Introduction
Partial nephrectomy (PN) has been a standard
treatment for small renal cell carcinoma (RCC) (1–3). The loss
of renal function is decreased following PN compared with that
following radical nephrectomy (RN), and oncological management is
reportedly equivalent between PN and RN (1,4). Although
patients following RN are not likely to exhibit severe renal
dysfunction requiring hemodialysis (5,6), 65% of
patients develop grade 3 chronic kidney disease, defined as an
estimated glomerular filtration rate (eGFR) <60 ml/min/1.73
m3, 3 years after nephrectomy (7) and the decrease in eGFR may lead to an
increased risk of cardiovascular-related death in the future
(8,9).
Therefore, the preservation of renal function as well as cancer
management should be considered in the treatment of RCC. Although
PN appeared to be the best treatment for patients with small RCC in
good general condition (10),
patients with RCC are typically elderly and exhibit comorbidities
that increase operative risks (11).
In particular, for patients of advanced age (>80 years) and
those with high-risk comorbidities, including cardiovascular and
severe pulmonary diseases, major operations requiring general
anesthesia should be avoided. Furthermore, for patients with
hereditary RCCs, including RCCs due to von Hippel-Lindau (VHL) or
Birt-Hogg-Dubé diseases, renal function gradually decreases if the
patients undergo repeated partial resection (12,13).
Furthermore, the difficulty of a surgical procedure increases
following multiple surgeries (12).
For small RCCs in high-risk patients or patients with hereditary
diseases, less invasive treatments with good cancer management and
minimal loss of renal function are ideal.
The efficacies of ablative therapies, including
radiofrequency (RF) ablation (RFA) and cryoablation, have been
reported previously: A number of authors have reported long-term
results following RFA for RCC and demonstrated excellent
oncological outcomes (14–20). In our institute, percutaneous RFA for
renal cancer was initiated in 2005. In the present study, patients
undergoing percutaneous RFA for renal cancer were evaluated with
respect to oncological management, invasiveness and renal
function.
Patients and methods
Patients
Between December 2005 and March 2015, percutaneous
RFA for renal tumors had been performed in 40 patients (30 male; 10
female) at the National Defense Medical College (Tokorozawa,
Japan). Written informed consent was obtained from all patients who
participated in the present study. In the 40 patients (41 tumors),
a total of 50 sessions of RFA were performed. The mean age was 69.7
years (range, between 23 and 88 years; median, 73 years). The mean
follow-up was 38 months (range, between 5.8 and 89.3 months). RFA
was indicated only for tumor 1 (T1) stage renal cancer. A total of
39 tumors were T1a (≤4 cm) and 2 tumors were T1b (4.6 and 4.7 cm).
For patients in good general condition for whom the use of general
anesthesia was possible, surgical resection was recommended. RFA
was indicated mainly for patients with high-risk comorbidities,
patients of advanced age and patients with hereditary RCC. Although
surgical resection was recommended even for patients with a
solitary kidney or renal dysfunction, RFA was indicated for certain
patients according to the wishes of the patient. The reasons for
indicating RFA are presented in Table
I. The mean diameter of ablated tumors was 2.5 cm (range, 1–4.7
cm; median, 2.4 cm). A total of 18 tumors were exophytic and 23
tumors were parenchymal. A total of 31 tumors had a Radius (tumor
size as maximal diameter), Exophytic/endophytic properties of the
tumor, Nearness of tumor deepest portion to the collecting system
or sinus, Anterior (a)/posterior (p) descriptor and the Location
relative to the polar line (R.E.N.A.L.) nephrometry score (21), of ≤7, and 10 tumors were scored as ≥8.
A total of 16 tumors were located anteriorly and 25 were located
posteriorly. The present study was approved by the Institutional
Review Board of the National Medical Defense College, Tokorozawa,
Japan (no. 549).
 | Table I.Reasons for selecting RFA. |
Table I.
Reasons for selecting RFA.
| Reason | Number of patients
(n=29) |
|---|
|
Comorbidities+advanced age | 8 |
| Comorbidities | 5 |
| Advanced age | 4 |
| Solitary
kidney | 3 |
| Severe cirrhosis of
the liver | 3 |
| Comorbidities+renal
dysfunction | 2 |
| VHL disease | 2 |
| Wishes of the
patienta | 2 |
RFA procedure
Percutaneous RFA was performed using an internally
cooled electrode (Cool-tip™ RF electrode; Radionics, Burlington,
MA, USA) or a multitined expandable electrode (LeVeen™ needle
electrode with an RF 3000 generator; Boston Scientific, Boston, MA,
USA). The type of electrode used was determined mainly by tumor
location, size, shape and the physician's preference. For the
Cool-tip™ electrode, RF energy was applied for 12 min under an
impedance control algorithm. For the LeVeen™ electrode, the tines
were expanded step-by-step in four steps, and RF energy was applied
at each step until a drastic increase in impedance (roll-off) was
achieved. Lidocaine (Xylocaine®; AstraZeneca plc.,
London, UK) was used for local anesthesia and fentanyl citrate
(0.1–0.2 mg; Daiichi Sankyo Co., Ltd., Tokyo, Japan) was used for
analgesia. For the majority of patients, the prone position was
used during RFA. All ablations were guided and monitored using
ultrasound (US; EUB7500; Hitachi Medical Systems, Tokyo, Japan) and
computed tomography (CT; Aquillion; Toshiba Medical Systems,
Tochigi-ken, Japan). On the basis of the size and shape,
overlapping ablations were applied by repositioning the electrode
to ablate the entire tumor. In certain cases, hydrodissection was
used to prevent thermal injury of neighboring organs by displacing
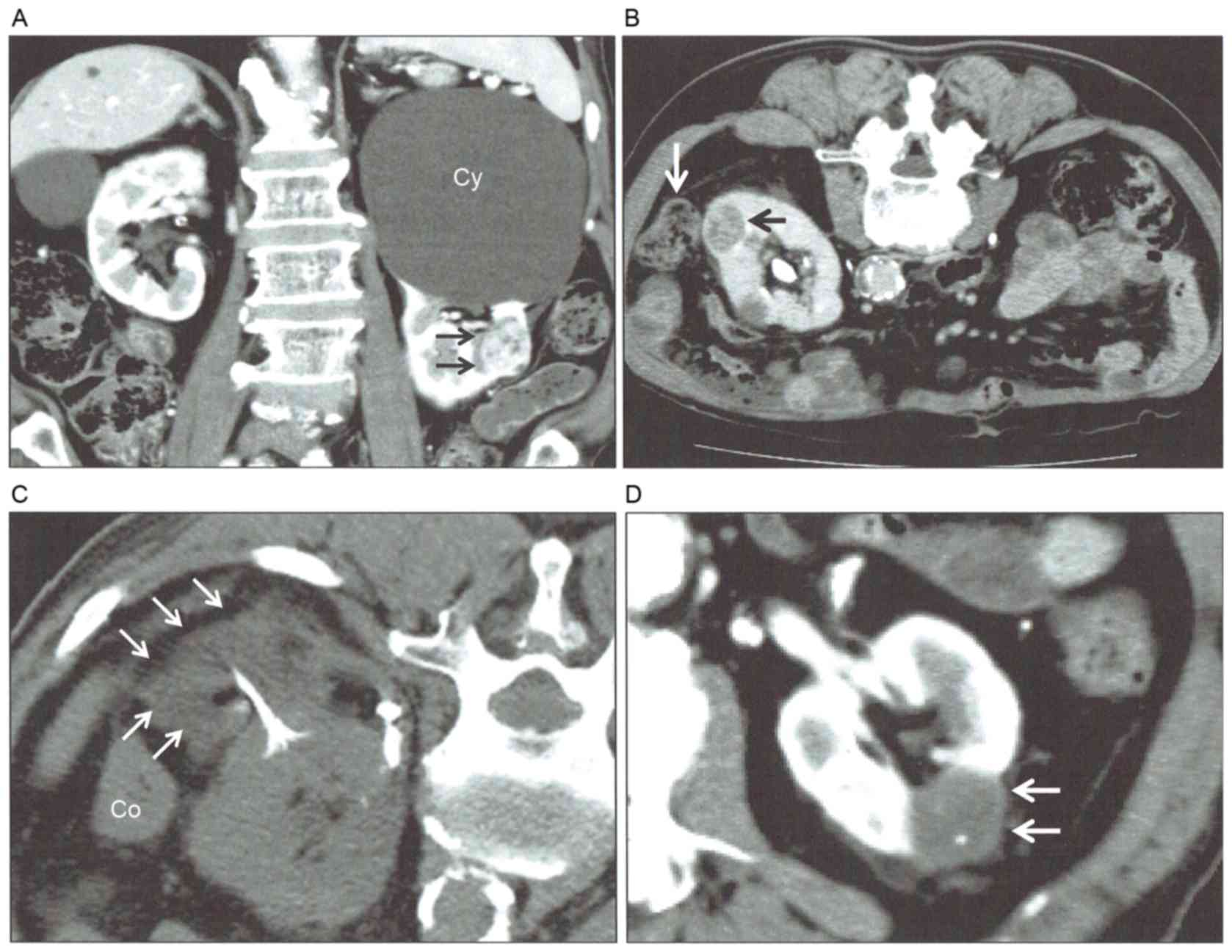
the tumor away from adjacent structures (Fig. 1). A maximum of 1,000 ml 5% dextrose
was infused into the space between the tumor and tissue to be
protected through a 19-guage needle placed under US or CT guidance.
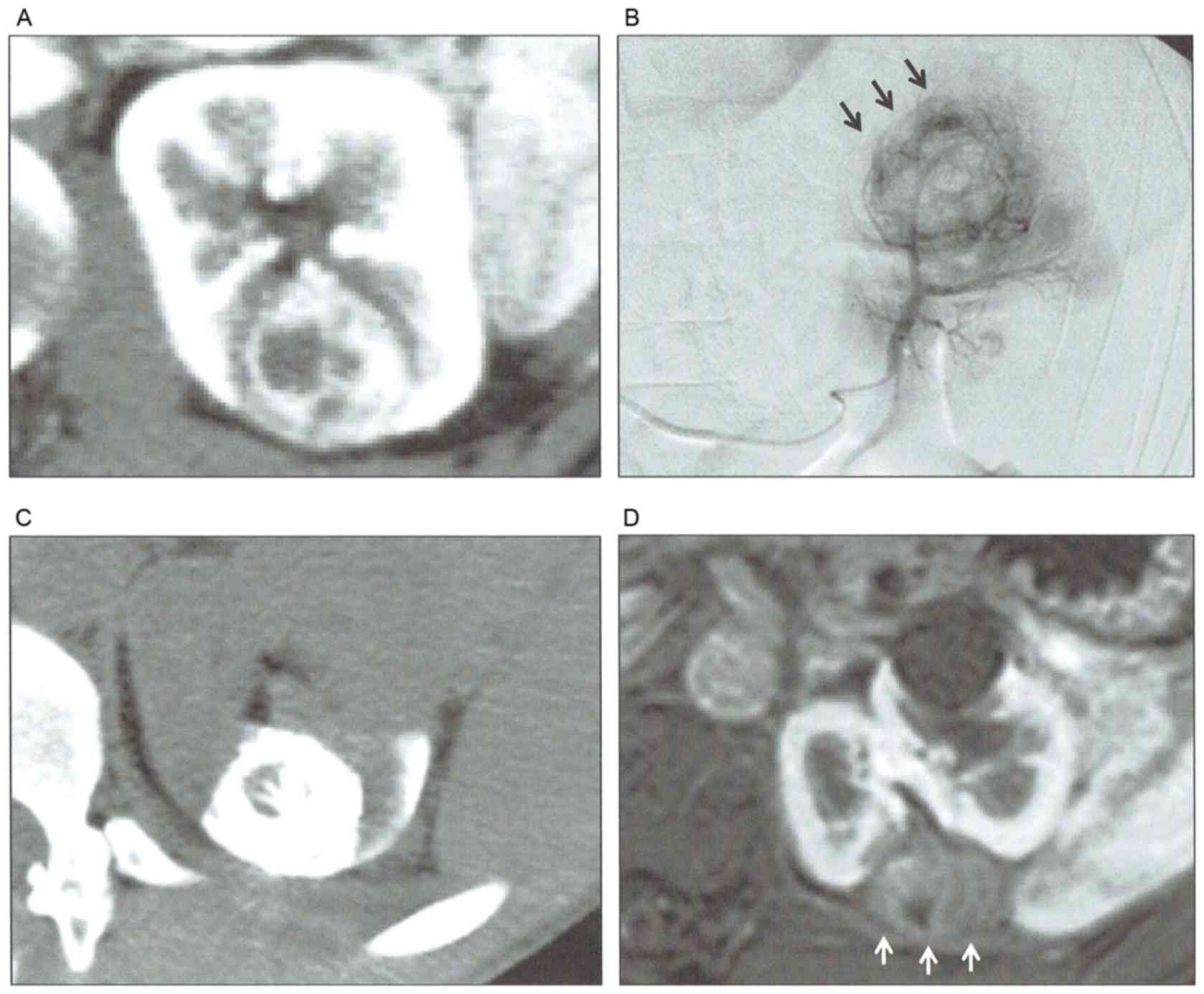
In certain cases, transarterial embolization was also performed a
number of days prior to RFA to decrease the vascular cooling effect
and to increase the complete ablation rate at initial RFA (Fig. 2). Lipiodol (Guerbet Japan, Tokyo,
Japan) was used for the transarterial embolization.
Since 2011, a biopsy of renal tumors was essentially
performed using RFA procedures. Prior to 2011, biopsy for renal
tumors was not performed in the majority of patients and RFAs were
performed for renal tumors for which RCC was strongly suspected by
imaging studies. Biopsy was performed for 12 tumors. In total, 8
tumors were diagnosed as clear-cell RCC, one as chromophobe RCC,
one as oncocytoma and pathological diagnosis could not be
determined in 2 tumors because of insufficient amounts of tissue
samples for pathological evaluation. These 2 tumors were enhanced
renal tumors and suggestive of RCC. A total of 8 tumors did not
undergo renal biopsies for the following reasons: Biopsy was not
performed for 3 renal tumors in patients with VHL disease because
these tumors were evidently enhanced by imaging studies. In
addition, biopsy was not performed for 3 renal tumors in 3 patients
who had bilateral renal tumors and whose contralateral tumors were
pathologically diagnosed as RCC. In total, 2 patients had a history
of RCC in contralateral kidneys and did not undergo renal biopsy
for renal tumors that were diagnosed as RCC by CT and magnetic
resonance imaging (MRI). For the remaining 21 tumors, biopsy was
not performed because RCC was suspected using CT and/or MRI.
Post-RFA assessment
Primary technical success of RF ablation was
evaluated using contrast-enhanced three-phase CT examinations,
immediately following or within 1 week of the procedure. Patients
were scheduled for follow-up imaging at 1, 3, 6 and 12 months and
semi-annually thereafter. In cases of impaired renal function,
unenhanced MRI was performed. Primary technical success was defined
as an absence of enhancement in the target tumor on the initial
post-RFA CT. Complete ablation was defined as an absence of
enhancement in the tumor determined using CT >3 months after
RFA. Residual tumor was defined as persistent enhancement in the
ablated tumor on the 3-month follow-up study. Local tumor
progression was defined as the appearance of enhancement around the
ablated tumor. Regarding unenhanced MRI, a high signal intensity
area on the T1 weighted image was considered an ablative zone,
according to a previous study in the liver (22). The treatment was considered to be
successful when the targeted lesion was covered by the hyperintense
area. If a residual or recurrent tumor was detected on imaging,
repeat ablation sessions were scheduled as required and as
appropriate.
Factors evaluated
The factors evaluated were age, sex, tumor size,
location (exophytic/parenchymal/central), R.E.N.A.L. nephrometry
score (21), and reasons for
indicating RFA. The oncological outcomes were evaluated by the rate
of complete ablation at initial RFA, rate of complete ablation
(including reablation of residual viable lesion), local
recurrence-free survival (LRFS) following complete ablation,
metastasis-free survival (MFS) following initial ablation,
RCC-specific survival (RCC-SS) and overall survival (OS). LRFS,
MFS, RCC-SS and OS were evaluated in 39 patients, excluding a
patient with oncocytoma. Invasiveness was evaluated by complication
and duration of hospital stay. Post-ablative renal function was
evaluated by the percentage decrease in eGFR between 1 and 6 months
after complete ablation. The eGFR was calculated using an equation
[eGFR ml/min/1.73 m2 = 194 (x 0.739 if female) × SCr-1.094 ×
age-0.287].
Statistical analysis
The results are expressed as the mean ± standard
deviation. The independence of the fit of the categorical data was
analyzed using the χ2 test. Survival curves were
constructed using the Kaplan-Meier estimator method. P<0.05 was
considered to indicate a statistically significant difference.
Results
Oncological outcome
The rate of complete ablation by single ablation was
85.4% (35/41 tumors). The rate of complete ablation at initial RFA
was increased (although not significantly) in exophytic tumors
compared with in parenchymal tumors (94.4 vs. 78.3%; P=0.1457;
Table II). The rate of complete
ablation at initial RFA was increased in tumors ≤3 cm compared with
in tumors >3 cm (90 vs. 72.7%; P=0.1656; Table II). In addition, complete ablation at
initial RFA was increased in tumors of R.E.N.A.L. nephrometry score
≤7 compared with in tumors of R.E.N.A.L. score ≥8 (90.3 vs. 70%;
P=0.1139; Table II). No significant
difference in the rate of complete ablation at initial RFA was
identified between anterior tumors and posterior tumors (76.9 vs.
88.0%; P=0.5508; Table II). However,
percutaneous RFA tended to be avoided for tumors with anterior
locations because of the risk of bowel injury.
 | Table II.Association between factors and
initial successful ablation. |
Table II.
Association between factors and
initial successful ablation.
| Factor (tumor
conditions) | Success rate (%)
(successful/not successful) |
P-valuea |
|---|
| Exophytic vs.
parenchymal | 94.4 (17/1) vs.
78.3 (18/5) | 0.1457 |
| ≤3 cm vs. >3
cm | 90 (27/3) vs. 72.7
(8/3) | 0.1656 |
| R.E.N.A.L. score ≤7
vs. ≥8 | 90.3 (28/3) vs. 70
(7/3) | 0.1139 |
| Anterior location
vs. posterior | 76.9 (13/3) vs. 88
(22/3) | 0.5508 |
In total, 5 patients with initially incomplete
ablation underwent reablation. Furthermore, 1 patient of advanced
age (>85 years) opted not to undergo reablation. The rate of
complete ablation following reablation for residual viable lesions
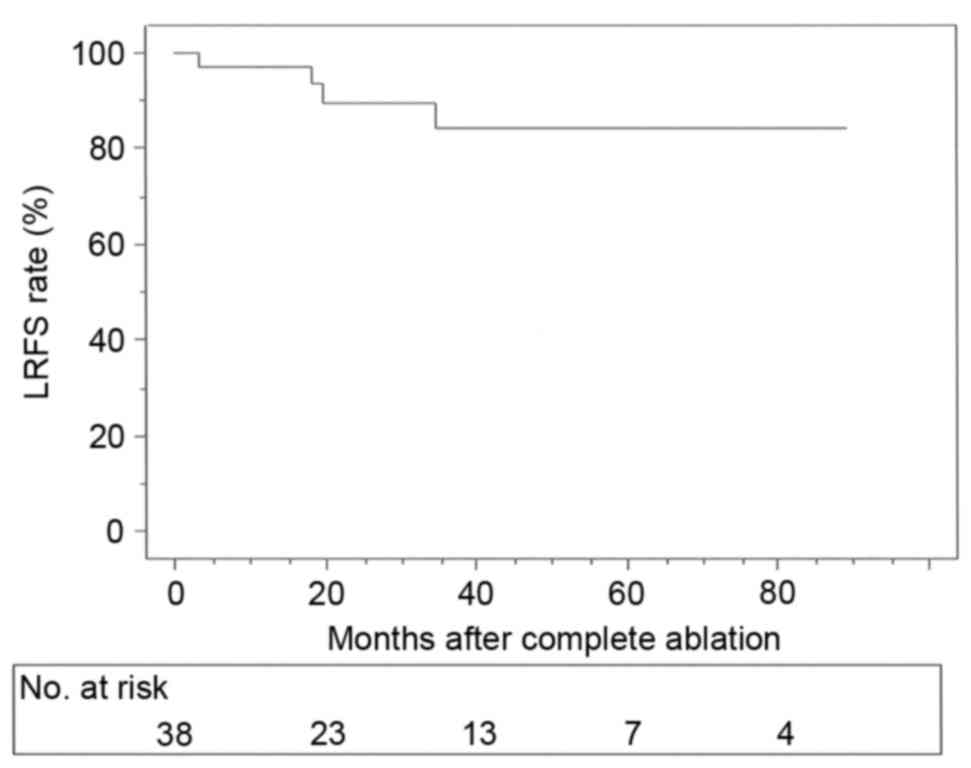
was 95.1%. The LRFS rates following complete ablation were 97.3,
89.8, 84.2 and 84.2% at 1, 2, 3 and 5 years, respectively (Fig. 3). Furthermore, 2 patients succumbed to
heart failure or cerebellar hemangioblastoma due to VHL disease.
The 3- and 5-year OS rates were 96.9 and 90%, respectively, and the
RCC-specific survival was 100%. Metastases developed following RFA
in 2 patients. A patient with a 4.7 cm RCC underwent RFA four times
until complete radiological ablation was achieved. Multiple lung
metastases and lymph node metastases, and local progression with
renal vein tumor thrombus were identified 7 months after the final
ablation (see the next section). In another patient with RCC (3
cm), the tumor once demonstrated complete ablation, but local
recurrence with small renal vein tumor thrombus was revealed 6
months after the RFA. The patient of advanced age was observed
without reablation as was his preference; however, repeated gross
hematuria occurred for 31 months after the RFA. Subsequently,
laparoscopic radical nephrectomy was performed 37 months after RFA.
However, multiple lung metastases developed 3 months after the
nephrectomy. MFS rates following complete ablation were 100, 95.7,
95.7 and 83.7 at 1, 2, 3 and 5 years, respectively.
Rapid progression following repeated
percutaneous RFA
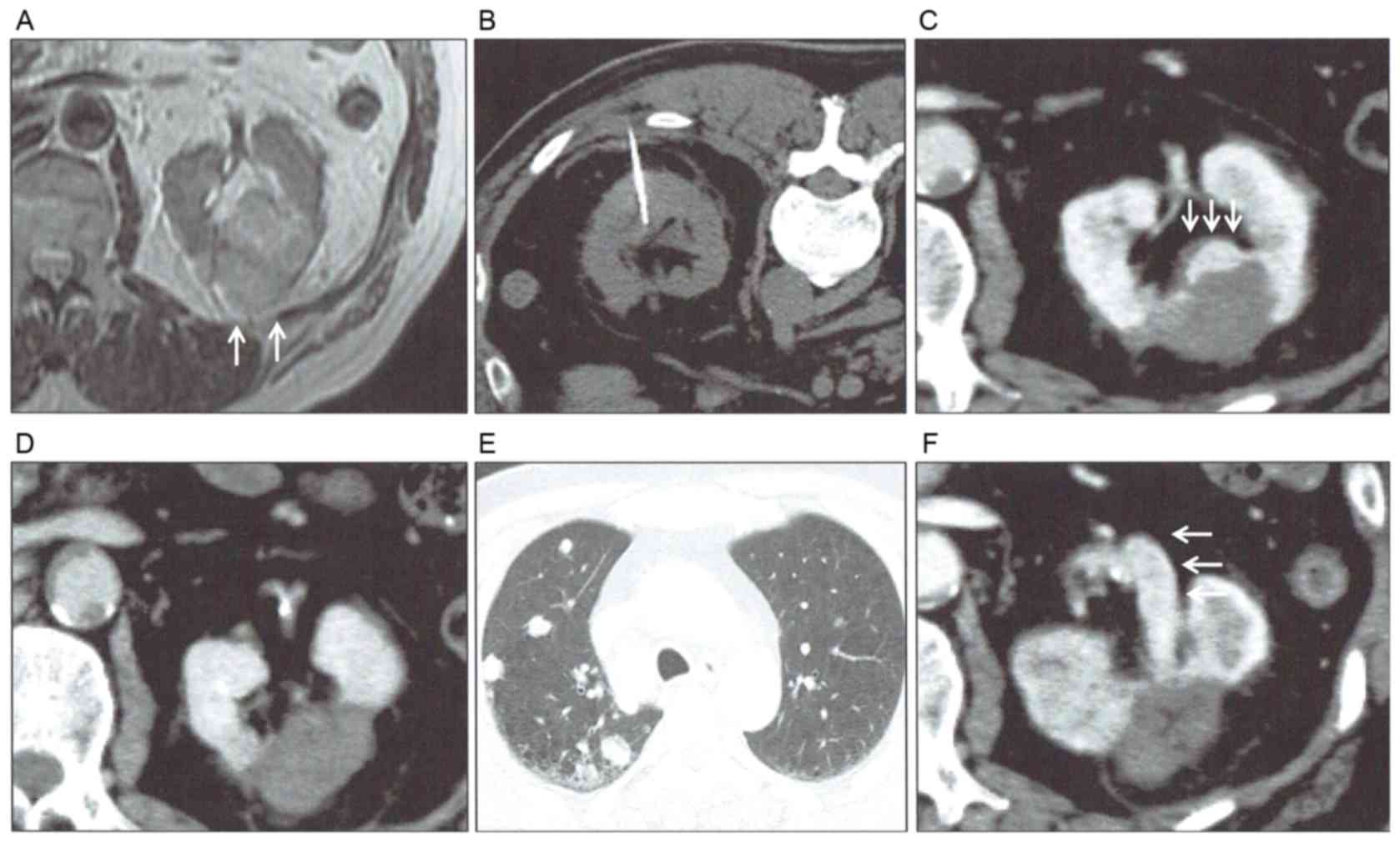
A 61-year-old male patient was diagnosed as left RCC
(4.7 cm) in August 2011 (Fig. 4A). As
the patient had chronic renal dysfunction, severe diabetes
mellitus, hypertension, hyperlipidemia and a history of myocardial
infarction, percutaneous RFA was performed in December 2011
(Fig. 4B). However, complete ablation
could not be achieved and an enhanced lesion remained near the
collecting system. Pathological diagnosis by needle biopsy was
clear-cell RCC (Fuhrman nucleolar grade 2). A second RFA was
performed in April 2012; however, complete ablation was also not
achieved. Growth of the remaining lesion was identified using CT 4
months after the second ablation, and a third ablation was
performed in December 2012. However, complete ablation could not be
achieved at that time (Fig. 4C). The
fourth ablation was performed in March 2013 and radiological
complete ablation was achieved. Although complete ablation was
confirmed 3 months after the final ablation (Fig. 4D), lymph node metastases in the
mediastinum, multiple lung metastases (Fig. 4E) and local progression with renal
vein tumor thrombus (Fig. 4F) were
presented 7 months after the final ablation. Subsequently,
interferon-α treatment was initiated for this patient.
Complication and post-operative
course
In a total of 50 sessions of RFA, complications were
observed in five sessions (10%). These were an arteriovenous
fistula, a perirenal hematoma, a high fever, a pneumothorax and a
temporal hypotension due to a sedative drug (midazolam). A patient
with arteriovenous fistula was embolized by transarterial approach
2 days after RFA. In 4 other patients, the complications were all
improved by conservative therapies. The average hospital stay was
3.2 days (range, between 1 and 20 days). The majority of patients
were able to resume dietary intake on the day of percutaneous RFA a
number of hours later.
Renal function
Postoperative eGFR was evaluated between 1 and 6
months after complete ablation in each patient. Compared with eGFR
prior to RFA, the average decrease in eGFR following complete
ablation was 2.7±9.0% (range: between −19.6 and 18.9%; median,
1%).
Discussion
Percutaneous RFA has become a viable option for the
treatment of small RCC. Excellent cancer management with rigorous
follow-up periods has been reported previously in hospitals with a
high volume of RFA (14–20). RFA appears to be an effective
treatment, particularly for patients of advanced age, patients
exhibiting comorbidities and patients with hereditary RCC. The
indications for RFA were considered to be comorbidity, age,
hereditary disease, solitary kidney and decreased renal function.
Although many patients who underwent RFA were at high risk for
surgery, the complication rates were low and oncological outcome
was acceptable. The RCC-SS rate was 100% (mean follow-up, 38
months) and the 3-year OS rate was 96.3%. Furthermore, the decrease
in eGFR was low following RFA. Percutaneous RFA appeared to be
beneficial for the majority of the patients in the present
study.
In the present study, the rate of complete ablation
at initial RFA was 85.4%. As the rate of complete ablation at
initial RFA was reportedly between 87 and 100% in a high-volume
hospital (14,15,17,18,20),
further improvement is required. In the present study the rate of
complete ablation at initial RFA tended to be decreased in patients
with parenchymal tumor, tumors >3 cm and tumors with R.E.N.A.L.
nephrometry scores ≥8, compared with their respective counterparts.
Schmit et al (23) reported a
significant association between R.E.N.A.L. nephrometry score and
local treatment failure. To improve oncological outcomes, we
recently performed transarterial embolization prior to ablation in
certain patients whose RCC was located near large vessels (Soga
et al, unpublished data). In addition, multiple RFA needles
were used for relatively large tumors. With these efforts, the rate
of complete ablation at initial ablation has gradually improved.
One of the merits of percutaneous RFA of residual lesions is that
reablation is possible. The rate of complete ablation following
reablation was 95.1%.
Multiple repeated ablation should be avoided because
of the possibility of rapid progression. In the present study, a
patient with T1b RCC (4.7 cm) was rapidly progressed following four
RFA treatments. Although rapid progression following RFA appears to
be a rare event in RCC, complete ablation should be achieved within
a minimal number of sessions (two sessions). Although rapid
progression in RCC has been rarely reported (24), rapid progression following RFA has
been reported in hepatocellular carcinoma (HCC) (25,26). Obara
et al (27) reported that
insufficient RFA may induce further malignant transformation of
HCC. Furthermore, Dong et al (28) demonstrated that insufficient RFA
promoted epithelial-mesenchymal transition of HCC cells through
protein kinase B and extracellular-signal-regulated kinase
signaling pathways. In contrast with rapid progression in HCC,
there are few reports regarding that in RCC. Kroeze et al
(29) reported that incomplete
thermal ablation stimulated proliferation of residual RCC cells in
a murine model. To the best of our knowledge, only one study has
previously demonstrated rapid progression following laparoscopic
RFA for T1b RCC (4.5 cm) (24). Rapid
progression appeared to be unlikely to occur in RCC compared with
HCC.
The oncological outcomes of RFA with durable
follow-up periods have previously been reported (14–20).
Ferakis et al (14) reported
the outcome with an average follow up of 61.2 months. In that
study, RFA was performed in 31 patients (39 tumors) and the rates
of initial complete ablation, complete ablation, 5-year LRFS and
RCC-SS were 90, 97, 89 and 100%, respectively. Psutka et al
(17) reported the results of
biopsy-proven RCC (median follow-up, 6.43 years; T1a, 143 tumors;
T1b, 42 tumors). In that study, when T1a tumors were focused, local
recurrence was observed in 6 patients (4.2%) and metastasis was
observed in 1 patient (0.7%). The 5-year RCC-SS was 100%. In
contrast, the 5-year OS was 74%, suggesting that many high-risk
patients were included in that study. Furthermore, Olweny et
al (16) compared the clinical
results of RFA with those of PN in patients who were followed up
for >5 years after treatment. They reported that the 5-year MFS,
5-year RCC-SS and 5-year OS were all comparable. Moreover, Ma et
al (18) reported results of RFA
for RCC in 52 healthy adults (average size, 2.2 cm; median
follow-up, 60 months) and local recurrence was observed in 3 tumors
(5.1%). The 10-year disease-free survival, 10-year RCC-SS and
10-year OS were 94.2, 100 and 91.1%, respectively. On the basis of
these results, the rate of local recurrence following complete
ablation appears to be low in T1a RCC and RCC-specific survival is
excellent. RFA may be one of the first choices for small RCC in
patients for whom surgery would be a high risk.
Although various complications have been reported,
the majority of those were minor and the complication rates were
low (30,31). In the present study, complications
were observed in five sessions following RFA (10%). However, only 1
patient required intervention (TAE). As that case was treated
because arteriovenous fistula occurred 2 days after RFA, enhanced
CT was routinely performed immediately following RFA (between 1 and
3 days after RFA). The average hospital stay following RFA was 3.2
days, and hospital stay may be reduced if the CT was performed in
an outpatient clinic. General patient condition was usually
excellent the day following RFA and dietary intake was usually able
to be resumed on the day of RFA. These results reflected the
minimal invasiveness of RFA. Early resumption of dietary intake and
maintaining daily activities appear to be advantageous for patients
of advanced age and patients exhibiting comorbidities.
One attractive advantage of RFA is the minimal
decrease in eGFR. In the present study, the mean decrease in eGFR
was only 2.7% following RFA. Lucas et al (32) reported a significantly decreased rate
of eGFR <60 following RFA, compared with those following RN or
PN. The decrease in eGFR following RFA was <10% in patients with
a solitary kidney (33,34). In view of renal function, RFA also
appears to be a viable option for patients with a solitary kidney,
renal dysfunction and hereditary disease, which carry a lifelong
risk for multiple RCC.
Although further improvements in oncological
outcomes and complication rates are required, RCC-SS and renal
function following RFA were excellent. Percutaneous RFA is a viable
option as a treatment for small RCC, especially in patients
exhibiting comorbidities, patients of advanced age, patients with
hereditary RCC and certain patients with decreased renal
function.
References
|
1
|
Belldegrun A, Tsui KH, deKernion JB and
Smith RB: Efficacy of nephron-sparing surgery for renal cell
carcinoma: Analysis based on the new 1997 tumor-node-metastasis
staging system. J Clin Oncol. 17:2868–2875. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lam JS, Shvarts O and Pantuck AJ: Changing
concepts in the surgical management of renal cell carcinoma. Eur
Urol. 45:692–705. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patard JJ, Tazi H, Bensalah K, Rodriguez
A, Vincendeau S, Rioux-Leclercq N, Guillé F and Lobel B: The
changing evolution of renal tumours: A single center experience
over a two-decade period. Eur Urol. 45:490–494. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pignot G, Bigot P, Bernhard JC, Bouliere
F, Bessede T, Bensalah K, Salomon L, Mottet N, Bellec L, Soulié M,
et al: Nephron-sparing surgery is superior to radical nephrectomy
in preserving renal function benefit even when expanding
indications beyond the traditional 4-cm cutoff. Urol Oncol.
32:1024–1030. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ito K, Nakashima J, Hanawa Y, Oya M,
Ohigashi T, Marumo K and Murai M: The prediction of renal function
6 years after unilateral nephrectomy using preoperative risk
factors. J Urol. 171:120–125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Süer E, Burgu B, Gökce Mİ, Türkölmez K,
Bedük Y and Baltaci S: Comparison of radical and partial
nephrectomy in terms of renal function: A retrospective cohort
study. Scand J Urol Nephrol. 45:24–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang WC, Levey AS, Serio AM, Snyder M,
Vickers AJ, Raj GV, Scardino PT and Russo P: Chronic kidney disease
after nephrectomy in patients with renal cortical tumours: A
retrospective cohort study. Lancet Oncol. 7:735–740. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zini L, Perrotte P, Capitanio U, Jeldres
C, Shariat SF, Antebi E, Saad F, Patard JJ, Montorsi F and
Karakiewicz PI: Radical versus partial nephrectomy: Effect on
overall and noncancer mortality. Cancer. 115:1465–1471. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zini L, Patard JJ, Capitanio U, Crepel M,
de La Taille A, Tostain J, Ficarra V, Bernhard JC, Ferrière JM,
Pfister C, et al: Cancer-specific and non-cancer-related mortality
rates in European patients with T1a and T1b renal cell carcinoma.
BJU Int. 103:894–898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lesage K, Joniau S, Fransis K and Poppel
HV: Comparison between open and radical nephrectomy for renal
tumors: Perioperative outcome and health-related quality of life.
Eur Urol. 51:614–620. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berdjis N, Hakenberg OW, Novotny V,
Froehner M and Wirth MP: Treating renal cell cancer in the elderly.
BJU Int. 97:703–705. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Steinbach F, Novick AC, Zincke H, Miller
DP, Williams RD, Lund G, Skinner DG, Esrig D, Richie JP, deKernion
JB, et al: Treatment of renal cell carcinoma in von Hippel-Lindou
disease: A multicenter study. J Urol. 153:1812–1816. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Metwalli AR and Linehan WM:
Nephron-sparing surgery for multifocal and hereditary renal tumors.
Curr Opin Urol. 24:466–473. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferakis N, Bouropoulos C, Granitsas T,
Mylona S and Poulias I: Long-term results after
computed-tomography-guided percutaneous radiofrequency ablation for
small renal tumors. J Endourol. 24:1909–1913. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tracy CR, Raman JD, Donnally C, Trimmer CK
and Cadeddu JA: Durable oncologic outcomes after radiofrequency
ablation: Experience from treating 243 small renal masses over 7.5
years. Cancer. 116:3135–3142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Olweny EO, Park SK, Tan YK, Best SL,
Trimmer C and Cadeddu JA: Radiofrequency ablation versus partial
nephrectomy in patients with solitary clinical T1a renal cell
carcinoma: Comparable oncologic outcomes at a minimum of 5 years of
follow-up. Eur Urol. 61:1156–1161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Psutka SP, Feldman AS, McDougal WS,
McGovern FJ, Mueller P and Gervais DA: Long-term oncologic outcomes
after radiofrequency ablation for T1 renal cell carcinoma. Eur
Urol. 63:486–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma Y, Bedir S, Cadeddu JA and Gahan JC:
Long-term outcomes in healthy adults after radiofrequency ablation
of T1a renal tumours. BJU Int. 113:51–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wah TM, Irving HC, Gregory W, Cartledge J,
Joyce AD and Selby PJ: Radiofrequency ablation (RFA) of renal cell
carcinoma (RCC): Experience in 200 tumours. BJU Int. 113:416–428.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramirez D, Ma YB, Bedir S, Antonelli JA,
Cadeddu JA and Gahan JC: Laparoscopic radiofrequency ablation of
small renal tumors: Long-term oncologic outcomes. J Endourol.
28:330–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kutikov A and Uzzo RG: The R.E.N.A.L.
nephrometry score: A comprehensive standardized system for
quantitating renal tumor size, location and depth. J Urol.
182:844–853. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koda M, Tokunaga S, Miyoshi K, Kishina M,
Fujise Y, Kato J, Matono T, Okamoto K, Murawaki Y and Kakite S:
Assessment of ablative margin by unenhanced magnetic resonance
imaging after radiofrequency ablation for hepatocellular carcinoma.
Eur J Radiol. 81:2730–2736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmit GD, Thompson RH, Kurup AN, Weisbrod
AJ, Boorjian SA, Carter RE, Geske JR, Callstrom MR and Atwell TD:
Usefulness of R.E.N.A.L. nephrometry scoring system for predicting
outcomes and complications of percutaneous ablation of 751 renal
tumors. J Urol. 189:30–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Uribe PS, Costabile RA and Peterson AC:
Progression of renal tumors after laparoscopic radiofrequency
ablation. Urology. 68:968–971. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seki T, Tamai T, Ikeda K, Imamura M,
Nishimura A, Yamashiki N, Nakagawa T and Inoue K: Rapid progression
of hepatocellular carcinoma after transcatheter arterial
chemoembolization and percutaneous radiofrequency ablation in the
primary tumour region. Eur J Gastroenterol Hepatol. 13:291–294.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ruzzenente A, Manzoni GD, Molfetta M,
Pachera S, Genco B, Donataccio M and Guglielmi A: Rapid progression
of hepatocellular carcinoma after radiofrequency ablation. World J
Gastroenterol. 10:1137–1140. 2004.PubMed/NCBI
|
|
27
|
Obara K, Matsumoto N, Okamoto M, Kobayashi
M, Ikeda H, Takahashi H, Katakura Y, Matsunaga K, Ishii T, Okuse C,
et al: Insufficient radiofrequency ablation therapy may induce
further malignant transformation of hepatocellular carcinoma.
Hepatol Int. 2:116–123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dong S, Kong J, Kong F, Kong J, Gao J, Ke
S, Wang S, Ding X, Sun W and Zheng L: Insufficient radiofrequency
ablation promotes epithelial-mesenchymal transition of
hepatocellular carcinoma cells through Akt and ERK signaling
pathways. J Transl Med. 11:2732013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kroeze SG, van Melick HH, Nijkamp MW,
Kruse FK, Kruijssen LW, van Diest PJ, Bosch JL and Jans JJ:
Incomplete thermal ablation stimulates proliferation of residual
renal carcinoma cells in a translational murine model. BJU Int.
110:E281–E286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hines-Peralta A and Goldberg SN: Review of
radiofrequency ablation for renal cell carcinoma. Clin Cancer Res.
10:6328S–6334S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lian H, Guo H, Zhang G, Yang R, Gan W, Li
X, Ji C and Liu J: Single-center comparison of complications in
laparoscopic and percutaneous radiofrequency ablation with
ultrasound guidance for renal tumors. Urology. 80:119–124. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lucas SM, Stern JM, Adibi M, Zeltser IS,
Cadeddu JA and Raj GV: Renal function outcomes in patients treated
for renal masses smaller than 4 cm by ablative and extirpative
techniques. J Urol. 179:75–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hoffmann RT, Jakobs TF, Kubisch CH, Trumm
C, Weber C, Siebels M, Helmberger TK and Reiser MF: Renal cell
carcinoma in patients with a solitary kidney after nephrectomy
treated with radiofrequency ablation: Mid term results. Eur J
Radiol. 73:652–656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Raman JD, Raj GV, Lucas SM, Williams SK,
Lauer EM, Ahrar K, Matin SF, Leveillee RJ and Cadeddu JA: Renal
functional outcomes for tumours in a solitary kidney managed by
ablative or extirpative techniques. BJU Int. 105:496–500. 2010.
View Article : Google Scholar : PubMed/NCBI
|