Introduction
Lung cancer has the highest mortality rate
worldwide. Although smoking has been established as the main cause
of lung cancer (1), smoking
prevalence has decreased on a global level (2). By contrast, the proportion of
non-smokers among lung cancer patients have been increasing through
the decades (3). Smoking-independent
lung cancer has been considered as a distinct disease from lung
cancer observed in smokers (4).
Therefore, it is important to understand the mechanisms behind the
carcinogenesis of patients with smoking-independent lung cancer in
order to treat lung cancer in the next generation.
Epidermal growth factor receptor (EGFR) gene
mutations are known as the main driver for oncogenic mutations in
smoking-independent lung cancer (5).
A total of >50% of lung cancer patients in Asian countries
harbor EGFR mutations (6), and these
patients benefit from treatment with EGFR tyrosine kinase
inhibitors (EGFR-TKIs).
The main EGFR mutations implicated in lung cancer
are deletions in exon 19 and a point mutation occurring at codon
858 (L858R) located at exon 21 (6).
These two mutations strongly predict the efficacy of EGFR tyrosine
kinase inhibitors (EGFR-TKIs), with response rates >70%
(5). The activating mutations of EGFR
lead to receptor dimerization and cause the activation of signaling
pathways. The main EGFR signaling pathways that mediate cancer
development and progression identified include the
phosphatidylinositol 3-kinase signaling pathway where activation
leads to Akt activation and suppression of apoptosis. Another
signaling pathway is via the proteins growth factor receptor-bound
protein 2 and Sos, which leads to the activation of
p21ras and cell cycle progression. Activation of
phospholipase C-γ1 phosphorylation leads to PIP2-induced actin
reorganization. EGFR-TKIs that target the receptor catalytic domain
of EGFR suppress the activation of signaling pathways caused by
EGFR dimerization (7). However, even
when other mutated oncogenic driver populations, including
activated anaplastic lymphoma kinase, are considered, the remaining
population without oncogenic driver mutations require optimal
therapy (5).
Several studies have focused on hormonal receptors
due to patients with smoking-independent lung cancers being mainly
female (3,8–21).
Estrogen exposure measured by an indirect method could only
demonstrate a weak association between female hormone-associated
factors and lung cancer (22).
Intratumoral estrogen expression has been demonstrated to increase
tumor proliferation (10) and worse
prognosis (8). However the
association between prognosis outcomes and expression of receptors
stimulated by increased intratumoral estrogen remain inconsistent
(11,14,21,23). The
present study investigates the expression of female
hormone-associated factors in attempt to elucidate this
epidemiological feature in the non-smoking population.
Materials and methods
Patients and specimens
Paraffin-embedded specimens and frozen specimens
were obtained from 38 patients who had never-smoked, who underwent
complete resection for primary lung cancer between January 2012 and
December 2013 at Nagoya City University Hospital (Nagoya, Japan).
Patient characteristics are summarized in Table I. Lung cancer staging was determined
according to the seventh edition of the TNM classification of the
lung and pleural tumors (24). The
pathological diagnosis was made according to the 2011 edition of
International Multidisciplinary Classification of Lung
Adenocarcinoma by the International Association for the Study of
Lung Cancer/American Thoracic Society/European Respiratory Society
(IASLC/ATS/ERS) (25). Pathological
invasiveness was determined according to the IASLC/ATS/ERS
definition. All frozen tumor samples were immediately frozen
subsequent to surgical resection and stored at −80°C until assayed.
The present study was approved by the ethics committee of Nagoya
City University Graduate School of Medicine (Nagoya, Japan).
Written consent was obtained from all patients prior to
surgery.
 | Table I.Clinicopathological characteristics
of 38 patients with lung adenocarcinoma with no smoking
history. |
Table I.
Clinicopathological characteristics
of 38 patients with lung adenocarcinoma with no smoking
history.
| Variable | Number |
|---|
| Age, years |
|
|
Range | 43–89 |
|
Average | 68.7±9.1 |
|
Median | 71 |
| Sex |
|
|
Male/Female | 7/31 |
| BMI |
|
|
Average | 23.1±2.8 |
| Invasiveness |
|
|
Non-invasive/Invasive | 17/21 |
| Grade |
|
|
G1/G2/G3 | 20/14/4 |
| pT |
|
|
T1/T2/T3/T4 | 25/10/2/1 |
| pN |
|
|
N0/N1/N2/Nx | 32/2/3/1 |
| pStage |
|
|
I/II/III | 31/2/5 |
| EGFR |
|
| Wild
type/Mutated | 23/15 |
| ALK |
|
|
Negative/Positive/N/A | 20/3/15 |
Immunohistochemistry (IHC)
Sections of 4 µm were sliced from paraffin blocks of
samples from the patients specified in Table I. The slides were treated twice with
xylene (Wako Pure Chemical Industries, Ltd., Osaka, Japan) for 10
min, and subsequently dehydrated twice in 100–70% ethanol (Wako
Pure Chemical Industries, Ltd.) for 5 min at each concentration,
respectively. Following thorough washing with running water,
antigen retrieval was performed using 10 mM sodium citrate buffer
adjusted to pH 6.0. Heating procedures for antigen retrieval and
blocking of the endogenous peroxidase differed according to the
antibodies. Paraffin slides for ERα and ERβ antibodies were
autoclaved for 15 min at 120°C and endogenous peroxidase was
blocked using methanol (Wako Pure Chemical Industries, Ltd.) with
0.3% H202 (Wako Pure Chemical Industries, Ltd.) for 30 min at room
temperature. Paraffin slides for aromatase (Cyp19) were heated by
microwave oven (500 W) for 10 min at 100°C, and endogenous
peroxidase was blocked with methanol (Wako Pure Chemical
Industries, Ltd.) with 3% H2O2 (Wako Pure Chemical Industries,
Ltd.). Non-specific antigens were blocked with Block Ace solution,
diluted according to manufacturer's instructions (DS Pharma
Biomedical Co., Ltd., Osaka, Japan) for 10 min at room temperature
with all slides. Primary antibodies used were as follows: Cyp19
(C16; dilution, 1:50; cat. no. sc14245; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA); ERα (HC-20; dilution, 1:50; cat. no.
sc-543; Santa Cruz Biotechnology, Inc.); and ERα (H-150; dilution,
1:10; cat. no. sc-8974; Santa Cruz Biotechnology, Inc.). All slides
were incubated with the primary antibodies overnight at 4°C.
EnVision kit (Dako; Agilent Technologies, Inc., Santa Clara, CA,
USA) and 3,3′-diaminobenzidine (DAB) substrate (Merck KGaA,
Darmstadt, Germany) were used to visualize ERα and β according to
the manufacture's instructions. Histofine kit (Nichirei
BioSciences, Inc., Tokyo, Japan) and DAB substrate (Merck KGaA)
were used to visualize Cyp19 according to the manufacture's
instructions. Slides were treated with DAB solution for 10 min at
room temperature and immediately washed under running water for 10
min. Chromogenic counterstains were performed using Mayer's
hematoxylin solution (Wako Pure Chemical Industries, Ltd.) for 30
sec and subsequently washed thoroughly under running water for 10
min. Following dehydration in 100% ethanol (Wako Pure Chemical
Industries, Ltd.) for 4 times, the coverslips were placed using
Malinol (MUTO PURE CHEMICALS Co., Ltd., Tokyo, Japan). Washing was
performed three times using phosphate-buffered saline between
procedures with a duration of 5 min for each step. Images were
captured using EVOS®XL (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with the objective scale set at ×40. Positive
staining of the tumor was determined as dense staining compared
with the stromal staining in >10% of the tumor cells.
Quantification was performed manually.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tumor tissues and
adjacent normal lung tissues using an ISOGEN RNA extracting kit
(Nippon Gene Co., Ltd., Tokyo, Japan) according to the
manufacturer's protocol. Complimentary (c)DNA was synthesized using
ReverTraAce qPCR RT MasterMix with gDNA remover (Toyobo Co., Ltd.,
Osaka, Japan) according to the manufacturer's protocol, and DNase
was used. RNA was denaturized by incubation at 65°C for 5 min.
DNase removal was performed at 37°C for 5 min. Reverse
transcription was performed by incubating at 37°C for 15 min, 50°C
for 5 min and 98°C for 5 min, respectively. A total of 50 ng RNA
was used for each RT-qPCR reaction. RT-qPCR amplification was
performed using the Applied Biosystems 7500 Fast Real-Time PCR
System (Thermo Fisher Scientific, Inc.). The thermocycling
conditions were set up as follows: denaturation for 20 sec at 95°C,
40 cycles of annealing and extension for 3 sec at 95°C and 30 sec
at 60°C. The Taqman® gene expression assay (Applied
Biosystems; Thermo Fisher Scientific, Inc.) for aromatase
(Hs00903413_m1), ERα exon 6 (Hs00174860_m1), ERα exon 7 (Custom
Taqman expressing assay AI70NU8; forward primer,
5′-GAGCTGGTTCACATGATCAACTG-3′, Reverse primer:
5′-AGAAGGTGGACCTGATCATGGA-3′; fluorescent probe,
5′-CAAAGCCTGGCACCCTC-3′) was used, and β-actin (Hs99999903-m1) was
used as an internal control. cDNA synthesized from MCF7 cell
culture mRNA were obtained from Dr. Tatsuya Toyama (Department of
Breast Surgery, Nagoya City University Graduate School of Medical
Sciences, Nagoya, Japan) and used as a reference for the
2−∆ΔCq method to evaluate the expression of each target
allele (26). A single replicate was
performed for each sample per probe due to the scarce resource of
the frozen specimens.
Statistical analysis
All statistical analysis was performed using JMP
statistical software ver.12.0.1 (SAS Institute, Inc., Cary, NC,
USA). The significance of extra-nuclear ERα staining was analyzed
using the χ2 test. Quantitative comparisons were
analyzed using the median test. The difference between normal
tissues and tumor tissues was analyzed using Wilcoxon's
matched-pair signed rank test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Extra-nuclear ERα staining is
associated with pathological invasiveness of tumors
IHC staining was performed for ERα, ERβ and
aromatase, which is the enzyme controlling the levels of
estradiol.
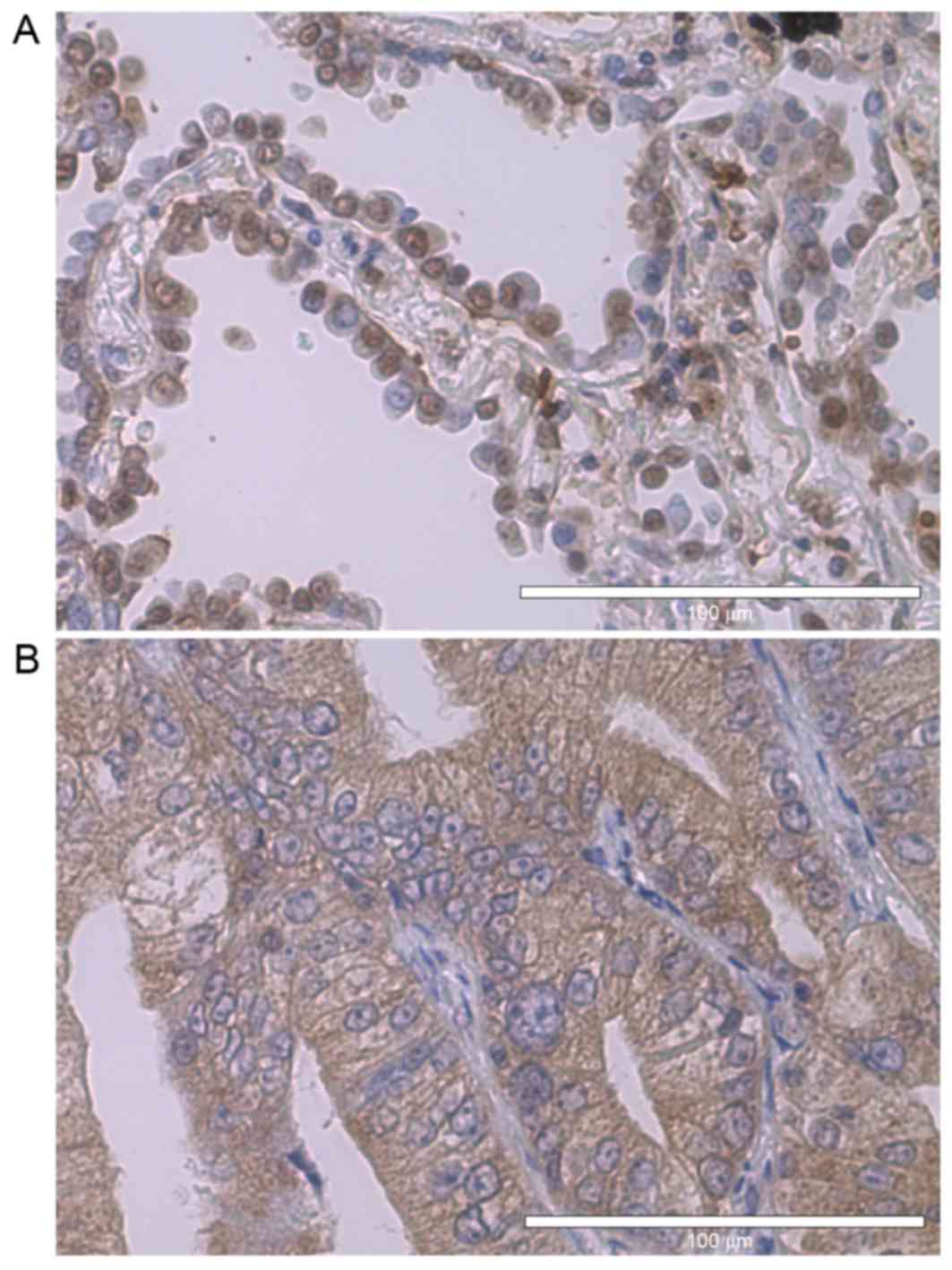
ERs are known as nuclear receptors (Fig. 1A), yet notably, extra-nuclear
expression of estrogen receptors was observed frequently (Fig. 1B). Taking this into consideration, the
nuclear expression and extra-nuclear expression of ERs were
analyzed individually. Cytosolic aromatase was expressed in 32%,
nuclear ERα in 21%, extra-nuclear ERα in 55%, nuclear ERβ in 92%
and extra-nuclear ERβ in 47% of the tumors. Sex and body-mass-index
exhibited no correlation with any of the female hormone-associated
factors.
Extra-nuclear ERα expression was significantly
associated with pathological invasiveness (P<0.001), lymph node
metastasis (P=0.04) and pathological stage (P=0.01) (Table II). Although it was also observed
that the positive expression of extra-nuclear ERβ was associated
with pathological invasiveness (P=0.05; data not shown), no
association between lymph node metastasis or pathological stage was
observed. Nuclear ERα expression, nuclear ERβ expression and
aromatase expression were not associated with any of the variables
considered. These IHC data led the present study to focus on the
extra-nuclear expression of ERα.
 | Table II.Clinicopathological
characteristics. |
Table II.
Clinicopathological
characteristics.
|
| Extra-nuclear ER-α
by IHC |
|
|---|
|
|
|
|
|---|
| Variable | Negative | Positive | χ2
(P-value) |
|---|
| Sex |
|
| 0.62 |
|
Male | 14 | 17 |
|
|
Female | 3 | 4 |
|
| BMI |
|
| 0.10 |
|
≤22.5 | 3 | 9 |
|
|
>22.5 | 14 | 12 |
|
| Invasiveness |
|
|
2.7×105 |
|
Non-invasive | 14 | 3 |
|
|
Invasive |
|
|
|
| pT | 3 | 18 | 0.06 |
| T1 | 15 | 10 |
|
| T2 | 2 | 8 |
|
| T3 | 0 | 2 |
|
| T4 | 0 | 1 |
|
| pN |
|
| 0.04 |
| N0 | 17 | 15 |
|
|
N1-2 | 0 | 5 |
|
| pStage |
|
| 0.01 |
| I | 17 | 14 |
|
|
II–III | 0 | 7 |
|
| EGFR |
|
| 0.25 |
|
Wild-type | 12 | 11 |
|
|
Mutated | 5 | 10 |
|
ERα exon 7 expression was lower in
more invasive tumors
A previous study indicated that ERα antibodies used
in the present study may recognize the spicing variant of exon 7 of
ERα (27). As a wide range of
splicing variants are known to be expressed in normal lung tissues
and lung cancer tissues (28), direct
sequencing was not adequate to assess the expression of a specific
splicing variant. Therefore, a RT-qPCR system was used in an
attempt to confirm the expression of this splicing variant.
Taqman probes for exon 6 and exon 7, each were
prepared. Of the 38 samples used in IHC, adequate RNA was retrieved
from 31, which were used in this experiment. The excluded RNA
samples from 7 patients exhibited too low a concentration to
assess. The 2−∆ΔCq method was used to assess the
expression of each exon, using β-actin as the housekeeping gene and
MCF7 cDNA as a reference sample.
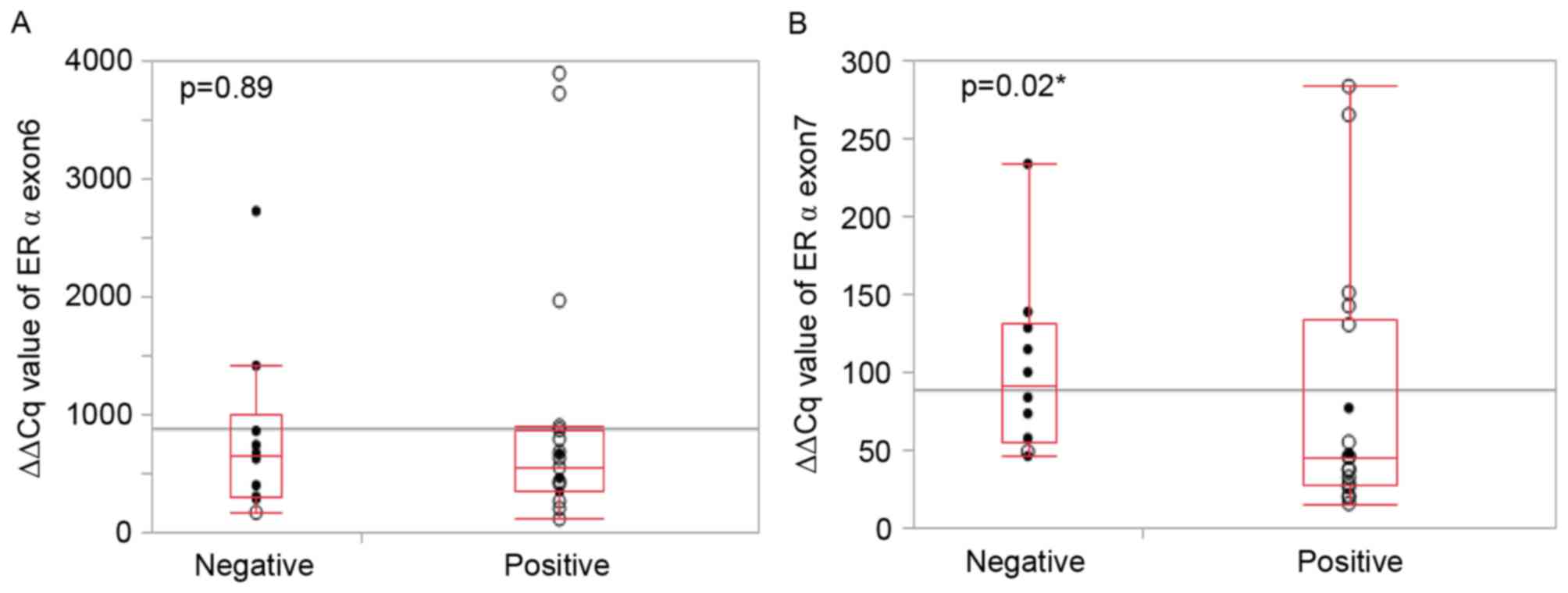
No statistically significant difference in the
expression of exon 6 between extra-nuclear ERα negative tumor
samples and extra-nuclear ERα positive samples was observed
(Fig. 2A). However, with expression
of exon 7, extra-nuclear ERα positive samples exhibited
significantly reduced expression levels compared with ERα negative
samples (P=0.02; Fig. 2B). Thus,
these data demonstrating a lower expression of exon 7 but not exon
6, which indicates the splicing of exon 7, is associated with
extra-nuclear ERα by IHC.
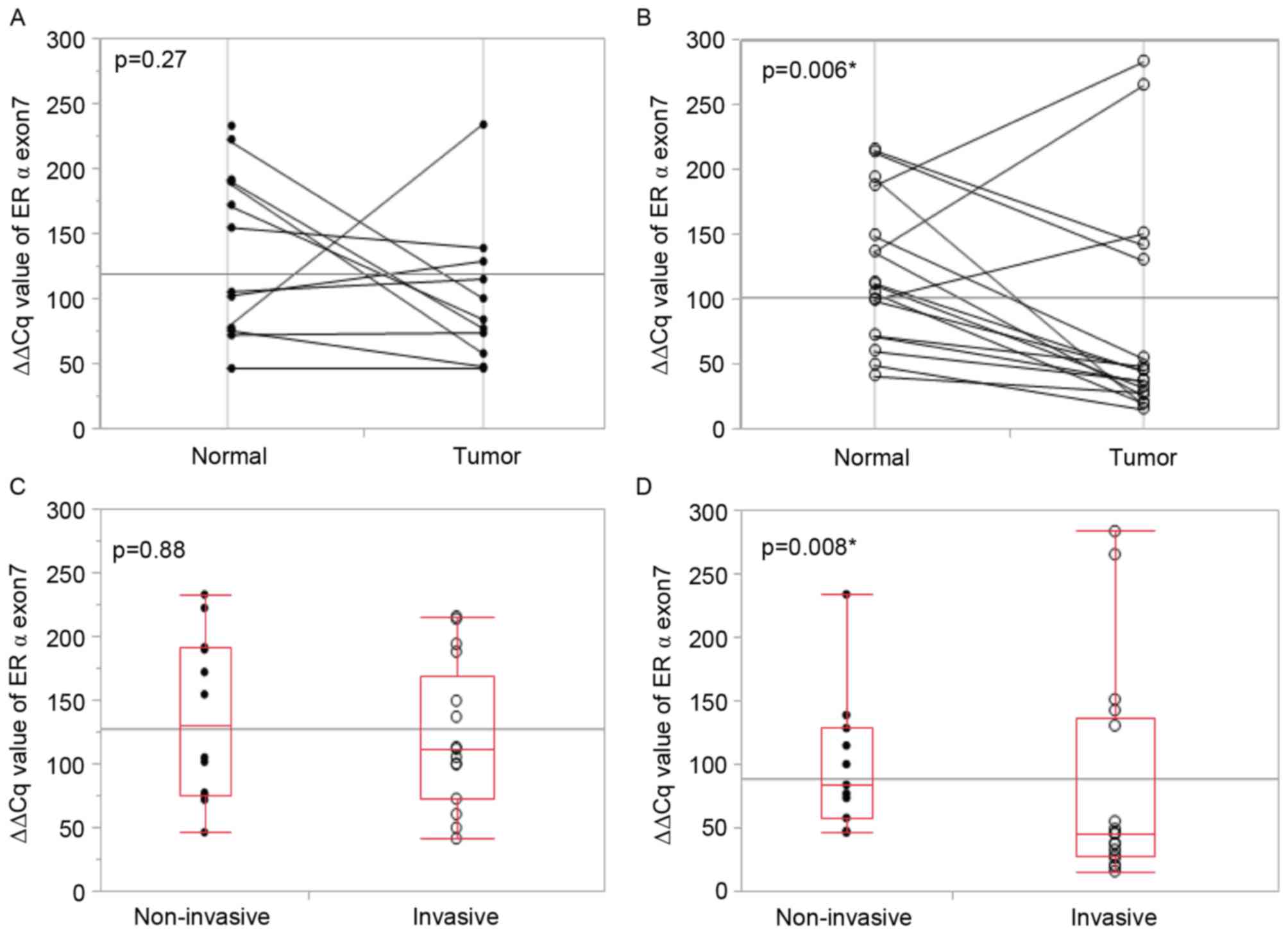
Extra-nuclear ERα by IHC revealed a significant
association with pathological invasiveness. Whether the lower
expression of exon 7 by RT-qPCR, which indicates the splicing of
exon 7, demonstrates an association with pathological invasiveness
was also investigated. In patients with non-invasive lung cancer,
the expression level of exon 7 did not differ between tumor tissues
and their adjacent normal tissues (Fig.
3A). However, in patients with invasive lung cancer, the exon 7
expression level was significantly lower in tumor tissues compared
with the adjacent normal tissue (Fig.
3B). The median expression level of exon 7 in normal tissues
did not differ between patients with non-invasive lung cancer and
patients with invasive lung cancer (Fig.
3C), whereas it was significantly reduced in tumor tissues from
patients with invasive lung cancer (Fig.
3D). The median expression of exon 6 did not differ between
patients with non-invasive lung cancer and patients with invasive
lung cancer in tumor tissues and their adjacent normal lung
tissues. These results indicate invasive lung cancer tumor tissues
are more likely to express ERα without exon 7 compared with
non-invasive tissues.
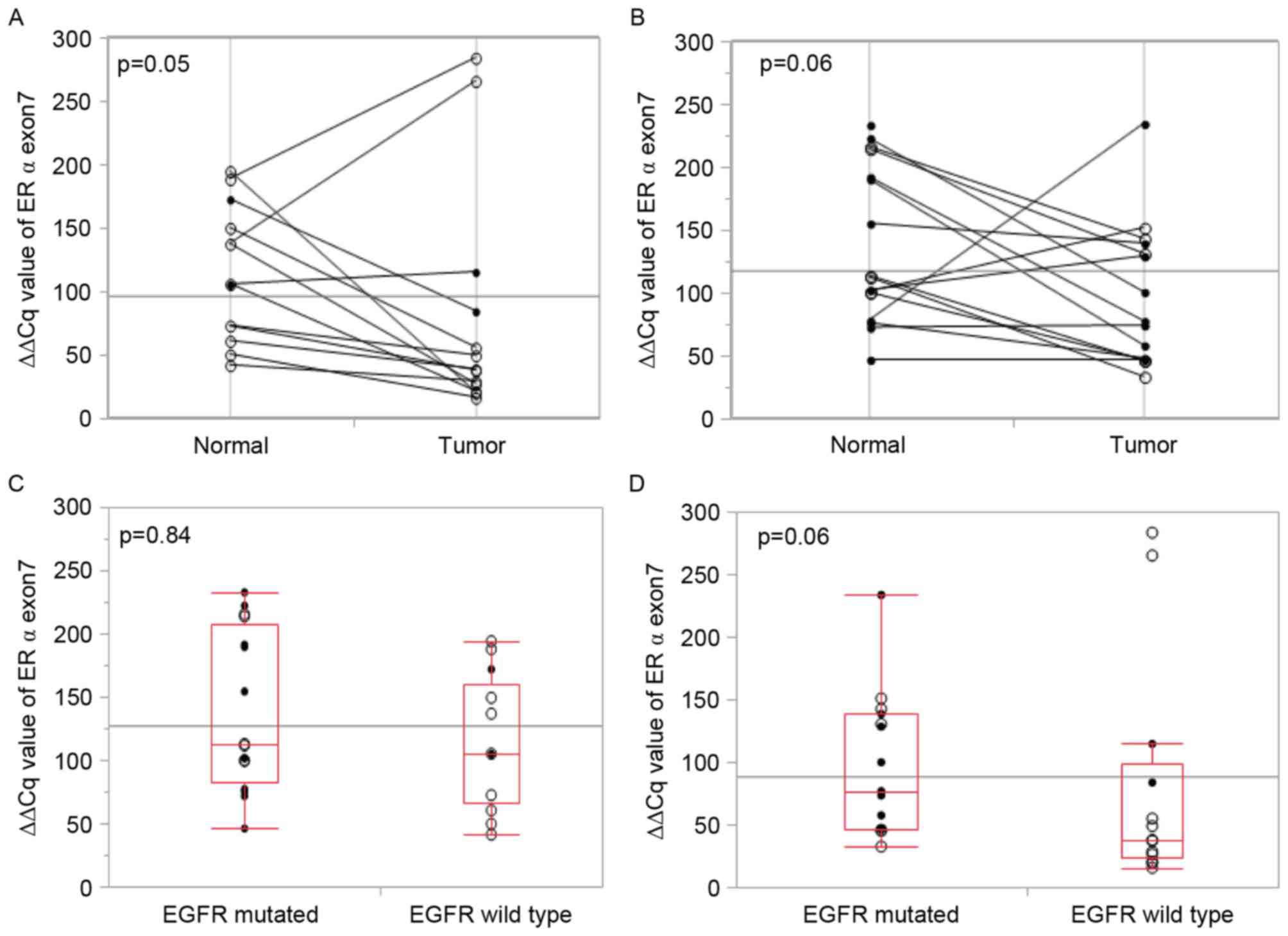
All tumor tissues from male patients demonstrated a
lower exon 7 expression level, yet they were all patients with
invasive lung cancer and therefore the present study is not able to
discuss whether this characteristic is due to sex or pathological
invasiveness. EGFR wild-type tumors tended to exhibit low
expression of exon 7, but this was not statistically significant
(Fig. 4A-D). The EGFR mutation status
demonstrated no association with the exon 7 expression level.
Discussion
The number of studies focusing on
smoking-independent lung cancer has increased since EGFR mutation
was identified as an oncogenic driver mutation. It is now common
knowledge that women are more likely to be affected by lung cancer
compared with men in the non-smoking population (5). This evidence has led the present study
to investigate the association between female hormone-associated
factors and lung cancer.
Although there is only weak evidence that estrogen
exposure to lung tissue induces lung cancer in clinical practice
(22), several studies have
demonstrated that intra-tumor aromatase expression (8,10,15,16,18,21)
exhibits an association with poorer prognoses. The present study
did not indicate statistically significant associations between
aromatase expression and pathological invasiveness. As the
antibodies mentioned in previous studies, which demonstrated an
association between aromatase expression and poorer prognosis by
IHC (15,21) were unavailable, human placenta tissues
were used as a positive control to test the antibodies used in the
present study. Sc14245 demonstrated good positive staining against
human placenta, however the positive detection rate, which was 32%,
was much lower compared with the previous studies (15,21). By
contrast, the study by Mah et al (16) used the same antibody as the present
study, and their positive detection rates for non-smoking women
with lung cancer was 42%, which was similar to the detection rate
of the present study. Therefore, the authors suggest that the
antibody selection for aromatase requires additional consideration.
The association between aromatase expression and smoking status
also requires additional investigation, which may be affecting this
discrepancy between studies.
There are a number of studies, which indicate that
ERα (9,11–13,23) and
ERβ expression (12,14–19,21) can be
used as markers to predict prognosis outcomes of lung cancer.
Antibody selection for IHC has been discussed for this difference.
The present study selected antibody (catalog no.) sc543 for the
detection of ERα and (catalog no.) sc8943 for the detection of ERβ
as these antibodies were used in several previous reports (11,12,19,29–33).
These two antibodies demonstrated good staining in human placenta
tissues. Positive detection rates for ERα have been reported in
previous studies (11,12,19,29–33).
In the present study, ERβ, particularly nuclear ERβ, revealed a
higher detection rate compared with previous studies (13,18,29).
Previous studies have reported an association between nuclear ERβ
expression and improved prognosis when all stages of lung cancer
were compared (13,18,29). This
discrepancy in ERβ data may be due to the population of the present
study, which consists mainly of patients with stage I lung
cancer.
The present study demonstrates that ERα is
associated with progressive pathological invasiveness, indicating
worse prognosis, compared with ERβ in lung cancer. This finding is
consistent with a number of previous reports (11,12,23).
Although the rate of ERα positive cases was within the range of
previous reports (11,12,19,29–33),
the present study demonstrated an improved association between ERα
and pathological invasiveness. The present study, which focused on
smoking-independent lung cancer, may contribute to the significance
of previous studies. However, the size of the present study was
smaller compared with previous studies investigating
hormone-associated factors. A study with a larger sample size is
required.
An in vitro experiment has demonstrated that
a number of extra-nuclear ERα stained against epitope HC-20 were
exon 7 splicing variants of ERα (27). As a wide range of alternative splicing
variants have been identified in lung cancer tissues (28), determining a specific splicing variant
from direct sequencing would have been quite challenging,
considering the limited amount of frozen specimens. The present
study attempted to confirm the splicing of ERα exon 7 by comparing
RT-qPCR results between exon 6 and exon 7. The data revealed that
lower expression levels of ERα exon 7 correlate with extra-nuclear
ERα expression and pathological invasiveness, indicating that exon
7 splicing variants of ERα perform a role in acquired invasiveness
in smoking-independent lung cancers.
Exon 7 splicing variants of ERα lack a part of the
ligand binding domain, indicating a dominant-negative phenotype
against estrogen signaling (27). A
previous study on endometrial cancer demonstrated an improved
prognosis with an increased expression of this splicing variant
(34). However, the findings of the
present study into lung cancer identified an association between
exon 7 splicing variants of ERα and a more invasive type of lung
cancer, which has potential for poorer prognoses. This discrepancy
in findings may be due to the difference in environmental estrogen
levels between the normal lung and uterus. The expression of
splicing variants differs between tissues (35), indicating that splicing variants
perform different roles depending on the tissue environment. Whole
length ERα are known to move dynamically from the membrane to the
nucleus, and to the membrane again. The reason why exon 7 splicing
variants of ERα appear to accumulate in the extra-nuclear space in
lung cancer tissues requires additional investigation.
At present, sex is hypothesized to be the sole risk
factor for EGFR mutation (6), which
therefore implies an association between EGFR mutation and hormonal
factors. The conclusions of whether there is a direct association
between these 2 pathways have not yet been determined (9,12,14,19,28). EGFR
and ER are known to interact downstream of the proliferating
cascade. A study by Garon et al (9) using human NSCLC xenografts demonstrated
that an anti-estrogen drug promoted the anti-proliferative effects
of an EGFR-TKI, which indicates that the ER signaling pathway is
able to direct interact with the EGFR signaling pathway. The data
of the present study demonstrated that patients with EGFR wild-type
lung cancer are likely to express ERα with lower exon 7 expression,
while patients with EGFR mutated lung cancer possessed a wide range
of ERα exon 7 expression levels. The association between EGFR
mutation status and lower ERα exon 7 expression demonstrates
possible interaction between these 2 pathways.
The mechanism underlying the decrease of ERα exon 7
may well be involved in the acquired invasiveness of lung cancer,
particularly with EGFR wild-type lung cancer. Whether the decrease
in the expression of ERα exon 7 is the trigger, or a different
trigger is inducing the splicing requires additional study. The
involvement of the splicing variants accumulating in the
extra-nuclear is another area which requires additional
investigation.
The present study suggests an association between
the expression of an exon 7 splicing variant of ERα and
pathological invasiveness in lung cancer tissues. It was also
indicated that a lower expression of ERα exon 7 may be associated
with EGFR wild-type lung cancer tissues compared with EGFR mutated
lung cancer tissues. The post-translational splicing mechanism of
ERα may be involved in the acquired invasiveness of
smoking-independent lung cancer. Additional investigation with a
larger sample, and in vitro experiments, are required.
Acknowledgements
The authors would like to thank Emeritus Professor
Yoshitaka Fujii, Department of Oncology, Immunology and Surgery,
Nagoya City University Graduate School of Medical Sciences for
helpful advice and discussions regarding the manuscript. The
abstract was presented at the IASLC 17th World Conference on Lung
Cancer 4 December 2016-7 December 2016 in Vienna, Austria and
published as abstract no. P3 01-044 in the Journal of Thoracic
Oncology Vol 12: no. 1S, 2017.
References
|
1
|
World Cancer Reports 2014. http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014September
27–2016
|
|
2
|
U.S. National Cancer Institute and World
Health Organization, . The Economics of Tobacco and Tobacco
Control. National Cancer Institute Tobacco Control Monograph 21.
NIH Publication No. 16-CA-8029A. U.S. Department of Health and
Human Services, National Institutes of Health, National Cancer
Institute. Bethesda, MD: World Health Organization, Geneva;
2016
|
|
3
|
Yano T, Miura N, Takenaka T, Haro A,
Okazaki H, Ohba T, Kouso H, Kometani T, Shoji F and Maehara Y:
Never-smoking nonsmall cell lung cancer as a separate entity:
Clinicopathologic features and survival. Cancer. 113:1012–1018.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Subramanian J and Govindan R: Lung cancer
in never smokers: A review. J Clin Oncol. 25:561–570. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pao W and Girard N: New driver mutations
in non-small-cell lung cancer. Lancet Oncol. 12:175–180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mitsudomi T: Molecular epidemiology of
lung cancer and geographic variations with special reference to
EGFR mutations. Transl Lung Cancer Res. 3:205–211. 2014.PubMed/NCBI
|
|
7
|
Brandt B, Meyer-Staeckling S, Schmidt H,
Agelopoulos K and Buerger H: Mechanisms of egfr gene transcription
modulation: Relationship to cancer risk and therapy response. Clin
Cancer Res. 12:7252–7260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kohno M, Okamoto T, Suda K, Shimokawa M,
Kitahara H, Shimamatsu S, Konishi H, Yoshida T, Takenoyama M, Yano
T and Maehara Y: Prognostic and therapeutic implications of
aromatase expression in lung adenocarcinomas with EGFR mutations.
Clin Cancer Res. 20:3613–3622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garon EB, Pietras RJ, Finn RS, Kamranpour
N, Pitts S, Márquez-Garbán DC, Desai AJ, Dering J, Hosmer W, von
Euw EM, et al: Antiestrogen fulvestrant enhances the
antiproliferative effects of epidermal growth factor receptor
inhibitors in human non-small-cell lung cancer. J Thorac Oncol.
8:270–278. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niikawa H, Suzuki T, Miki Y, Suzuki S,
Nagasaki S, Akahira J, Honma S, Evans DB, Hayashi S, Kondo T and
Sasano H: Intratumoral estrogens and estrogen receptors in human
non-small cell lung carcinoma. Clin Cancer Res. 14:4417–4426. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimizu K, Hirami Y, Saisho S, Yukawa T,
Maeda A, Yasuda K and Nakata M: Membrane-bound estrogen receptor-α
expression and epidermal growth factor receptor mutation are
associated with a poor prognosis in lung adenocarcinoma patients.
World J Surg Oncol. 10:1412012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raso MG, Behrens C, Herynk MH, Liu S,
Prudkin L, Ozburn NC, Woods DM, Tang X, Mehran RJ, Moran C, et al:
Immunohistochemical expression of estrogen and progesterone
receptors identifies a subset of NSCLCs and correlates with EGFR
mutation. Clin Cancer Res. 15:5359–5368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rouquette I, Lauwers-Cances V, Allera C,
Brouchet L, Milia J, Nicaise Y, Laurent J, Delisle MB, Favre G,
Didier A and Mazières J: Characteristics of lung cancer in women:
Importance of hormonal and growth factors. Lung Cancer. 76:280–285.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Z, Li Z, Ding X, Shen Z, Liu Z, An T,
Duan J, Zhong J, Wu M, Zhao J, et al: ERbeta localization
influenced outcomes of EGFR-TKI treatment in NSCLC patients with
EGFR mutations. Sci Rep. 5:113922015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Verma MK, Miki Y, Abe K, Nagasaki S,
Niikawa H, Suzuki S, Kondo T and Sasano H: Co-expression of
estrogen receptor beta and aromatase in Japanese lung cancer
patients: Gender-dependent clinical outcome. Life Sci. 91:800–808.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mah V, Marquez D, Alavi M, Maresh EL,
Zhang L, Yoon N, Horvath S, Bagryanova L, Fishbein MC, Chia D, et
al: Expression levels of estrogen receptor beta in conjunction with
aromatase predict survival in non-small cell lung cancer. Lung
Cancer. 74:318–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nose N, Uramoto H, Iwata T, Hanagiri T and
Yasumoto K: Expression of estrogen receptor beta predicts a
clinical response and longer progression-free survival after
treatment with EGFR-TKI for adenocarcinoma of the lung. Lung
Cancer. 71:350–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abe K, Miki Y, Ono K, Mori M, Kakinuma H,
Kou Y, Kudo N, Koguchi M, Niikawa H, Suzuki S, et al: Highly
concordant coexpression of aromatase and estrogen receptor beta in
non-small cell lung cancer. Hum Pathol. 41:190–198. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nose N, Sugio K, Oyama T, Nozoe T, Uramoto
H, Iwata T, Onitsuka T and Yasumoto K: Association between estrogen
receptor-beta expression and epidermal growth factor receptor
mutation in the postoperative prognosis of adenocarcinoma of the
lung. J Clin Oncol. 27:411–417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Omoto Y, Kobayashi Y, Nishida K, Tsuchiya
E, Eguchi H, Nakagawa K, Ishikawa Y, Yamori T, Iwase H, Fujii Y, et
al: Expression, function, and clinical implications of the estrogen
receptor beta in human lung cancers. Biochem Biophys Res Commun.
285:340–347. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanaka K, Shimizu K, Kakegawa S, Ohtaki Y,
Nagashima T, Kaira K, Horiguchi J, Oyama T and Takeyoshi I:
Prognostic significance of aromatase and estrogen receptor beta
expression in EGFR wild-type lung adenocarcinoma. Am J Transl Res.
8:81–97. 2016.PubMed/NCBI
|
|
22
|
Schwartz AG, Ray RM, Cote ML, Abrams J,
Sokol RJ, Hendrix SL, Chen C, Chlebowski RT, Hubbell FA, Kooperberg
C, et al: Hormone use, reproductive history, and risk of lung
cancer: The women's health initiative studies. J Thorac Oncol.
10:1004–1013. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mazieres J, Rouquette I, Lepage B, Milia
J, Brouchet L, Guibert N, Beau-Faller M, Validire P, Hofman P and
Fouret P: Specificities of lung adenocarcinoma in women who have
never smoked. J Thorac Oncol. 8:923–929. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
International Union Against Cancer (UICC),
. TNM Classification of Malignant Tumours. Sobin LH, Gospodarowicz
MK and Wittekind C: 7th. Wiley-Blackwell; Oxford: 2009
|
|
25
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
van Schil PE, et al: International association for the study of
lung cancer/american thoracic society/european respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ivanova MM, Mazhawidza W, Dougherty SM and
Klinge CM: Sex differences in estrogen receptor subcellular
location and activity in lung adenocarcinoma cells. Am J Respir
Cell Mol Biol. 42:320–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fasco MJ, Hurteau GJ and Spivack SD:
Gender-dependent expression of alpha and beta estrogen receptors in
human nontumor and tumor lung tissue. Mol Cell Endocrinol.
188:125–140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kawai H, Ishii A, Washiya K, Konno T, Kon
H, Yamaya C, Ono I, Minamiya Y and Ogawa J: Estrogen receptor alpha
and beta are prognostic factors in non-small cell lung cancer. Clin
Cancer Res. 11:5084–5089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schwartz AG, Prysak GM, Murphy V, Lonardo
F, Pass H, Schwartz J and Brooks S: Nuclear estrogen receptor beta
in lung cancer: Expression and survival differences by sex. Clin
Cancer Res. 11:7280–7287. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Márquez-Garbán DC, Chen HW, Fishbein MC,
Goodglick L and Pietras RJ: Estrogen receptor signaling pathways in
human non-small cell lung cancer. Steroids. 72:135–143. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stabile LP, Dacic S, Land SR, Lenzner DE,
Dhir R, Acquafondata M, Landreneau RJ, Grandis JR and Siegfried JM:
Combined analysis of estrogen receptor beta-1 and progesterone
receptor expression identifies lung cancer patients with poor
outcome. Clin Cancer Res. 17:154–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun HB, Zheng Y, Ou W, Fang Q, Li P, Ye X,
Zhang BB, Yang H and Wang SY: Association between hormone receptor
expression and epidermal growth factor receptor mutation in
patients operated on for non-small cell lung cancer. Ann Thorac
Surg. 91:1562–1567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hirschfeld M, Ouyang YQ, Jaeger M, Erbes
T, Orlowska-Volk M, Zur Hausen A and Stickeler E: HNRNP G and
HTRA2-BETA1 regulate estrogen receptor alpha expression with
potential impact on endometrial cancer. BMC Cancer. 15:862015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen M and Manley JL: Mechanisms of
alternative splicing regulation: Insights from molecular and
genomics approaches. Nat Rev Mol Cell Biol. 10:741–754.
2009.PubMed/NCBI
|