Introduction
Hepatocellular carcinoma (HCC) is the third most
lethal cancer worldwide, with the highest incidence in East Asia
(1). Radical resection is the
principal treatment for HCC, but the recurrence rate is very high
(2). Radiofrequency ablation (RFA)
and transcatheter arterial chemoembolization (TACE) may be
performed to treat intrahepatic recurrence, but only systemic
chemotherapy can be employed for extrahepatic recurrence (3). In a phase III trial, sorafenib was the
only drug that prolonged the overall survival (OS) time of patients
with HCC (4,5). However, the difference in median OS time
between the patients treated with sorafenib, which is very
expensive, and best supportive care was only 2 months.
S-1 is a novel, orally administered drug that
combines the components (in a molar concentration ratio of 1:0.4:1)
as follows: Tegafur, a metabolically activated prodrug of
5-fluorouracil (5-FU); 5-chloro-2,4-dihydropyridine, a reversible
dihydropyrimidine dehydrogenase (DPYD) inhibitor; and oteracil
potassium (6). Several case studies
show a marked response of patients with HCC to S-1 (7–9). Although
a phase I/II study of S-1 in patients with advanced HCC showed
promising antitumor activity with acceptable toxicities (10), a phase III randomized study of S-1 in
patients with sorafenib refractory advanced HCC failed to show
clinical advantage (11). Thus,
selection of the patients with HCC who will gain clinical benefit
from S-1 treatment is required.
Thymidylate synthase (TYMS) is the rate-limiting
enzyme in the synthesis of thymine nucleotides (12). TYMS expression levels are
associated with response of patients to 5-FU therapy and their
prognosis, and high levels of TYMS expression in most cases
are associated with worse responses and shorter survival times
(12–14). DPYD, which degrades pyrimidines,
uracil and thymine and inactivates 5-FU, is associated with the
response to 5-FU-based therapies (15,16).
Therefore, it was hypothesized that TYMS and DPYD
are potential biomarkers for predicting the efficacy of S-1 for
treating patients with HCC. In the present study, 30 patients with
HCC who were treated with S-1 subsequent to having relapsed
following surgical resection were assessed. The levels of
TYMS and DPYD mRNAs in surgically resected specimens
were measured using reverse transcription-quantitative polymerase
chain reaction assays (RT-qPCR) to investigate the association of
TYMS and DPYD expression with the efficacy of S-1
treatment and OS time.
Materials and methods
Characteristics of patients
A total of 55 patients with relapsed HCC were
studied. All patients underwent curative liver resection at the
Institute of Gastroenterology, Tokyo Women's Medical University
Hospital (Tokyo, Japan) between September 1997 and January 2007.
All patients subsequently relapsed following surgery. In total, 14
of these patients had hepatitis B virus, 24 had hepatitis C and 11
had alcoholic liver cirrhosis. A total of 35 patients received S-1
upon recurrence (S-1 group), and 20 patients never received S-1 or
any fluoropyrimidine derivative (control group). S-1 was generally
administrated at 80 mg/m2 of body surface area per day
for 4 weeks followed by 2 weeks of rest, and this 6-week cycle was
repeated until the disease progressed. The dose and schedule of S-1
administration was modified based on liver function and the general
condition of each patient. The patients with control group had
never received systemic chemotherapy. S-1 was used following
failure of local control therapy, including TACE and/or RFA. In the
S-1 group, patients experienced relapses as follows: Liver, 30
(85.7%); lung, 18 (51.4%); lymph node, 5 (14.2%); brain, 6 (17.1%);
bone, 8 (22.9%); and adrenal gland, 2 (5.7%). The characteristics
of patients in the S-1 and control groups are shown in Table I. Clinicopathological characteristics
of the S-1 group according to TYMS and DPYD mRNA
levels are shown in Table II, and
those of the control group are shown in Table III. Biochemical data such as serum
albumin, indocyanine green retention test (ICG R15), prothrombin
time, total bilirubin and cholinesterase were collected from each
patient to compare the liver function between TYMS high and low
expression patients, as well as dihydropyrimidine dehydrogenase
(DYPD). Clinicopathological data including tumor differentiation,
number of tumors, portal vein invasion, hepatic vein invasion and
intrahepatic metastasis were also assessed from each patient to
compare the background between TYMS high and low expression
patients, as well as DYPD. Child-Pugh score was used to assess the
liver function (17).
 | Table I.Characteristics of patients. |
Table I.
Characteristics of patients.
| Characteristic | S-1 group | Control group |
|---|
| Total number of
patients | 35 | 20 |
| Host factors |
|
|
| Age,
years (median) | 59.6 | 65.6 |
| Sex
(M/F) | 29/6 | 16/4 |
| Child
Pugh Score | 5.4 | 5.6 |
| Viral
hepatitis (B/C) | 12/14 | 2/14 |
|
Alcoholic hepatitis | 5 | 6 |
| Liver
status (Chronic hepatits/liver cirrhosis) | 23/4 | 13/7 |
| Recurrent site |
|
|
|
Liver | 30 (85.7%) | 18 (90%) |
|
Lung | 18 (51.4%) | 8 (40%) |
|
Bone | 8 (22.9%) | 6 (30%) |
|
Brain | 6 (17.1%) | 2 (10%) |
| Lymph
node | 5 (14.2%) | 3 (15%) |
| Adrenal
gland | 1 (5.7%) | 0 (0%) |
| Blood serum
test |
|
|
| AFP
(ng/ml) | 8,502
(2–83,069) | 7,827
(2–113,156) |
| PIVKA
II (U/ml) | 12,345
(5–277,820) | 2,117
(14–18,500) |
 | Table II.Clinicopathological characteristics
of the S-1 group. |
Table II.
Clinicopathological characteristics
of the S-1 group.
| Characteristic | TYMS high | TYMS low | P-value | DPYD high | DPYD low | P-value |
|---|
| Age,
yearsa | 65 (42–76) | 60 (31–72) | 0.57 | 65 (42–76) | 59 (31–72) | 0.24 |
| Liver function
parameters |
|
|
|
|
|
|
| Child
Pugh score |
|
|
|
|
|
|
|
A | 13 | 13 | 0.77 | 15 | 15 | 0.68 |
|
B | 4 | 5 |
| 3 | 2 |
|
|
Albumin, g/dla | 4.0 (3.3–4.5) | 3.8 (2.8–4.8) | 0.28 | 3.95 (2.8–4.5) | 3.8 (2.8–4.8) | 0.64 |
| Cholinesterase,
IU/la | 221 (116–321) | 226 (65–316) | 0.77 | 225 (102–321) | 226 (65–316) | 0.85 |
| ICG R15,
%a | 13 (4–28) | 8.5 (3–32) | 0.08 | 14 (4–28) | 9 (3–32) | 0.10 |
| Prothrombin time,
%a | 88.3
(40.6–100) | 87.7
(59.4–100) | 0.89 | 84.4
(40.6–100) | 91.1
(71.1–100) | 0.36 |
| Tumor
pathology |
|
|
|
|
|
|
|
Differentiation |
|
|
|
|
|
|
|
Well | 0 | 2 | 0.41 | 0 | 2 | 0.32 |
|
Moderate | 13 | 14 |
| 15 | 12 |
|
|
Poor | 3 | 3 |
| 3 | 3 |
|
| Number
of tumors |
|
|
|
|
|
|
|
Solitary | 11 | 12 | 0.73 | 13 | 11 | 0.33 |
|
Multiple | 5 | 7 |
| 4 | 7 |
|
| Portal
vein invasion |
|
|
|
|
|
|
|
Yes | 4 | 7 | 0.38 | 5 | 6 | 0.71 |
|
No | 11 | 10 |
| 11 | 10 |
|
| Hepatic
vein invasion |
|
|
|
|
|
|
|
Yes | 5 | 0 | 0.02 | 5 | 0 | 0.04 |
|
No | 10 | 17 |
| 11 | 16 |
|
|
Intrahepatic metastasis |
|
|
|
|
|
|
|
Yes | 4 | 7 | 0.39 | 4 | 7 | 0.26 |
|
No | 11 | 10 |
| 12 | 9 |
|
 | Table III.Clinicopathological characteristics
of the control group (n=20). |
Table III.
Clinicopathological characteristics
of the control group (n=20).
| Characteristic | TYMS high, n | TYMS low, n | P-value | DPYD high, n | DPYD low, n | P-value |
|---|
| Age,
yearsa | 66 (59–81) | 57.5 (53–78) | 0.05 | 64.5 (53–80) | 63.5 (53–81) | 0.90. |
| Liver function
parameters |
|
|
|
|
|
|
| Child
Pugh score |
|
|
|
|
|
|
|
A | 9 | 8 | 0.53 | 8 | 9 | 0.53 |
|
B | 1 | 2 |
| 2 | 1 |
|
|
Albumin, g/dla | 4 (3.2–4.2) | 3.65 (3.1–4.2) | 0.20 | 3.8 (3.1–4.2) | 3.65 (3.1–4.2) | 0.59 |
| Cholinesterase,
IU/la | 243 (82–318) | 178 (45–282) | 0.15 | 190.5 (82–318) | 243 (45–296) | 0.31 |
| ICG R15,
%a | 10 (2.2–47) | 20 (7–59) | 0.07 | 19 (2.2–30) | 13 (5–59) | 0.71 |
| Prothrombin time,
%a | 84.9
(81.9–100) | 94.7
(60.4–100) | 0.68 | 90.15
(66.3–100) | 85 (60.4–100) | 0.70 |
| Tumor
pathology |
|
|
|
|
|
|
|
Differentiation |
|
|
|
|
|
|
|
Well | 0 | 0 | 1.00 | 0 | 0 | 1.00 |
|
Moderate | 8 | 8 |
| 8 | 8 |
|
|
Poor | 2 | 2 |
| 2 | 2 |
|
| Numbers
of tumor |
|
|
|
|
|
|
|
Solitary | 9 | 8 | 0.53 | 7 | 10 | 0.06 |
|
Multiple | 1 | 2 |
| 3 | 0 |
|
| Portal
vein invasion |
|
|
|
|
|
|
|
Yes | 2 | 2 | 1.00 | 2 | 2 | 1.00 |
|
No | 8 | 8 |
| 8 | 8 |
|
| Hepatic
vein invasion |
|
|
|
|
|
|
|
Yes | 1 | 1 | 1.00 | 0 | 2 | 0.14 |
|
No | 9 | 9 |
| 10 | 8 |
|
|
Intrahepatic metastasis |
|
|
|
|
|
|
|
Yes | 1 | 1 | 1.00 | 0 | 2 | 0.14 |
|
No | 9 | 9 |
| 10 | 8 |
|
The Ethics Committee of the Tokyo Women's Medical
University (Tokyo, Japan) approved the present study, which was
performed in accordance with the Declaration of Helsinki. Patients
granted their informed consent to be involved in the present
study.
Microdissection
Formalin-fixed paraffin-embedded (FFPE) tumor
specimens were cut into 10 µm thick serial sections Manual
microdissection was performed using a scalpel if the histology was
homogeneous and the tissue contained >90% cancer cells. For all
other samples, laser-capture microdissection (P.A.L.M. Microlaser
Technologies AG, Munich, Germany) was performed to ensure that only
tumor cells were acquired.
RNA isolation and cDNA synthesis
RNA isolation from FFPE specimens was performed
using an RNeasy FFPE kit (Qiagen, Inc., Valencia, CA, USA)
according to the manufacturer's protocol. The cDNAs were
synthesized using a High Capacity cDNA Reverse Transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
RT-qPCR
The cDNAs were amplified using a TaqMan PreAmp
Master Mix kit (Applied Biosystems; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Quantification of target
genes TYMS and DPYD and the internal reference gene
(β-actin, ACTB) was performed using a
fluorescence-based real-time detection method (StepOne Real-time
Polymerase Chain Reaction System; Applied Biosystems; Thermo Fisher
Scientific, Inc.). The primers and probes used were from TaqMan
Gene Expression Assays (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with assay IDs: Hs00426591_m1 for TYMS;
Hs00559278_m1 for DPYD; and Hs01060665_g1 for
ACTB.
The PCR reaction mixture consisted of 10 µl of
TaqMan Fast Universal PCR Master Mix without uracil-N-glycosylase
(Applied Biosystems; Thermo Fisher Scientific, Inc.), 5 µl of
amplified cDNA, 1 µl of each of the TaqMan Gene Expression Assay
primers and probe (20X) and 3 µl of nuclease-free water. Cycling
conditions were 95°C for 20 sec, followed by 40 cycles at 95°C for
1 sec and 60°C for 20 sec. The threshold cycle (Cq) value for each
gene was determined using SDS software v1.2 (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The ∆-Cq (∆Cq) value, which is the
difference between the Cq value of the target gene and that of the
endogenous reference gene, was also calculated using the same
software. Δ-∆Cq (∆∆Cq), which is the difference between the DCq
value for each sample and the highest ∆Cq value as a calibrator,
was also calculated. The value of 2−∆∆Cq was used for
quantitation of relative mRNA levels (18).
Statistical analysis
Comparisons of clinicopathological backgrounds were
assessed using the χ2 test. The Kaplan-Meier method was
used to generate survival curves, and the log-rank test was used to
compare survival between groups. The Cox proportional hazard
regression model was used in multivariate analysis. In the S-1
group, OS was defined as the time from the first day of S-1
administration to mortality from any cause. In the control group,
OS was defined as the time from the day of operation to mortality
from any cause. Median values were used as the cutoff values to
divide high and low expression levels. P<0.05 was considered to
indicate a statistically significant difference. All values were
two-sided. Statistical analyses were performed using JMP 10 (SAS
Institute Inc., Cary, NC, USA).
Results
Treatment of the S-1 group
A total of 35 patients with HCC in the S-1 group
received S-1 upon recurrence of disease. Median treatment time was
12 weeks, and the mean dose administered was 89.6 mg/day. Grade 1
or 2 gastrointestinal adverse events were most common, but grade 3
or 4 never occurred. A total of 3 patients stopped receiving S-1
due to fatigue, appetite loss or diarrhea.
Gene expression and survival time of
the S-1 group
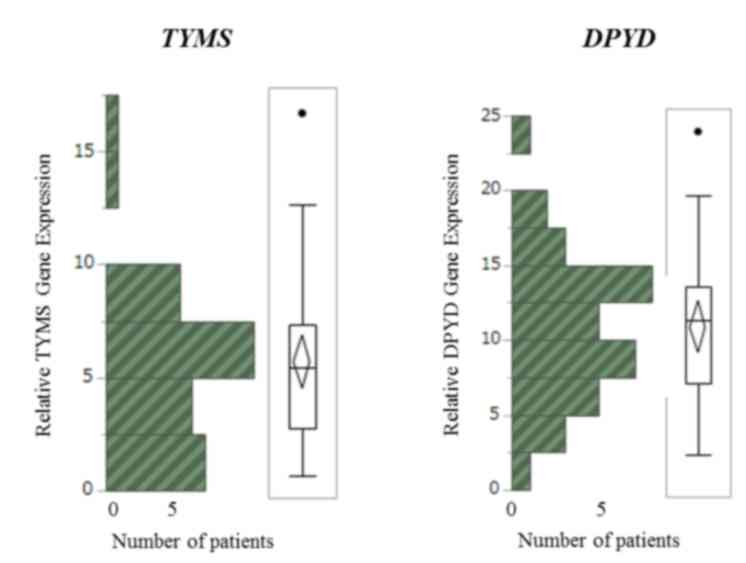
The median level of DPYD mRNA was 11.31
(range, 2.36–23.92), and the median level of TYMS mRNA was
5.46 (range, 0.67–16.68; Fig. 1),
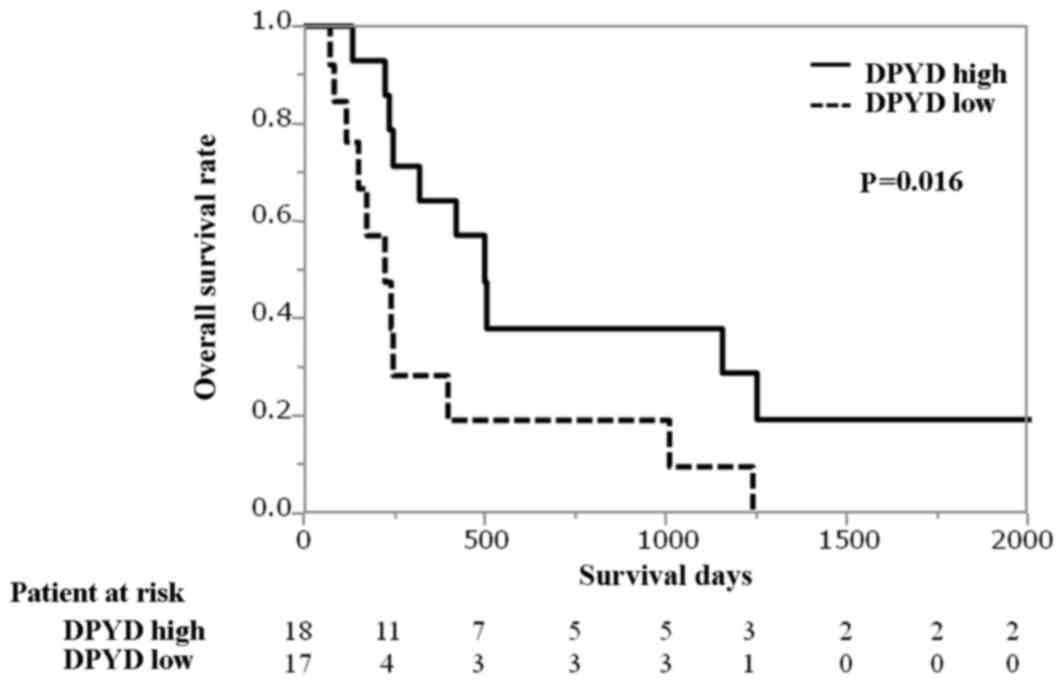
relative to ACTB. Using the median value as a cutoff, S-1
group patients with high levels of DPYD mRNA were associated
with longer overall survival time in contrast to those with low
levels of DPYD mRNA [median 501 days vs. 225 days; hazard
ratio (HR), 0.35 (95% confidence interval [CI]: 0.14–0.85);
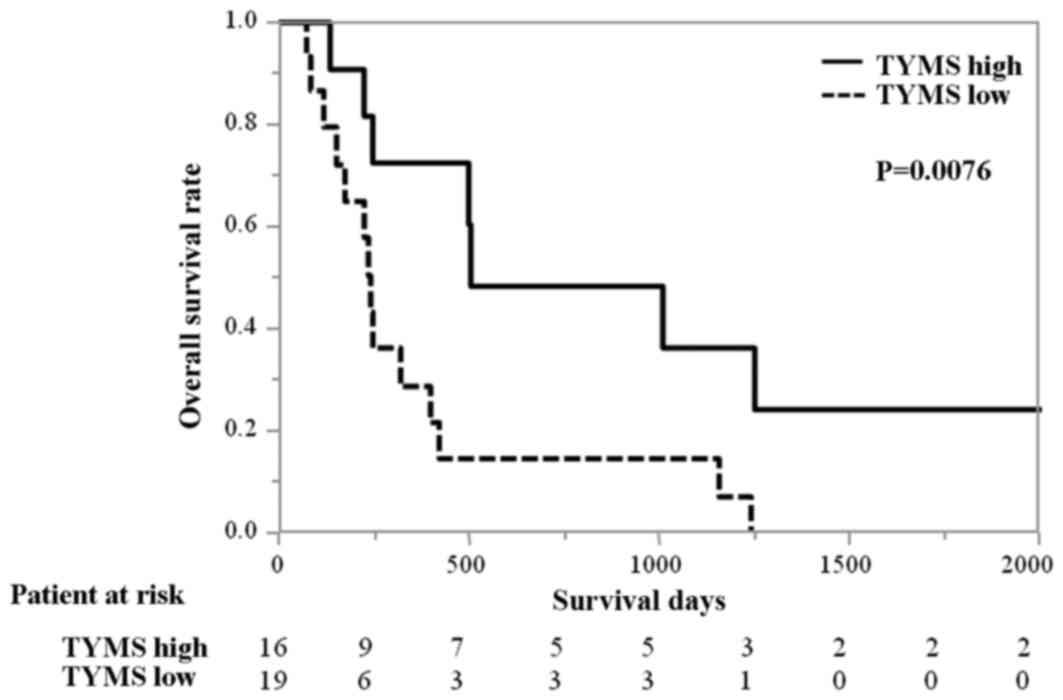
P=0.016; Fig. 2]. Similarly, the OS
time of patients with high levels of TYMS mRNA was
significantly longer compared with those with low levels of
TYMS mRNA (median 503 days vs. 239 days; HR 0.29 [95% CI,
0.100–0.726) P=0.0076; Fig. 3]. The
results of multivariate analysis indicated that the levels of
TYMS and DPYD mRNA were significant independent
prognostic variables (TSYD, P=0.013; DPYD, P=0.0171)
in contrast to age, number of tumors and Child-Pugh score (17).
Gene expression and survival time of
the control group
The gene expression data raised the question of
whether the levels of TYMS and DPYD mRNA are
predictive markers for the efficacy of S-1 therapy or prognostic
markers regardless of administration of S-1. To answer this
question, the levels of TYMS and DPYD mRNAs were
determined in 20 patients with relapsed HCC who never received S-1
or any fluoropyrimidine as a control group. Overall survival time
of this group was calculated from the day of curative surgery to
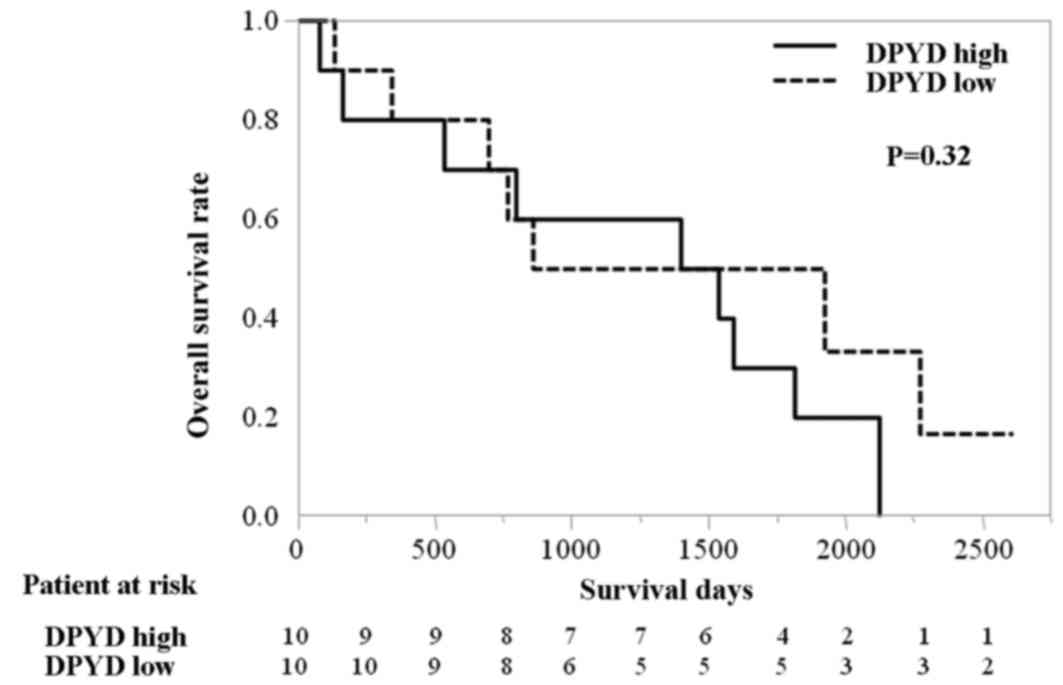
mortality. There was no significant difference in OS time between
patients in the control group with high or low levels of
DPYD mRNA [median, 1,466 vs. 1,391 days; HR 1.69 (95% CI:
0.60–5.11); P=0.32; Fig. 4],
indicating that the levels of DPYD mRNA are a predictive
marker of S-1 efficacy and not a prognostic marker of HCC. There
was a tendency for longer survival time in the high group compared
with the low group that was not statistically significant [median
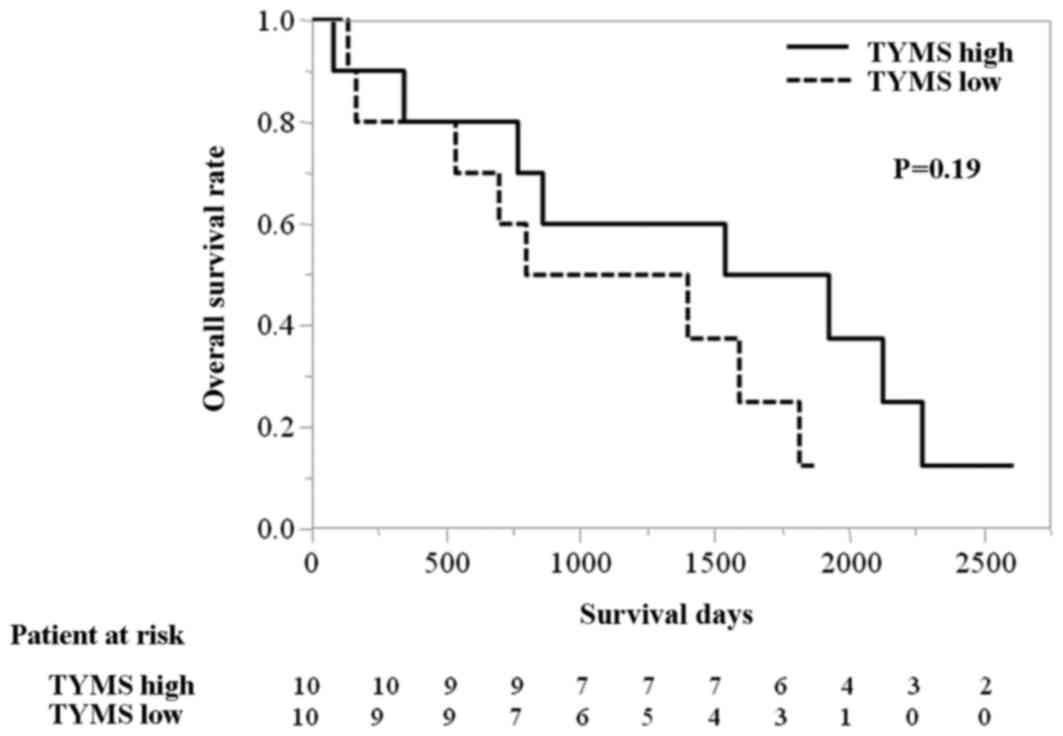
1,729 vs. 1,094 days; HR 0.48 (95% CI: 0.14–1.44), P=0.19; Fig. 5].
Analysis of clinicopathological
parameters according to TYMS and DPYD mRNA expression in each
group
The survival time of the patients with HCC depends
not only on cancer progression but also on liver dysfunction, since
the majority of patients with HCC have liver cirrhosis (17). To determine whether the levels of TYMS
and DPYD mRNA were associated with liver function, patients'
values of serum albumin, indocyanine green retention test (ICG
R15), prothrombin time, total bilirubin and cholinesterase were
analyzed. No significant association was observed between each of
these variables and levels of either of the mRNAs (Tables II and III).
Tumor differentiation, number of tumors, portal vein
invasion, hepatic vein invasion and intrahepatic metastasis were
assessed. A significant difference was observed in the incidence of
hepatic vein invasion in the S-1 group between patients with high
and low levels of TYMS mRNA (P=0.015) and high and low
levels of DPYD mRNA (P=0.043) (Table II), although the difference was
increased in the TYMS high and DPYD high patients, which showed
favorable outcomes. The values of all other parameters were not
significantly different between the TYMS high and low or the DPYD
high and low in S-1 and control groups (Tables II and III).
Discussion
In the present study, high levels of TYMS and
DPYD mRNAs were associated with a significantly longer OS
time in patients with HCC treated with S-1. Using qPCR, Nii et
al (19) measured TYMS and
DPYD mRNA levels in 74 patients with HCC and demonstrated
that the OS time of patients with high levels of DPYD mRNA
was significantly longer compared with patients with low levels of
DPYD mRNA. These findings are in complete agreement with
those of the present study. They also reported that the prognosis
of patients with high levels of TYMS mRNA is more favorable
compared with those with low levels, although the difference was
not statistically significant. They showed that low levels of
DPYD mRNA associate significantly with advanced clinical
stage, undifferentiated histology, microscopic intrahepatic
metastasis and a high Ki-67 labeling index, which lead to poor
prognosis. The present study demonstrated that there was no
significant association between the expression of each mRNA and
differentiation of the tumor, vessel invasion, intrahepatic
metastasis and liver cirrhosis severity (Child-Pugh score).
Although, DPYD mRNA expression is lower in HCC tissues
compared with that of normal liver tissue (20), there is no consensus on whether the
grade of HCC is associated with DPYD mRNA expression.
To determine whether the levels of TYMS and
DPYD mRNA were associated with effectiveness of S-1
chemotherapy (predictive marker) or tumor biology (prognostic
marker), the levels of TYMS and DPYD were determined
in patients with HCC who did not receive S-1 or therapy with any
fluoropyrimidine. There was no significant association between the
levels of DPYD mRNA and prognosis of the control group,
indicating that DPYD mRNA levels are a predictive marker for
S-1 therapy, but not as a prognostic marker. However, the present
study was limited due to its small sample size.
Evidence supports the theory that high levels of
TYMS expression contribute to resistance to 5-FU and that
patients with low levels of TYMS mRNA respond favorably to
treatment with fluoropyrimidines (12,14,21,22).
This is explained by the theory that the 5-FU metabolite
5-fluorodeoxyuridine monophosphate forms a ternary complex with
TYMS and folic acid, which depletes TYMS, leading to the inhibition
of DNA synthesis by tumor cells.
By contrast, several studies demonstrate the
opposite result that high levels of TYMS expression predict
a favorable outcome for patients who are treated with
fluoropyrimidines (23,24). TYMS expression levels predict
the efficacy of fluoropyrimidine therapy and serve as a prognostic
marker of various cancers in advanced stages (24,25). In
the present study, high levels of TYMS mRNA in the control
group indicated a tendency for longer survival time, although the
data were not statistically significant due to the limited number
of samples. Thus, TYMS mRNA levels may have potential to be
a prognostic marker of HCC independent of whether patients undergo
chemotherapy. However, further larger studies were warranted.
The main cytotoxic component of S-1 is tegafur,
which is a prodrug of 5-FU (6). 5-FU
is mainly metabolized in the liver and may therefore be difficult
to administer to patients with liver dysfunction (6). However, previous studies show promising
efficacy of S-1 for treating patients with HCC (7–9). In a
phase I/II study of S-1 therapy administered to patients with HCC,
the response rate was 21.7%, and progression-free survival and OS
times were 3.7 months and 16.6 months, respectively (10). The survival data are comparable with
the outcomes of sorafenib treatment of a cohort of Japanese
patients with HCC (26). There is no
cytotoxic drug with convincing evidence of efficacy when used
systemically to treat HCC. A previous study reported that phase III
randomized study of S-1 in patients with sorafenib refractory
advanced HCC failed to show clinical advantage (11). Therefore, TYMS, DPD or other
biomarkers are anticipated to select the patients who may obtain
clinical benefit.
In conclusion, the present assessed the utility of
TYMS and DPYD mRNAs as potential biomarkers for
patients with HCC who were treated with S-1. These data require
confirmation by conducting a large clinical trial.
Acknowledgements
The present study was checked by Edanz English
Language Services. The authors thank Dr Shunichi Ariizumi for his
careful review of the manuscript. The present study was financially
supported by departmental funds from the Tokyo Women's Medical
University.
References
|
1
|
Altekruse SF, McGlynn KA and Reichman ME:
Hepatocellular carcinoma incidence, mortality, and survival trends
in the United States from 1975 to 2005. J Clin Oncol. 27:1485–1491.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cha C, Fong Y, Jarnagin WR, Blumgart LH
and DeMatteo RP: Predictors and patterns of recurrence after
resection of hepatocellular carcinoma. J Am Coll Surg. 197:753–758.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo W, He X, Li Z and Li Y: Combination of
transarterial chemoembolization (TACE) and radiofrequency ablation
(RFA) vs. surgical resection (SR) on survival outcome of early
hepatocellular carcinoma: A meta-analysis. Hepatogastroenterology.
62:710–714. 2015.PubMed/NCBI
|
|
4
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, De Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shirasaka T, Shimamato Y, Ohshimo H,
Yamaguchi M, Kato T, Yonekura K and Fukushima M: Development of a
novel form of an oral 5-fluorouracil derivative (S-1) directed to
the potentiation of the tumor selective cytotoxicity of
5-fluorouracil by two biochemical modulators. Anticancer Drugs.
7:548–557. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hatano H, Kobayashi S, Nagano H, Tomokuni
A, Tomimaru Y, Murakami M, Marubashi S, Eguchi H, Takeda Y,
Tanemura M, et al: A case of successful multimodal treatment for
combined hepatocellular and cholangiocarcinoma with portal venous
tumor thrombus. Gan To Kagaku Ryoho. 36:2374–2376. 2009.(In
Japanese). PubMed/NCBI
|
|
8
|
Nakamura M, Nagano H, Wada H, Noda T, Ota
H, Damdinsuren B, Marubashi S, Miyamoto A, Takeda Y, Umeshita K, et
al: A case of hepatocellular carcinoma with multiple lung, spleen,
and remnant liver metastasis successfully treated by combination
chemotherapy with the novel oral DPD-inhibiting chemotherapeutic
drug S-1 and interferon-alpha. J Gastroenterol. 41:1120–1125. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suganuma T, Terauchi R, Shikina A, Tanaka
M, Aozasa S, Utsunomiya K, Fujino K, Ito H, Okada K, Tsuda T and
Hase K: A case of hepatocellular carcinoma with bone metastasis
responding to concurrent TS-1/low-dose cisplatin (CDDP) therapy and
radiotherapy. Gan To Kagaku Ryoho. 31:781–784. 2004.(In Japanese).
PubMed/NCBI
|
|
10
|
Furuse J, Okusaka T, Kaneko S, Kudo M,
Nakachi K, Ueno H, Yamashita T and Ueshima K: Phase I/II study of
the pharmacokinetics, safety and efficacy of S-1 in patients with
advanced hepatocellular carcinoma. Cancer Sci. 101:2606–2611. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kudo M, Moriguchi M, Numata K, Hidaka H,
Tanaka H, Ikeda M, Kawazoe S, Ohkawa S, Sato Y, Okusaka T, et al: A
randomized, double-blind, placebo-controlled phase III study of S-1
in patients with sorafenib-refractory advanced hepatocellular
carcinoma (S-CUBE). J Clin Oncol. 33 Supple:abstr 40182015.
|
|
12
|
Salonga D, Danenberg KD, Johnson M,
Metzger R, Groshen S, Tsao-Wei DD, Lenz HJ, Leichman CG, Leichman
L, Diasio RB and Danenberg PV: Colorectal tumors responding to
5-fluorouracil have low gene expression levels of dihydropyrimidine
dehydrogenase, thymidylate synthase, and thymidine phosphorylase.
Clin Cancer Res. 6:1322–1327. 2000.PubMed/NCBI
|
|
13
|
Leichman CG, Lenz HJ, Leichman L,
Danenberg K, Baranda J, Groshen S, Boswell W, Metzger R, Tan M and
Danenberg PV: Quantitation of intratumoral thymidylate synthase
expression predicts for disseminated colorectal cancer response and
resistance to protracted-infusion fluorouracil and weekly
leucovorin. J Clin Oncol. 15:3223–3229. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lenz HJ, Danenberg KD, Leichman CG,
Florentine B, Jonston PG, Groshen S, Zhou L, Xiong YP, Danenberg PV
and Leichman LP: p53 and thymidylate synthase expression in
untreated stage II colon cancer: Associations with recurrence,
survival, and site. Clin Cancer Res. 4:1227–1234. 1998.PubMed/NCBI
|
|
15
|
Kuramochi H, Hayashi K, Uchida K, Miyakura
S, Shimizu D, Vallbohmer D, Park S, Danenberg KD, Takasaki K and
Danenberg PV: 5-fluorouracil-related gene expression levels in
primary colorectal cancer and corresponding liver metastasis. Int J
Cancer. 119:522–526. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnston SJ, Ridge SA, Cassidy J and
McLeod HL: Regulation of dihydropyrimidine dehydrogenase in
colorectal cancer. Clin Cancer Res. 5:2566–2570. 1999.PubMed/NCBI
|
|
17
|
Peng Y, Qi X and Guo X: Child-pugh versus
MELD score for the assessment of prognosis in liver cirrhosis: A
systematic review and meta-analysis of observational studies.
Medicine (Baltimore). 95:e28772016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nii A, Shimada M, Ikegami T, Harino Y,
Imura S, Morine Y, Kanemura H, Arakawa Y and Sugimoto K:
Significance of dihydropyrimidine dehydrogenase and thymidylate
synthase mRNA expressions in hepatocellular carcinoma. Hepatol Res.
39:274–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takahashi T, Yoshida H, Mamada Y, Taniai
N, Mizuguchi Y, Shimizu T, Kakinuma D, Ishikawa Y, Akimaru K,
Sugisaki Y and Tajiri T: Profiling of fluorouracil-related genes by
microdissection technique in hepatocellular carcinoma.
Hepatogastroenterology. 54:1612–1616. 2007.PubMed/NCBI
|
|
21
|
Lenz HJ, Hayashi K, Salonga D, Danenberg
KD, Danenberg PV, Metzger R, Banerjee D, Bertino JR, Groshen S,
Leichman LP and Leichman CG: p53 point mutations and thymidylate
synthase messenger RNA levels in disseminated colorectal cancer: An
analysis of response and survival. Clin Cancer Res. 4:1243–1250.
1998.PubMed/NCBI
|
|
22
|
Metzger R, Leichman CG, Danenberg KD,
Danenberg PV, Lenz HJ, Hayashi K, Groshen S, Salonga D, Cohen H,
Laine L, et al: ERCC1 mRNA levels complement thymidylate synthase
mRNA levels in predicting response and survival for gastric cancer
patients receiving combination cisplatin and fluorouracil
chemotherapy. J Clin Oncol. 16:309–316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Edler D, Glimelius B, Hallström M,
Jakobsen A, Johnston PG, Magnsson I, Ragnhammar P and Blomgren H:
Thymidylate synthase expression in colorectal cancer: A prognostic
and predictive marker of benefit from adjuvant fluorouracil-based
chemotherapy. J Clin Oncol. 20:1721–1728. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koumarianou A, Tzeveleki I, Mekras D,
Eleftheraki AG, Bobos M, Wirtz R, Fountzilas E, Valavanis C,
Xanthakis I, Kalogeras KT, et al: Prognostic markers in early-stage
colorectal cancer: Significance of TYMS mRNA expression. Anticancer
Res. 34:4949–4962. 2014.PubMed/NCBI
|
|
25
|
Zhao HY, Ma GW, Zou BY, Li M, Lin SX, Zhao
LP, Guo Y, Huang Y, Tian Y, Xie D and Zhang L: Prognostic
significance of thymidylate synthase in postoperative non-small
cell lung cancer patients. Onco Targets Ther. 7:1301–1310. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakano M, Tanaka M, Kuromatsu R, Nagamatsu
H, Sakata K, Matsugaki S, Kajiwara M, Fukuizumi K, Tajiri N,
Matsukuma N, et al: Efficacy, safety, and survival factors for
sorafenib treatment in Japanese patients with advanced
hepatocellular carcinoma. Oncology. 84:108–114. 2013. View Article : Google Scholar : PubMed/NCBI
|