERT were significantly higher
in patients with stage T3 or T4 compared with those in stage T1 or
T2 (33.3 vs. 59.2%; P<0.05; Fig.
1).
Discussion
Numerous genetic markers have been used to detect
CTCs by RT-qPCR, including cytokeratin 19, carcinoembryonic
antigen, hTERT, c-Met and survivin (10,11) and
positive expression levels varied from 9.6 (CK19, CK20) to 71.2%
(MUC1) in patients with gastric cancer, according to the genetic
markers used (16,17). microRNA has previously been used to
detect tumor cells induced by epithelial-to-mesenchymal transition
(18). Pearl et al (19) combined antigen and molecular markers
to improve CTC detection. A previous study captured tumor cells
using epithelial antigens and were able to detect tumor cells using
tumor progenitor genes; however, the result was negative rather
than positive for the enrichment of tumor cells because CD45
depletion of leukocytes induced significantly greater recovery of
spiked hepatocellular carcinoma cells (14).
The present study developed another type of CTC
detection system which utilized the known CD45 leukocyte depletion
system along with the novel genetic markers, MAGE A1-6 and hTERT.
hTERT mRNA has been used as a genetic marker for CTCs in previous
studies (10,11,20);
however, the majority of these studies used blood MNCs that
contained activated lymphocytes, and these may have induced
false-positive hTERT gene expression. In the CD45-depleted cells it
was possible to remove the activated lymphocytes, theoretically
reducing the rates of false-positive hTERT expression.
The MAGE gene family has previously been studied due
to its specific expression in cancer cells (21). Certain studies have used MAGE genes
for detecting CTCs (5,6). The present study hypothesized that with
CD45-depleted blood cells; a combination of hTERT and MAGE genes
would be the most effective markers for CTC detection. MAGE A1-6
and hTERT expression was detected in 12 and 14 cancer cell lines,
respectively; only two gastric cancer cell lines (NCI-N87 and
SNU688) did not express the MAGE gene. Therefore, it was possible
to use these genes as CTC markers.
When comparing RNA extraction efficiency, it was
revealed that the TRIzol and RNeasy Mini methods produced the most
sensitive results. In a study by Kim et al (22), NucleoSpin was identified to be the
most effective kit for obtaining high-quality RNA. These authors
compared yield and purity of RNA, RNA integrity and cycle threshold
values of housekeeping genes, but they did not compare the results
of rare gene amplification among normal blood cells (22). In the present study, NucleoSpin failed
to amplify the rare target RNA in the blood, whereas the TRIzol and
RNeasy Mini methods successfully amplified the MAGE A1-6 and hTERT
genes. The TRIzol reagent demonstrated the highest mean RNA yield
(22,23) of the RNA extraction kits investigated,
with good purity. Furthermore, to amplify rare genes, RNA yields
may be the most important factor.
The TRIzol and RNeasy Mini methods revealed similar
detection rates for the SNU1 cells in the blood; however, the
RNeasy Mini method was more reliable. The fact that the RNA
extraction procedure with TRIzol was susceptible to technical
variation may explain the variable detection rates for the rare
SNU1 cells.
Reported specificities for detection of the hTERT
gene in malignant disease have ranged from 67 to 100% (12,20,24,25),
but in the present study the specificity was 93%. Healthy
individuals and patients with benign disease were included in the
control group, whereas previous studies evaluated hTERT specificity
based on results in healthy volunteers (12,20,24). Even
though the rates of hTERT specificity did not differ greatly
between the present study and previous studies, the results of the
present study may be efficiently utilized in the clinic.
The sensitivity for the MAGE and hTERT combination
in the group with malignant diseases was 58.9%. The expression
level of MAGE was lower compared with that of hTERT in the cancer
cell lines, but the sensitivities for MAGE were similar to those
for hTERT as MAGE expression was amplified using nested PCR. The
reported sensitivities of RT-qPCR have varied, including 39 and 70%
in breast cancer (21,25), 82% in lung cancer (26), 59% in gastric cancer (27) and 25% in colorectal cancer (28). The majority of studies used 10 ml
blood and 3–8 genes as CTC markers, whereas the present study used
3 ml blood and 2 genes. With reduced blood volume and number of
genetic markers, the MAGE and hTERT combination should be a more
practical approach in the clinical laboratory.
Positive expression levels of the MAGE and hTERT
genes in the patients with colorectal cancer are summarized in
Fig. 1. Positive expression levels
reported using RT-qPCR in patients with colorectal cancer have
varied from 25 (28) to 87% (2); the positive expression level of 87% was
observed in a study in which blood was obtained from tumor drainage
veins from 23 patients, whereas the 25% positive rate was obtained
in a study of 735 blood samples from patients with colorectal
cancer. Thus, the result of 51.4% revealed in the present study was
satisfactory to detect CTCs in the patients with colorectal cancer;
however, the number of cases in the present study was not enough to
directly compare these results with those in a previous study by
Iinuma et al (28).
In conclusion, the methods used for blood
processing, RNA extraction, and MAGE A1-6 and hTERT reverse
transcription resulted in good rates of sensitivity and specificity
in the detection of CTCs. The MAGE A1-6 and hTERT genes may serve
as markers in this practical approach for CTC detection in the
clinical setting.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education, Science and Technology (grant
no. NRF-2013R1A1A2007189). The present study was also partially
supported by the Research Institute of Medical Science, Catholic
University of Daegu (Daegu, Korea; 2014).
References
|
1
|
Trzpis M, McLaughlin PM, de Leij LM and
Harmsen MC: Epithelial cell adhesion molecule: More than a
carcinoma marker and adhesion molecule. Am J Pathol. 171:386–395.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Linuma H, Okinaga K, Adachi M, Suda K,
Sekine T, Sakagawa K, Baba Y, Tamura J, Kumagai H and Ida A:
Detection of tumor cells in blood using CD45 magnetic cell
separation followed by nested mutant allele-specific amplification
of p53 and K-ras genes in patients with colorectal cancer. Int J
Cancer. 89:337–344. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balic M, Lin H, Williams A, Datar RH and
Cote RJ: Progress in circulating tumor cell capture and analysis:
Implications for cancer management. Expert Rev Mol Diagn.
12:303–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bostick PJ, Chatterjee S, Chi DD, Huynh
KT, Giuliano AE, Cote R and Hoon DS: Limitations of specific
reverse-transcriptase polymerase chain reaction markers in the
detection of metastases in the lymph nodes and blood of breast
cancer patients. J Clin Oncol. 16:2632–2640. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sang M, Wu X, Fan X, Sang M, Zhou X and
Zhou N: Multiple MAGE-A genes as surveillance marker for the
detection of circulating tumor cells in patients with ovarian
cancer. Biomarkers. 19:34–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Joosse SA, Müller V, Steinbach B, Pantel K
and Schwarzenbach H: Circulating cell-free cancer-testis MAGE-A RNA
BORIS RNA, let-7b and miR-202 in the blood of patients with breast
cancer and benign breast diseases. Br J Cancer. 26:909–917. 2014.
View Article : Google Scholar
|
|
7
|
Jheon S, Hyun DS, Lee SC, Yoon GS, Jeon
CH, Park JW, Park CK, Jung MH, Lee KD and Chang HK: Lung cancer
detection by a RT-nested PCR using MAGE A1-6 common primers. Lung
Cancer. 43:29–37. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ries J, Schultze-Mosgau S, Neukam F,
Diebel E and Wiltfang J: Investigation of the expression of
melanoma antigen-encoding genes (MAGE-A1 to -A6) in oral squamous
cell carcinomas to determine potential targets for gene-based
cancer immunotherapy. Int J Oncol. 26:817–824. 2005.PubMed/NCBI
|
|
9
|
Kwon S, Kang SH, Ro J, Jeon CH, Park JW
and Lee ES: The melanoma antigen gene as a surveillance marker for
the detection of circulating tumor cells in patients with breast
carcinoma. Cancer. 104:251–256. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu N, Zhou J, Cui F and Tang X:
Circulating tumor cells in lung cancer: Detection methods and
clinical applications. Lung. 193:157–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang S, Zheng G, Cheng B, Chen F, Wang Z,
Chen Y, Wang Y and Xiong B: Circulating tumor cells (CTCs) detected
by RT-PCR and its prognostic role in gastric cancer: A
meta-analysis of published literature. PLoS One. 9:e992592014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yadegarazari R, Hassanzadeh T, Majlesi A,
Keshvari A, Esfahani A Monsef, Tootoonchi A, Shabab N and Saidijam
M: Improved real-time RT-PCR assays of two colorectal cancer
peripheral blood mRNA biomarkers: A pilot study. Iran Biomed J.
17:15–21. 2013.PubMed/NCBI
|
|
13
|
Chebel A, Rouault JP, Urbanowicz I,
Baseggio L, Chien WW, Salles G and Ffrench M: Transcriptional
activation of hTERT, the human telomerase reverse transcriptase, by
nuclear factor of activated T cells. J Biol Chem. 284:35725–35734.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu HY, Qian HH, Zhang XF, Li J, Yang X,
Sun B, Ma JY, Chen L and Yin ZF: Improved method increases
sensitivity for circulating hepatocellular carcinoma cells. World J
Gastroenterol. 21:2918–2925. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th. Springer
Corp.; New York, NY: pp. 117–376. 2010
|
|
16
|
Majima T, Ichikura T, Takayama E, Chochi K
and Mochizuki H: Detecting circulating cancer cells using reverse
transcriptase-polymerase chain reaction for cytokeratin mRNA in
peripheral blood from patients with gastric cancer. Jpn J Clin
Oncol. 30:499–503. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uen YH, Lin SR, Wu CH, Hsieh JS, Lu CY, Yu
FJ, Huang TJ and Wang JY: Clinical significance of MUC1 and c-Met
RT-PCR detection of circulating tumor cells in patients with
gastric carcinoma. Clin Chim Acta. 367:55–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ortega FG, Lorente JA, Puche JL Garcia,
Ruiz MP, Sanchez-Martin RM, de Miguel-Pérez D, Diaz-Mochon JJ and
Serrano MJ: miRNA in situ hybridization in circulating tumor
cells-MishCTC. Sci Rep. 5:92072015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pearl ML, Dong H, Tulley S, Zhao Q,
Golightly M, Zucker S and Chen WT: Treatment monitoring of patients
with epithelial ovarian cancer using invasive circulating tumor
cells (iCTCs). Gynecol Oncol. 2:229–238. 2015. View Article : Google Scholar
|
|
20
|
Wang HY, Ahn S, Kim S, Park S, Jung D,
Park S, Han H, Sohn J, Kim S and Lee H: Detection of circulating
tumor cell-specific markers in breast cancer patients using the
quantitative RT-PCR assay. Int J Clin Oncol. 20:878–890. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Strati A, Kasimir-Bauer S, Markou A,
Parisi C and Lianidou ES: Comparison of three molecular assays for
the detection and molecular characterization of circulating tumor
cells in breast cancer. Breast Cancer Res. 15:R202013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim JH, Jin HO, Park JA, Chang YH, Hong YJ
and Lee JK: Comparison of three different kits for extraction of
high-quality RNA from frozen blood. Springerplus. 3:762014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang Z, Uboh CE, Chen J and Soma LR:
Isolation of RNA from equine peripheral blood cells: Comparison of
methods. Springerplus. 2:4782013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Man Y, Cao J, Jin S, Xu G, Pan B, Shang L,
Che D, Yu Q and Yu Y: Newly identified biomarkers for detecting
circulating tumor cells in lung adenocarcinoma. Tohoku J Exp Med.
234:29–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen C, Hu L, Xia L and Li Y: The
detection of circulating tumor cells of breast cancer patients by
using multimarker (Survivin, hTERT and hMAM) quantitative real-time
PCR. Clin Biochem. 42:194–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu Y, Xu G, Cao J, Jin S, Man Y and Shang
L: Combination of four gene markers to detect circulating tumor
cells in the peripheral blood of patients with advanced lung
adenocarcinoma using real-time PCR. Oncol Lett. 5:1400–1406.
2013.PubMed/NCBI
|
|
27
|
Kolostova K, Matkowski R, Gürlich R,
Grabowski K, Soter K, Lischke R, Schützner J and Bobek V: Detection
and cultivation of circulating tumor cells in gastric cancer.
Cytotechnology. 68:1095–1102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iinuma H, Watanabe T, Mimori K, Adachi M,
Hayashi N, Tamura J, Matsuda K, Fukushima R, Okinaga K, Sasako M
and Mori M: Clinical significance of circulating tumor cells,
including cancer stem-like cells, in peripheral blood of recurrence
and prognosis in patients with Dukes' stage B and C colorectal
cancer. J Clin Oncol. 29:1547–1555. 2011. View Article : Google Scholar : PubMed/NCBI
|