Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common malignancy and the third cause of tumor associated deaths
worldwide (1). The curative therapies
currently available for HCC are liver resection, local ablative
treatments and liver transplantation (2). Due to limited donors, rigorous
indication for local ablative treatments, liver tumor resection is
the main curative treatment for HCC in clinical practices (3). However, the recurrence rate after
hepatic resection was over 70% in 5 years (4). The high recurrence and metastasis are
closely related to poor prognosis of HCC patients after radical
treatment. Currently, there is no effective way to radically
prevent recurrence and metastasis of HCC. Therefore, it is of great
clinical significance to accurately determine the prognosis and
take the corresponding individualized treatment after surgery,
which will be helpful to improve the long-term survival of HCC
patients.
Human urotensin II (UII), isolated from the
urophysis of teleost fish, is an undecapeptide (H-Glu-Thr-Pro-Asp-c
[Cys-Phe-Trp-Lys-Tyr-Cys]-Val-OH). The orphan G-protein coupled
receptor 14 was identified as the urotensin II receptor (UTR)
(5). As a powerful vasoactive peptide
in mammals, initially UII/UTR is reported to play an important role
in portal hypertension, renal disease and heart failure (5). Recent studies demonstrated that UII/UTR
may involve in tumorigenesis and tumor progression in many
malignant tumors (6), such as adrenal
gland neoplasms (7), breast carcinoma
(8), pulmonary adenocarcinoma
(9), renal cell carcinoma and colon
carcinoma (10). Our previous study
demonstrated that UII and UTR are up-regulated in rat HCC model and
human HCC tissue, exogenous UII can increase hepatic oval cell and
HCC cell proliferation in vitro (11–13). All
these results indicated that UII played an important role in
initiation and progression of HCC. And in our preliminary
experiment, we also found that the intensity of UTR expression on
HCC tissues varies from patient to patient. So, we wonder whether
UTR has a clinical significance in HCC patients and whether UTR
plays a role on HCC development.
The aim, in the present study, was to determine
whether UTR could be as a biomarker to predict the outcomes of HCC
patients underwent radical treatment. The effects of UTR on HCC
cell motility and invasion was also explored.
Materials and methods
Patients and tissue samples
HCC patients underwent curative resection at Beijing
Youan Hospital (Beijing, China) from January 2010 to March 2013
were enrolled. Patients had a history of malignancy, or previous
anticancer therapy, or detectable distant metastases, or carrying
tumor residual after surgery, or received special treatment (such
as gene therapy, molecular targeted drug therapy) during follow-up,
were excluded from the present study. HCC were staged according to
the TNM staging system of the Union for International Cancer
Control/American Joint Committee on Cancer (AJCC, 7th edition), and
Barcelona Clinic Liver Cancer (BCLC) staging system. The
clinicopathological characteristics of patients were retrieved from
the medical records and summarized in Table I. The study was approved by the Ethics
Committee of Beijing Youan Hospital, Capital Medical University.
Written informed consent was obtained from each patient.
 | Table I.Correlation between UTR expressions
and clinicopathologic variables of patients with HCC. |
Table I.
Correlation between UTR expressions
and clinicopathologic variables of patients with HCC.
|
|
| Expression of
UTR |
|
|---|
|
|
|
|
|
|---|
| Variables | Total (n=83) | Low (n=44) | High (n=39) | P-valuea |
|---|
| Sex (%) |
|
|
| 0.948 |
| Male | 70 (84.34) | 37 (84.09) | 33 (84.62) |
|
|
Female | 13 (15.66) | 7
(15.91) | 6
(15.38) |
|
| Age (years) (%) |
|
|
| 0.089 |
| ≤50 | 45 (54.22) | 20 (45.45) | 25 (64.10) |
|
|
>50 | 38 (45.78) | 24 (54.55) | 14 (35.90) |
|
| Underlying liver
disease (%) |
|
|
| 0.432 |
| HBV | 76 (91.57) | 39 (88.64) | 37 (94.87) |
|
| HCV | 5 (6.02) | 4 (9.09) | 1 (2.56) |
|
|
Others | 2 (2.41) | 1 (2.27) | 1 (2.56) |
|
| Tumor location
(%) |
|
|
| 0.271 |
| Right
lobe | 61 (73.49) | 32 (72.73) | 29 (74.36) |
|
| Left
lobe | 16 (19.28) | 9
(20.45) | 7
(17.95) |
|
|
Bilateral | 4 (4.82) | 1 (2.27) | 3 (7.69) |
|
|
Caudate | 2 (2.41) | 2 (4.55) | 0
(0) |
|
| Tumor number (%) |
|
|
| 0.002b |
|
Single | 60 (72.29) | 38 (86.36) | 22 (56.41) |
|
|
Multiple | 23 (29.71) | 6
(13.64) | 17 (43.59) |
|
| Tumor size (cm) |
|
|
| 0.017b |
|
<3 | 30 (36.14) | 22 (50.00) | 8
(20.51) |
|
| 3–5 | 31 (37.35) | 14 (31.82) | 17 (43.59) |
|
|
>5 | 22 (26.51) | 8
(18.18) | 14 (35.90) |
|
| AFP (ng/ml)
(%) |
|
|
| 0.296 |
|
<20 | 36 (43.37) | 21 (47.73) | 15 (38.46) |
|
|
20–400 | 22 (26.51) | 13 (29.55) | 9
(23.08) |
|
|
>400 | 25 (30.12) | 10 (22.73) | 15 (38.46) |
|
| TNM stage (%) |
|
|
| 0.000b |
|
I+II | 57 (68.67) | 41 (93.18) | 16 (41.03) |
|
|
III+IV | 26 (31.33) | 3 (6.82) | 23 (58.97) |
|
| BCLC HCC stage
(%) |
|
|
| 0.000b |
| A | 42 (50.60) | 30 (68.18) | 12 (30.77) |
|
| B | 22 (26.51) | 12 (27.27) | 10 (25.64) |
|
| C | 19 (22.89) | 2 (4.55) | 17 (43.59) |
|
| Child-Pugh class
(%) |
|
|
| 0.139 |
| A | 75 (90.36) | 42 (95.45) | 33 (84.62) |
|
| B | 8 (9.64) | 2 (4.55) | 6
(15.38) |
|
| Histologic grade
(%) |
|
|
| 0.021b |
|
Poorly | 18 (21.69) | 5
(11.36) | 13 (33.33) |
|
|
Moderate | 52 (62.65) | 29 (65.91) | 23 (58.97) |
|
|
Well | 13 (15.66) | 10 (22.73) | 3 (7.69) |
|
| Tumor recurrence
(%) |
|
|
| 0.000b |
| No | 43 (51.81) | 31 (74.45) | 12 (30.77) |
|
|
Yes | 40 (48.19) | 13 (29.55) | 27 (69.23) |
|
| Mortality (%) |
|
|
| 0.000b |
| No | 65 (78.31) | 41 (93.18) | 24 (61.54) |
|
|
Yes | 18 (21.69) | 3 (6.82) | 15 (38.46) |
|
Follow-up and endpoints
All enrolled patients were follow-up in real-life
clinical practice by outpatient clinic or telephone. The primary
endpoints were death or 3 years (36 months) follow-up, and the
secondary endpoint was HCC recurrence.
In total, 14 (16.87%) patients were lost to
follow-up. Forty patients (48.19%) recurrence and 18 patients
(21.69%) death were observed during 3 years follow-up.
Immunohistochemistry (IHC)
Formalin-fixed, paraffin-embedded HCC tissues
samples were collected from the 83 patients above-mentioned. The
immunohistostaining (IHS) was routinely done. In briefly, the
sections were incubated with a specific antibody against UTR 1:200
[GPR14 (M-250): sc-28998; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA] at 4°C for overnight and then incubated with a
second antibody with broad spectrum (Invitrogen, Carlsbad, CA, USA)
at 37°C for 20 min. After washed by PBS for three times, the
visualization signal was used by a 3,3′-diaminobenzidine and
counterstained with hematoxylin. The result of IHS was separately
determined by two experienced pathologists. UTR scores were
determined by assessing both staining intensity and the proportion
of positively stained tumor cells. IHS intensity was divided into
0, no positive staining; 1, positive staining was weak yellow; 2,
positive staining was yellow and 3, strong positive staining was
brown. The mean percentage of positive tumor cells was determined
in five fields under ×400 magnification. UTR positive expression
was estimated as staining intensity × mean percentage of positive
tumor cells. The scores ≤4 was regarded as UTR low expressions and
5–12 as UTR high expressions.
Western blot analysis
The UTR protein concentration was determined using a
BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA)
as described in the manufacturer's manual. Lysate protein (80 µg)
was separated on 10% SDS-PAGE gels and transferred to a
polyvinylidene fluoride membrane. After blotting, the membrane was
blocked in 5% skim milk in TBST for 1 h at room temperature and
then incubated with the specific primary antibody against UTR
(1:500) and GAPDH (1:5,000) at 4°C overnight. After well washed by
TBST, membranes were then incubated with horseradish
perxoidase-conjugated secondary antibody (1:5,000). The membrane
was developed by enhanced chemiluminescence detecting reagents. And
densities of specific proteins were normalized according to the
amount of total protein and GAPDH.
Cell lines
Human hepatic cell line QSG-7701, human HCC cell
line BEL-7402 (obtained from the Cell Bank of the Chinese Academy
of Sciences, Shanghai, China), and MHCC-97H cells (human HCC cell
lines with high metastatic potential, established at the Liver
Cancer Institute, Zhongshan Hospital, Fudan University, Shanghai,
China) were maintained in Dulbecco's modified Eagle's medium
containing 10% of fetal calf serum and antibiotics (100 U/ml
penicillin and 100 µg/ml streptomycin). The cells were harvested in
the logarithmic phase of growth and serum-starved for 8 h before
used in experiments outlined below.
Small interfering RNA-mediated UTR
gene silencing
The expression of human UTR was knocked down using
small interfering RNA (siRNA) duplexes (two sequences and one
control siRNA). The two pre-designed siRNA (1-sense, 5′CCA UGU ACG
UCU ACG UGG UTR T-3′ and antisense, 5′-ACCACGUAGACGUACAUGGAG-3′;
2-sense, 5′ACG CAA CCC UCA ACA GCU Ctt-3′ and antisense,
5′-GAGCUGUUGAGGGUUGCGUtg-3′) were bought from Life Technologies
Corp., (Carlsbad, CA, USA). Fluorescein Conjugate (A: sc-36869,
Santa Cruz Biotechnology, Inc.) was used as control. Cells (105) in
the exponential growth phase were inoculated in 6-well plates and
cultured for 24 h. According to the manufacturer's recommended
protocol, cells were serum-starved for 8 h before transfected with
10 µM siRNA in serum free medium (Opti-MEM; Gibco, Grand Island,
NY, USA). The result of transfection was analyzed by western blot
analysis.
In vitro invasion and motility
assays
Transfected cells (2×104) in 200 µl serum free
medium were added to the upper compartment of MilliCell (12 mm
diameter with 8 µm pores) chambers which were pre-coated with
Matrigel (Corning Inc., Corning, NY, USA), and the chambers were
placed into 24-well plates with 0.5 ml complete medium. After 24 h
cultivation at 37°C, chambers were taken out and washed with PBS (5
times, each for 5 min) after the medium was discarded. Immediately,
the chambers were fixed in 24-well plates with 1 ml absolute ethyl
alcohol for 15 min. washed with PBS for 3 times, the chambers were
then stained with 1 ml crystal violet (0.1%) for another 15 min.
Cells were well washed with PBS buffer. Then cells in the upper
compartment of chambers were wiped away, and which in the lower
compartment of chambers were observed by light microscope. The
amount of five high power fields per chamber were counted and mean
value was calculated. The invasive activity was quantified from at
least three individual chambers. The migration assay is similarly
performed using invasion assay excepting that no Matrigel was
used.
Statistical analysis
Data were analyzed with SPSS Statistics software,
version 22.0 (IBM Corporation, Armonk, NY, USA). Differences among
the categorical variables and quantitative variables were analyzed
using Chi-square and the paired Wilcoxon signed rank test/unpaired
t-test, respectively. Univariate and multivariate Cox proportional
hazards analyses were used to assess the effects of various factors
on prognosis. Kaplan-Meier analysis was used to assess
recurrence-free survival (RFS)/overall survival (OS), and log-rank
tests were used to compare them between the subgroups. All P-values
were two-sided, and P<0.05 was considered to be statistically
significant.
Results
The clinical characteristics of
enrolling patients
In a total of 83 enrolled HCC patients, 77 (92.77%)
patients underwent lobectomy and 8 (7.23%) patients underwent
hemihepatectomy. There were 70 men and 13 women (84.34% vs.
15.66%). The age of 45 (54.22%) patients were under 50 years. A
total of 76 (91.57%) patients had chronic hepatitis B, and 5
(6.02%) had chronic hepatitis C. Majority (73.49%) of the tumor was
located at right lobe. A total of 23 (29.71%) patients had more
than one tumor in their liver. The tumor sizes in 30 (36.14%)
patients were no more than 3 cm, while in 22 (26.51%) patients were
more than 5 cm. Serum α-fetoprotein (AFP) levels in 36 (43.37%)
patients were less than 20 ng/ml, that in 22 (26.51%) patients
range between 20 to 400 ng/ml, and in 25 (30.12%) patients were
above 400 ng/ml. According to TNM stage classification, 57 (68.67%)
patients were I or II stage. While according to BCLC staging, 42
(50.60%) patients were stage A, 22 (26.51%) stage B, 19 (22.89%)
stage C. Poor differentiation was found in 18 (21.69%) patients,
and moderate differentiation was found in 52 (62.65%) patients, and
well differentiation was found in 13 (15.66%) patients.
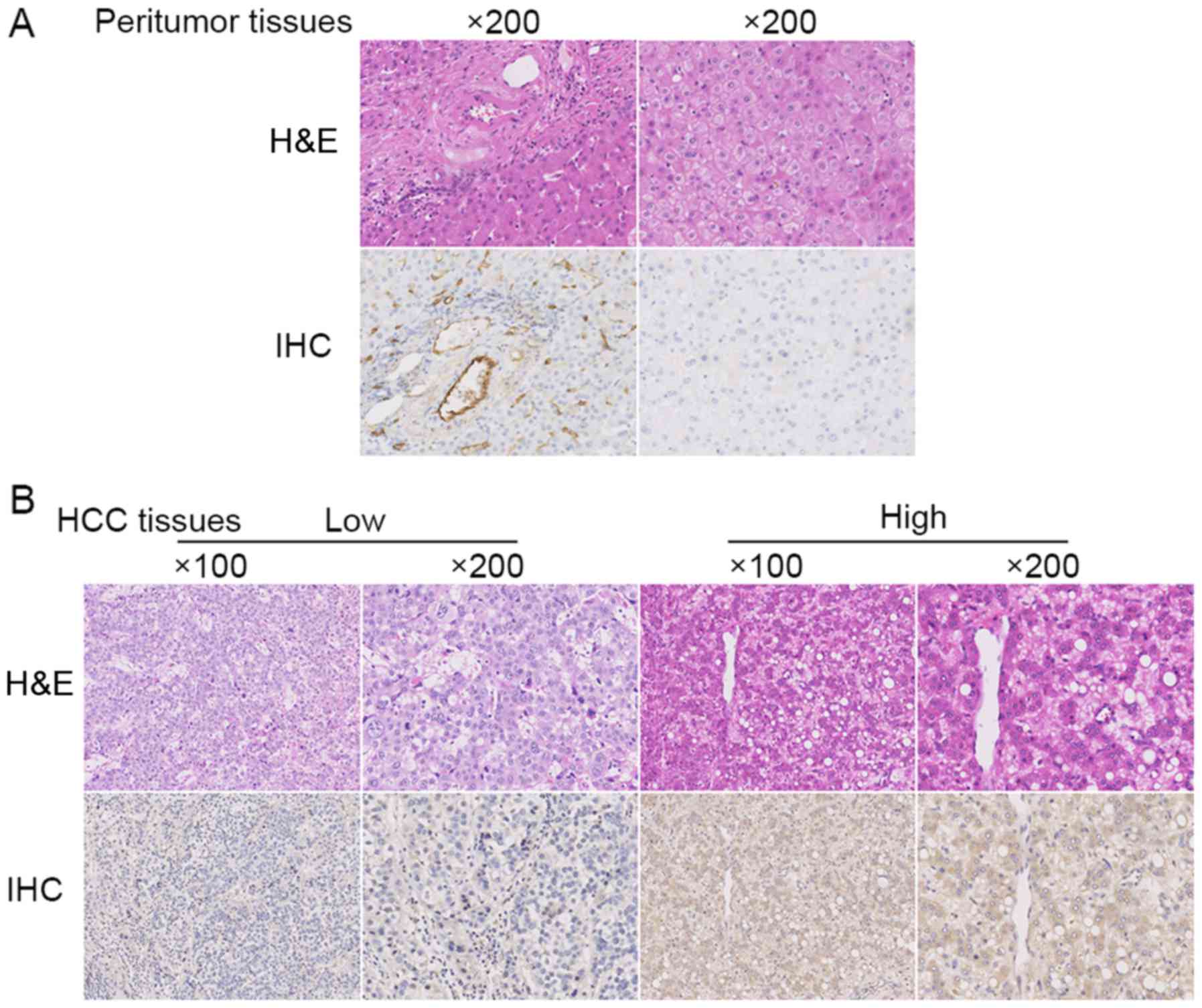
UTR expression characteristic on
tissues
In the peritumor tissues, UTR staining was positive
in the vessels and portal area, and the little staining or negative
in the normal liver cells (Fig. 1A).
Whereas in HCC tissues, UTR staining was detected positive in the
cytomembrane and cytoplasm of HCC cells (Fig. 1B).
Relationship between expressions of
UTR and clinicopathological characteristics
Based on UTR expressions determined by IHC, the HCC
patients were divided into two subgroups: low UTR expressions group
(N=44) and high UTR expressions group (N=39). The
clinicopathological characteristics between the two subgroups were
shown in Table I. Those with high
expression levels of UTR had higher stage of HCC progression, such
as higher TNM stage and BCLC stage (P=0.000, P=0.000), more
multiple tumor, bigger tumor size, poorer histologic grade, high
tumor recurrence and mortality (P=0.004, 0.026, 0.021, 0.000 and
0.000, respectively). There were no correlation between UTR
expressions and sex, age, chronic liver disease, tumor location,
serum AFP, and Child-Pugh class (P>0.05).
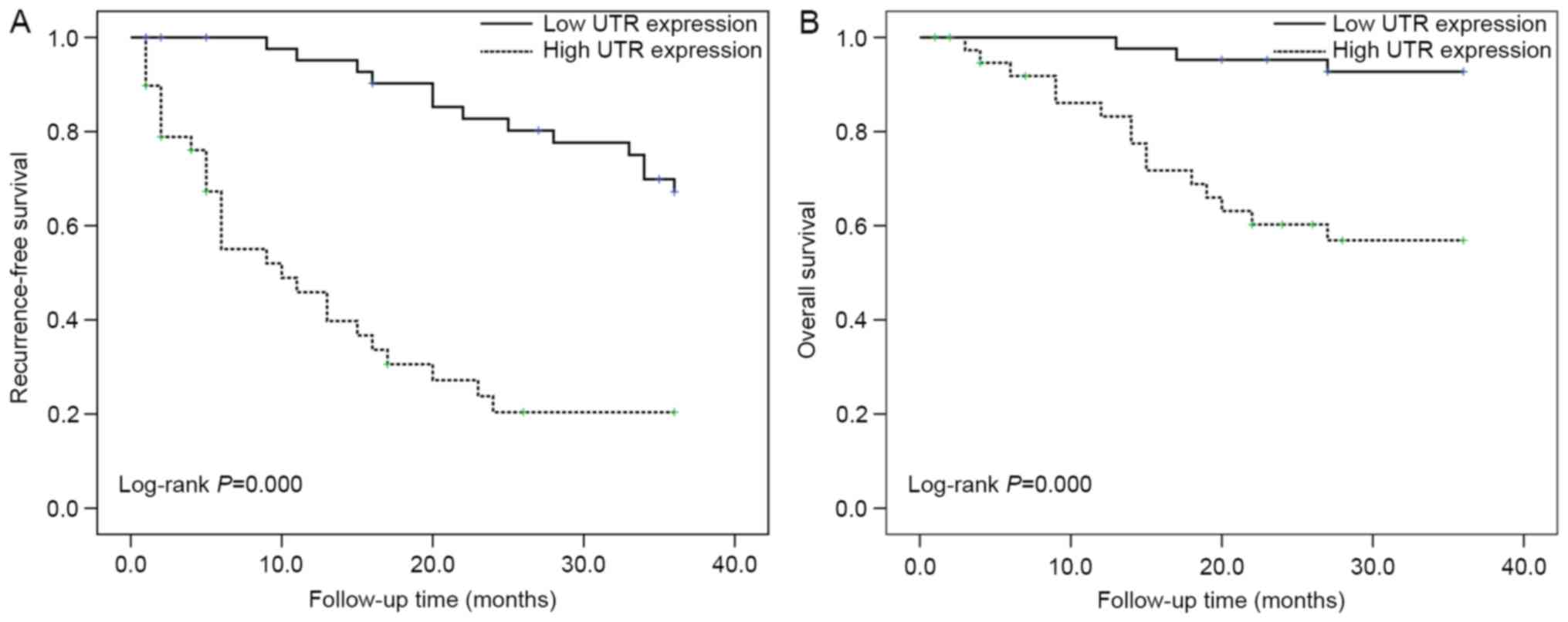
RFS and OS
The relationship between RFS/OS and UTR expressions
were summarized in Table II. Three
parameters including tumor number (P=0.009), TNM stage (P=0.000),
and high expression of UTR (P=0.000) were significantly related to
RFS. The TNM stage, Child-pugh class and UTR high expressions
(P=0.002, P=0.013 and P=0.001) were also significantly related to
OS. It found that UTR high expressions were an independent
prognostic factor for RFS and OS (P=0.004 and 0.038, respectively).
The Kaplan-Meier curve and log-rank test also indicated that UTR
expressions was associated with RFS and OS in HCC patients (P=0.000
and P=0.000; Fig. 2A and B).
 | Table II.Univariate and multivariate Cox
proportional hazards analyses of recurrence-free survival and
overall survival. |
Table II.
Univariate and multivariate Cox
proportional hazards analyses of recurrence-free survival and
overall survival.
|
|
| Recurrence-free
survival | Overall
survival |
|---|
|
|
|
|
|
|---|
|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|
|---|
| Variables | Categories | Risk ratio | 95% CI | P-value | Risk ratio | 95% CI | P-value | Risk ratio | 95% Cl | P-value | Risk ratio | 95% CI | P-value |
|---|
| Sex | Male | Reference
group |
|
|
|
|
| Reference
group |
|
|
|
|
|
|
| Female | 0.653 | 0.256–1.668 | 0.373 |
|
|
| 0.574 | 0.132–2.496 | 0.459 |
|
|
|
| Age (years) | ≤50 | Reference
group |
|
|
|
|
| Reference
group |
|
|
|
|
|
|
| >50 | 1.030 | 0.554–1.917 | 0.925 |
|
|
| 0.711 | 0.276–1.836 | 0.481 |
|
|
|
| Liver disease | HBV | Reference
group |
|
|
|
|
| Reference
group |
|
|
|
|
|
|
| HCV/others | 0.611 | 0.147–2.534 | 0.497 |
|
|
| 1.319 | 0.303–5.737 | 0.712 |
|
|
|
| Tumor location | Right lobe | Reference
group |
|
|
|
|
| Reference
group |
|
|
|
|
|
|
| Other lobes | 0.865 | 0.423–1.770 | 0.691 |
|
|
| 0.514 | 0.149–1.778 | 0.293 |
|
|
|
| Tumor nubmer | Single | Reference
group |
|
|
|
|
| Reference
group |
|
|
|
|
|
|
| multiple | 2.356 | 1.235–4.495 | 0.009a | 1.403 | 0.706–2.787 | 0.333 | 1.860 | 0.720–4.802 | 0.200 |
|
|
|
| Tumor size | <3 | Reference
group |
|
|
|
|
| Reference
group |
|
|
|
|
|
|
| 3–5 | 1.409 | 0.621–3.196 | 0.412 |
|
|
| 4.135 | 0.859–19.913 | 0.077 |
|
|
|
|
| >5 | 4.128 | 1.911–8.918 | 0.000a |
|
|
| 8.025 | 1.731–37.216 | 0.008a |
|
|
|
| AFP | <20 | Reference
group |
|
|
|
|
| Reference
group |
|
|
|
|
|
|
| 20–400 | 1.367 | 0.612–3.051 | 0.446 |
|
|
| 0.781 | 0.195–3.123 | 0.727 |
|
|
|
|
| >400 | 2.419 | 1.158–5.055 | 0.019a |
|
|
| 2.617 | 0.930–7.360 | 0.068 |
|
|
|
| TNM stage | I–II | Reference
group |
|
|
|
|
| Reference
group |
|
|
|
|
|
|
| III | 5.604 | 2.870–10.942 | 0.000a | 2.756 | 1.275–5.957 | 0.010a | 4.663 | 1.802–12.066 | 0.002a | 1.912 | 0.647–5.650 | 0.241 |
| BCLC HCC stage | A | Reference
group |
|
|
|
|
| Reference
group |
|
|
|
|
|
|
| B | 1.785 | 0.857–3.719 | 0.122 |
|
|
| 1.181 | 0.282–4.943 | 0.820 |
|
|
|
|
| C | 3.312 | 1.518–7.226 | 0.003a |
|
|
| 6.360 | 2.163–18.701 | 0.001a |
|
|
|
| Child-Pugh
class | A | Reference
group |
|
|
|
|
| Reference
group |
|
|
|
|
|
|
| B | 0.930 | 0.286–3.025 | 0.904 |
|
|
| 3.705 | 1.316–10.432 | 0.013a | 1.822 | 0.627–5.293 | 0.270 |
| Histologic
grade | Poorly | Reference
group |
|
|
|
|
| Reference
group |
|
|
|
|
|
|
| Moderate/well | 0.841 | 0.386–1.832 | 0.663 |
|
|
| 0.518 | 0.194–1.382 | 0.189 |
|
|
|
| UTR expression | Low | Reference
group |
|
|
|
|
| Reference
group |
|
|
|
|
|
|
| High | 5.480 | 2.771–10.839 | 0.000a | 3.275 | 1.454–7.376 | 0.004a | 7.603 | 2.195–26.334 | 0.001a | 4.578 | 1.087–19.277 | 0.038a |
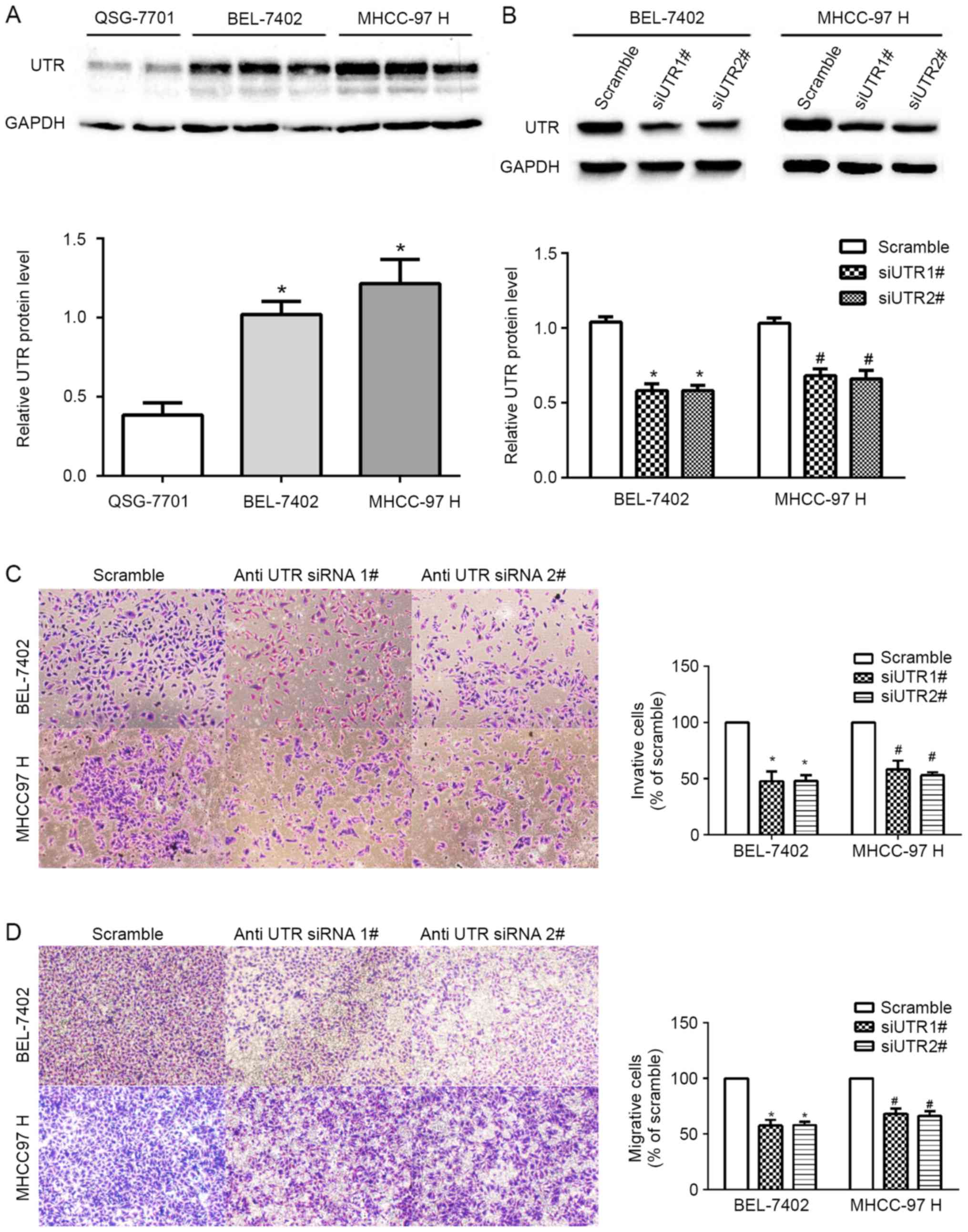
siRNA-mediated UTR gene silencing
First, we determined the expression of UTR in two
HCC cell lines and one normal cell line using western blot
analysis. We found higher UTR expressions in the HCC cell lines
(BEL-7402 and MHCC-97H) than in normal cell lines (QSG-7701)
(Fig. 3A). Then, we knocked down UTR
gene using two defined siRNAs (methods above-mentioned) in BEL-7402
and MHCC-97H cells, respectively. The level of UTR protein,
normalized by GAPDH was obviously reduced compared with that in the
negative control cells (Fig. 3B).
Furthermore, Transwell invasion assays revealed that silencing UTR
expression decreased the invasion of the BEL-7402 and MHCC-97H
cells compared to the control cells (P<0.05; Fig. 3C). Silencing UTR expression was also
decreased the migration of HCC cells (P<0.05; Fig. 3D). These results indicated the
positive role of UTR in migration and invasion of human HCC cell
lines in vitro.
Discussion
Our study shows that UTR is the potential biomarker
to predict the prognosis in HCC patients after radical liver
resection, and patient with a higher expression of UTR always has a
worse prognosis than that with a lower expression of UTR. The
evidence are as follows: Firstly, we demonstrate that UTR
expression was associated with HCC malignant features, such as HCC
stage, tumor number and tumor size; secondly, patients with a
higher UTR expression level tend towards a high recurrence and
mortality rate after resection, and survival curves (RFS and OS)
showed significant difference between the two subgroups; finally,
univariate and multivariate analysis found that UTR was an
independent risk factor for predicting RFS and OS. In the light of
these results, we suggest those patients with high UTR expression
should be closely monitored or taken prophylactic treatments, such
as molecular targeted drug therapy.
In several other malignancies, upregulation of UTR
has been observed to have a relationship with poor prognosis
(6). Federico et al (10) reported that UTR may play a role in
colon carcinogenesis, when they found that UTR is expressed at a
higher positive rate in colon adenocarcinomas than in adenomatous
polyps and normal epithelial cells (65–90, 30–48 and 5–30%,
respectively). De Cobelli et al (14) and Grieco et al (15) suggested that UTR could be considered
as prognostic marker in human prostate carcinoma patients. Franco
et al (16) reported that UTR
expression determines prognosis of bladder cancer, through
discriminating non-muscle-invasive bladder transitional cancer
(NMIBC) from muscle-invasive bladder transitional cancer and
predicting the risk of relapses in NMIBCs. Consistent with these
findings, we found that high UTR expression level was associated
with poor prognosis in HCC patients after radical liver
resection.
Migration and invasion are the embodiment of tumor
metastasis ability. Evidence shows that tumor metastasis occurs at
an early stage (17–19), and suggests that early metastasis is
one of the major causes of recurrence and poor prognosis after
surgical resection (20). Moreover,
several studies have reported that UTR can stimulate the migration
and invasion of many malignant cell lines. For example, it is
reported that UTR is involved in the regulation of motility and
invasion of colon cancer (10),
bladder cancer cells (16) and
prostate adenocarcinoma cells (15).
Furthermore, we found a positive correlation between UTR expression
and HCC cells metastatic potential (UTR levels, MHCC-97H >
BEL-7402 >> QSG-7701) in vitro study. Therefore, we
speculate UTR may mediate cell invasion and migration in HCC cells.
In order to investigate our speculation, we used two siRNAs to
downregulate UTR level and then monitored cell motility behavior
in vitro. Consistent with our speculation, the results
showed that UTR knockdown in HCC cells reduced migration and
invasion. Our results are complementary to our previous studies, in
which UII/UTR system is found expressing differences between tumor
and peri-tumor, and promoting tumorigenesis in hepatic progenitor
cell (9). These studies together
suggest UTR as a target for future HCC therapies because it plays
an important role in tumorigenesis and tumor progression.
In our study, information of the caval/portal
thrombosis during the follow-up were not included. It would be
better to correlate them to UTR expression at the moment of the HCC
recurrence. In clinical practice, it is difficult to evaluate
wheather or not caval/portal thrombosis exist, especially in
patients followed-up by telephone, without obtaining all-around
medical information. And in future study, it is possible to carry
out the correlation between UTR and caval/portal thrombosis in
selected patients with good compliance.
The drawbacks of this study cannot be ignored. Due
to small sample size and retrospective study, a more comprehensive
analysis was not done for the risk factors determining poor OS and
RFS, such as platelet count, microscopic vascular invasion,
anatomic resection, grade of inflammation and antiviral
therapy.
In conclusion, our novelty findings indicate that
UTR may be regarded as a novel biomarker to predict outcomes after
radical liver resection. It is also suggested that UTR as a
potential therapeutic target inhibited invasion and metastasis of
HCC.
Poor prognosis of hepatocellular carcinoma (HCC)
patients is closely related to high recurrence, invasion and
metastasis after radical treatment. In this study, we reported some
novelty findings that urotensin II receptor (UTR) overexpression
was associated with tumor number and size, histology, TNM/BCLC HCC
stage, recurrence and mortality, and also correlated with
recurrence-free survival and overall survival in HCC patients
underwent curative liver resection. Furthermore, siRNA-mediated
silencing UTR expression inhibited HCC cell motility and Invasion.
Our novelty findings indicate that UTR may be regarded as a novel
biomarker to predict outcomes after radical liver resection and as
a potential therapeutic target for HCC.
Acknowledgements
This study was supported by grants from National
Natural Science Foundation of China (grant no. 81272757 and
81672725), the Project of Construction of Innovative Teams and
Teacher Career Development for Universities and Colleges under
Beijing Municipality (grant no. IDHT20150502), the Capital Science
and Technology Development Fund (2014-1-2181), Beijing Municipal
Administration of Hospitals Clinical Medicine Development of
Special Funding (ZYLX201610) and Beijing Municipal Administration
of Hospitals' Ascent Plan (DFL20151602).
Glossary
Abbreviations
Abbreviations:
|
AFP
|
α-fetoprotein
|
|
HCC
|
hepatocellular carcinoma
|
|
OS
|
overall survival
|
|
RFS
|
recurrence-free survival
|
|
UII
|
urotensin II
|
|
UTR
|
urotensin II receptor
|
|
IHC
|
Immunohistochemistry
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Wallace MC, Preen D, Jeffrey GP and Adams
LA: The evolving epidemiology of hepatocellular carcinoma: A global
perspective. Expert Rev Gastroenterol Hepatol. 9:765–779.
2015.PubMed/NCBI
|
|
2
|
Au JS and Frenette CT: Management of
hepatocellular carcinoma: Current status and future directions. Gut
Liver. 9:437–448. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balogh J, Victor D III, Asham EH,
Burroughs SG, Boktour M, Saharia A, Li X, Ghobrial RM and Monsour
HP Jr: Hepatocellular carcinoma: A review. J Hepatocell Carcinoma.
3:41–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mlynarsky L, Menachem Y and Shibolet O:
Treatment of hepatocellular carcinoma: Steps forward but still a
long way to go. World J Hepatol. 7:566–574. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Proulx CD, Holleran BJ, Lavigne P, Escher
E, Guillemette G and Leduc R: Biological properties and functional
determinants of the urotensin II receptor. Peptides. 29:691–699.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Federico A, Zappavigna S, Dallio M, Misso
G, Merlino F, Loguercio C, Novellino E, Grieco P and Caraglia M:
Urotensin-II receptor: A double identity receptor involved in
vasoconstriction and in the development of digestive tract cancers
and other tumors. Curr Cancer Drug Targets. 17:109–121. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takahashi K, Totsune K, Murakami O and
Shibahara S: Expression of urotensin II and urotensin II receptor
mRNAs in various human tumor cell lines and secretion of urotensin
II-like immunoreactivity by SW-13 adrenocortical carcinoma cells.
Peptides. 22:1175–1179. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Balakan O, Kalender ME, Suner A, Cengiz B,
Oztuzcu S, Bayraktar R, Borazan E, Babacan T and Camci C: The
relationship between urotensin II and its receptor and the
clinicopathological parameters of breast cancer. Med Sci Monit.
20:1419–1425. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu YQ, Song Z, Zhou CH, Xing SH, Pei DS
and Zheng JN: Expression of urotensin II and its receptor in human
lung adenocarcinoma A549 cells and the effect of urotensin II on
lung adenocarcinoma growth in vitro and in vivo.
Oncol Rep. 24:1179–1184. 2010.PubMed/NCBI
|
|
10
|
Federico A, Zappavigna S, Romano M, Grieco
P, Luce A, Marra M, Gravina AG, Stiuso P, D'Armiento FP, Vitale G,
et al: Urotensin-II receptor is over-expressed in colon cancer cell
lines and in colon carcinoma in humans. Eur J Clin Invest.
44:285–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Dong K, Xue X, Feng P and Wang X:
Elevated expression of urotensin II and its receptor in
diethylnitrosamine-mediated precancerous lesions in rat liver.
Peptides. 32:382–387. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu X, Wang P, Shi Z, Dong K, Feng P, Wang
H and Wang X: Urotensin-II-mediated reactive oxygen species
generation via NADPH oxidase pathway contributes to hepatic oval
cell proliferation. PLoS One. 10:e01444332015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu XT, Wang PY, Shi ZM, Dong K, Feng P,
Wang HX and Wang XJ: Up-regulation of urotensin II and its receptor
contributes to human hepatocellular carcinoma growth via activation
of the PKC, ERK1/2, and p38 MAPK signaling pathways. Molecules.
19:20768–20779. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Cobelli O, Buonerba C, Terracciano D,
Bottero D, Lucarelli G, Bove P, Altieri V, Coman I, Perdonà S,
Facchini G, et al: Urotensin II receptor on preoperative biopsy is
associated with upstaging and upgrading in prostate cancer. Future
Oncol. 11:3091–3098. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grieco P, Franco R, Bozzuto G, Toccacieli
L, Sgambato A, Marra M, Zappavigna S, Migaldi M, Rossi G, Striano
S, et al: Urotensin II receptor predicts the clinical outcome of
prostate cancer patients and is involved in the regulation of
motility of prostate adenocarcinoma cells. J Cell Biochem.
112:341–353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Franco R, Zappavigna S, Gigantino V, Luce
A, Cantile M, Cerrone M, Facchini G, Perdonà S, Pignata S, Di
Lorenzo G, et al: Urotensin II receptor determines prognosis of
bladder cancer regulating cell motility/invasion. J Exp Clin Cancer
Res. 33:482014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harper KL, Sosa MS, Entenberg D, Hosseini
H, Cheung JF, Nobre R, Avivar-Valderas A, Nagi C, Girnius N, Davis
RJ, et al: Mechanism of early dissemination and metastasis in
Her2+ mammary cancer. Nature. Dec 14–2016.(Epub ahead of
print). View Article : Google Scholar
|
|
18
|
Hosseini H, Obradović MM, Hoffmann M,
Harper KL, Sosa MS, Werner-Klein M, Nanduri LK, Werno C, Ehrl C,
Maneck M, et al: Early dissemination seeds metastasis in breast
cancer. Nature. Dec 14–2016.(Epub ahead of print). View Article : Google Scholar
|
|
19
|
Hu Y, Yu X, Xu G and Liu S: Metastasis: An
early event in cancer progression. J Cancer Res Clin Oncol.
143:745–757. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aufhauser DD Jr, Sadot E, Murken DR,
Eddinger K, Hoteit M, Abt PL, Goldberg DS, DeMatteo RP and Levine
MH: Incidence of occult intrahepatic metastasis in hepatocellular
carcinoma treated with transplantation corresponds to early
recurrence rates after partial hepatectomy. Ann Surg. Jan
12–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|