Introduction
Glioblastoma multiforme (GBM), one of the most
aggressive human malignancies, is a brain cancer that originates
from glial cells (1). GBM is
characterized by diffuse infiltration of the brain tissue
surrounding the bulk of the tumor (2,3). The
standard treatment option is typically surgical resection followed
by radiotherapy and chemotherapy (4).
Due to the highly diffuse infiltration, achieving complete surgical
resection is impractical and the efficiency of radiotherapy is
reduced. Thus, examining the mechanisms that affect the invasive
behavior of glioma cells may help to establish novel effective
therapies and develop novel treatment strategies.
Parkinson protein 2 E3 ubiquitin protein ligase
(PARK2) is a key factor in the regulation of the development of
numerous diseases, including multiple human malignancies (5). Previous studies have demonstrated that
PARK2 deficiency promotes the initiation of colorectal adenoma and
hepatocellular carcinoma and accelerates the progression of
tumorigenesis (6,7). Conversely, PARK2 overexpression
mitigates cell proliferation and suppresses the progression of
breast and lung cancer cell cycles (8,9). Although
somatic alterations of PARK2 are frequently observed in GBM cells
(10), the consequences of
inactivating PARK2 in the invasion-metastasis cascade of glioma
cells remain to be fully understood. Therefore, the function of
PARK2 in the metastasis of GBM cells and the corresponding
molecular mechanisms require further assessment.
The present study revealed that PARK2 overexpression
mitigated the metastasis and invasion of GBM cells, while PARK2
knockdown promoted the invasion-metastasis cascade of GBM cells.
Furthermore, PARK2 negatively regulated the expression of zinc
finger E-box-binding homeobox 1 (ZEB1). The promoter effects of
PARK2 knockdown on metastasis and epithelial-mesenchymal transition
(EMT) were attenuated by silencing ZEB1 expression. These results
revealed an important mechanism underlying the regulation of the
invasion-metastasis cascade of GBM cells, which may be a potential
treatment target for GBM.
Materials and methods
Cell line preparation and culture
U87, U251, U373, A172 and LN444 were obtained from
the American Type Culture Collection (Manassas, VA, USA). All cell
lines were maintained in Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc.) and supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), L-glutamine, 100 IU/ml penicillin, 100 mg/ml
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.), sodium
pyruvate and nonessential amino acids. All cells were cultured in a
5% CO2 incubator at 37°C.
PARK2 overexpression lentivirus and
short hairpin RNA (shRNA) lentivirus construction
The human PARK2 overexpression lentivirus
(containing the whole coding sequence; https://www.ncbi.nlm.nih.gov/nuccore/NM_004562.2)
was purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China).
The blank vector lentivirus, acting as a control, an shRNA
lentivirus targeting human PARK2 (shPARK2 forward,
5′-GATCCTCCAAAGAAACCATCAAGAACTTCCTGTCAGATTCTTGATGGTTTCTTTGGATTTTTG-3′
and reverse,
5′-AATTCAAAAATCCAAAGAAACCATCAAGAATCTGACAGGAAGTTCTTGATGGTTTCTTTGGAGG-3′)
and a scrambled shRNA lentivirus, acting as a negative control,
were also designed and synthesized by Shanghai GenePharma Co.,
Ltd.
Small interfering RNA (siRNA) design
and transfections
A172 cells and A172/shPARK2 cells were transfected
with ZEB1 siRNA (siZEB1; sense, 5′-CAGUGUUCCAUGCUUAAGAdTdT-3′ and
anti-sense, 5′-UCUUAAGCAUGGAACACUGdTdT-3′) and a negative
non-targeted control siRNA (siControl sense,
5′-TTCTCCGAACGTGTCACGTdTdT-3′ and anti-sense,
5′-ACGTGACACGTTCGGAGAAdTdT-3′), which were designed and synthesized
by Shanghai GenePharma Co., Ltd. The cells were cultured until
30–50% confluence was attained and then 2.0 µg siRNA and 10.0 µl
Lipofectamine® 2000 transfection reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) were separately diluted in serum-free Opti-MEM-1
medium (Gibco; Thermo Fisher Scientific, Inc.) and then mixed
together. The mixture was subsequently incubated at room
temperature for 20 min and then added directly onto the cells for 6
h at 37 °C.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA was isolated from 1×106 U87 or A172
cells using TRIzol reagent (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) according to manufacturer's protocol. Equal quantities of
RNA (500 ng) were reverse transcribed into cDNA using a QuantiTect
reverse transcription kit according to the manufacturer's protocol
(Qiagen Inc., Valencia, CA, USA). The resulting cDNA was used as
the template for qPCR. Oligonucleotide primers were synthesized
(Invitrogen; Thermo Fisher Scientific, Inc.), and qPCR was
performed in a 20 µl volume containing 2 µl template cDNA, 2X
SYBR-Green master mix (Roche Diagnostics GmbH, Mannheim, Germany)
and 10 pM of each primer. The primer sequences were as follows:
E-cadherin forward, 5′-TTGACGCCGAGAGCTACAC-3′ and reverse,
5′-GTCGACCGGTGCAATCTT-3′; vimentin forward,
5′-TACAGGAAGCTGCTGGAAGG-3′ and reverse, 5′-ACCAGAGGGAGTGAATCCAG-3′;
and β-actin forward, 5′-TTGTTACAGGAAGTCCCTTGCC-3′; and reverse,
5′-ATGCTATCACCTCCCCTGTGTG-3′. Amplification was performed using the
Light Cycler 480 PCR system (Roche Diagnostics GmbH) under the
following thermocycling conditions: 95°C for 30 sec followed by 40
cycles of 95°C for 5 sec and 60°C for 20 sec. Quantity values for
gene expression were generated by the relative quantification
(2−ΔΔCq) method (11);
fluorescence generated by each sample was normalized to the β-actin
product for each gene of interest. The experiments were repeated
three times.
Migration assay
The migration of U87 and A172 cells was assayed
using 24-well collagen-coated Boyden chambers (Chemicon; EMD
Millipore, Billerica, MA, USA) with 8 µm pores (12). A total of 4×104 cells from
indicated groups (NC and PARK2 or shControl and shPARK2) were
seeded in the upper chamber (0.2 ml DMEM in the upper chamber) and
0.8 ml DMEM with 10% FBS was added in the lower chamber. Following
an incubation period of 48 h at 37 °C, migrating cells were
quantified according to the manufacturer's protocol. Briefly, the
cells that migrated to the basal side of the membrane were fixed
with 4% paraformaldehyde for 5 min at 25°C and then stained with 1%
crystal violet for 10 min at 25°C. The cells were subsequently
visualized and photographed with a CKX41 light microscope (Olympus
Corporation, Tokyo, Japan) at ×200 magnification. Images of three
random fields from three replicate wells were obtained and the
number of migratory or invasive cells was counted.
Invasion assay
The invasion of U87 and A172 cells was also assayed
using 24-well collagen-coated Boyden chambers (Chemicon; EMD
Millipore) with 8 µm pores. Following resuspension in 200 µl
serum-free DMEM, 6×104 U87 and A172 cells were seeded on
Matrigel-coated chamber inserts (0.2 ml DMEM in the upper chamber
and 0.8 ml DMEM with 10% FBS in the lower chamber) and incubated at
37°C for 48 h (BD Biosciences, San Jose, CA, USA). Wells were
subsequently washed with PBS. The cells that migrated to the basal
side of the membrane were fixed with 4% paraformaldehyde for 5 min
at 25°C and then stained with 1% crystal violet for 10 min at 25°C.
The cells were subsequently visualized and photographed with a
CKX41 light microscope (Olympus Corporation) at ×200 magnification.
Images of three random fields from three replicate wells were
obtained and migratory or invasive cells were counted.
Statistical analysis
GraphPad Prism version 5.0 for Windows (GraphPad
Software Inc., San Diego, CA, USA) was applied for the statistical
analyses. Results were expressed as the mean ± standard error of
the mean. A Student's t-test (unpaired) was used to evaluate the
statistical significance of the results. P<0.05 was considered
to indicate a statistically significant difference.
Results
Overexpression of PARK2 mitigates
metastasis and invasion of GBM cells
Since the invasion-metastasis cascade may induce
mortality in patients with GBM, the present study determined
whether PARK2 regulated GBM progression by influencing metastasis.
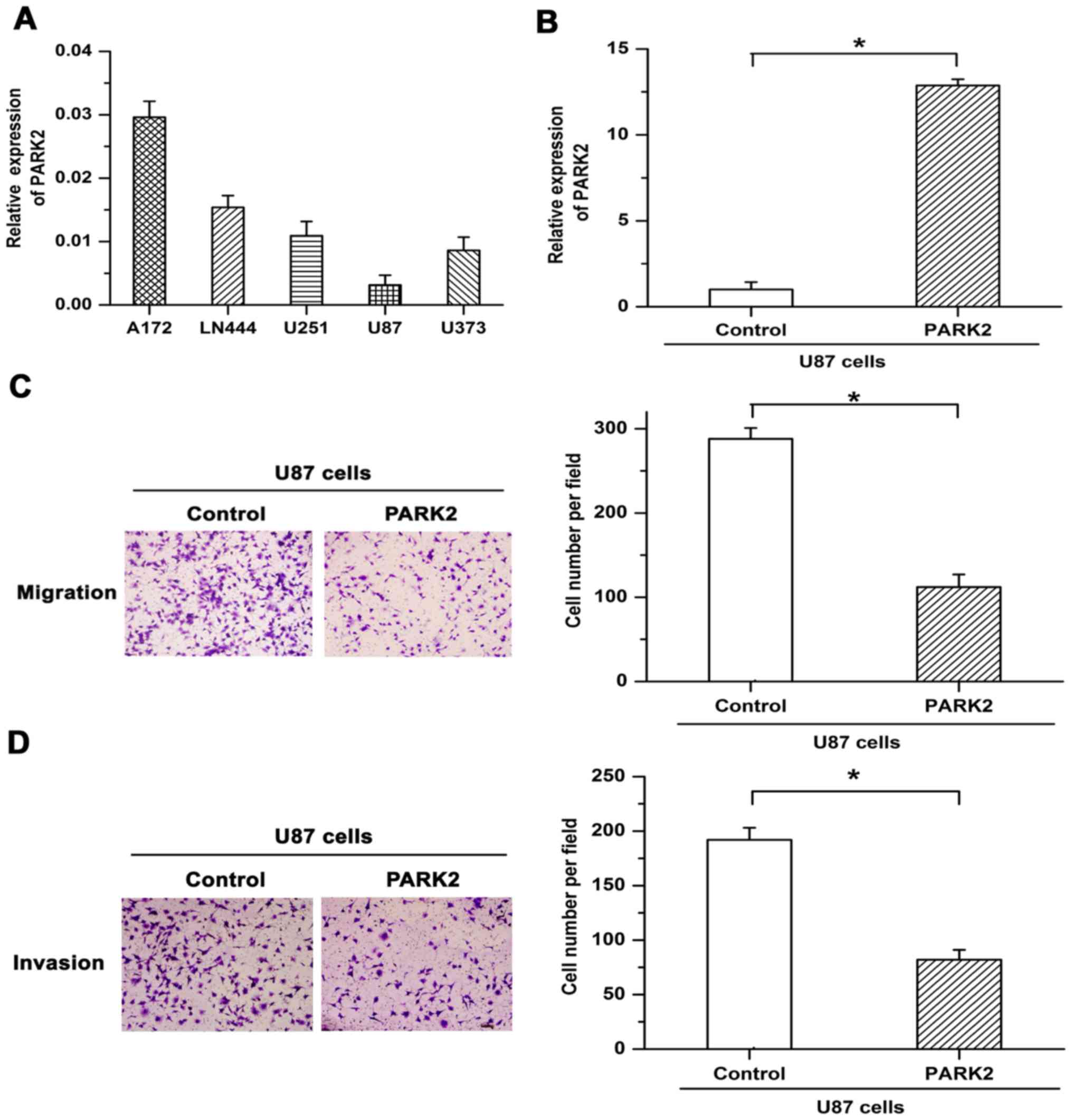
To select appropriate cell lines for further functional
examination, PARK2 mRNA expression was assessed in five GBM cell
lines. Since PARK2 mRNA expression in U87 cells was lower than in
other cell lines (Fig. 1A), stable
overexpression of PARK2 mRNA in U87 cells was induced via
lentiviral infection. Overexpression efficiency was confirmed by
RT-qPCR (Fig. 1B). Transwell
migration and Matrigel invasion chamber assays were used to
determine the effect of PARK2 on the metastasis of GBM cells.
Migratory and invasive potential was revealed to be attenuated by
PARK2 overexpression in U87 cells (Fig.
1C and D). The results suggested that overexpression of PARK2
repressed the metastasis of GBM cells.
PARK2 knockdown promotes migration and
invasion of GBM cells
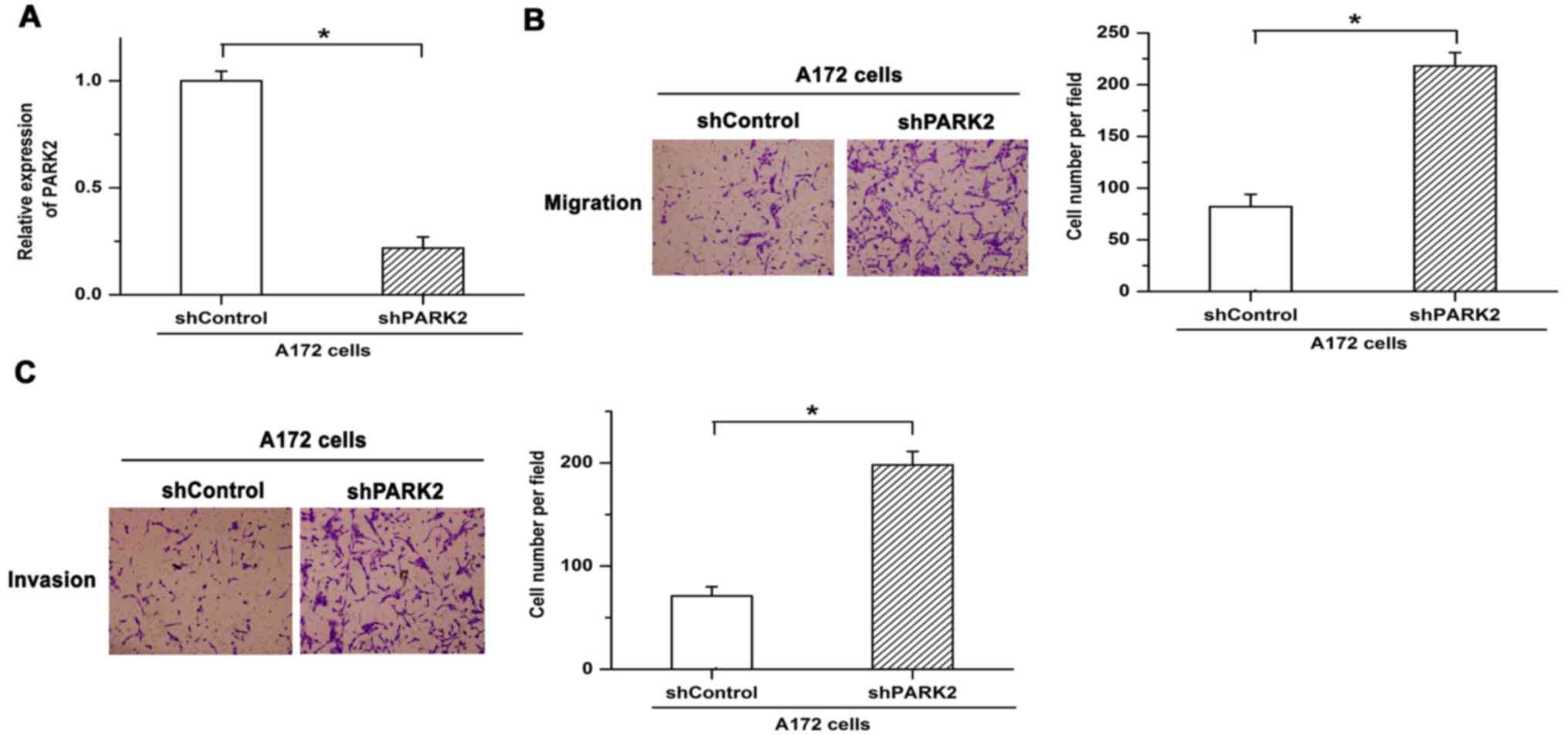
To reveal the function of PARK2 in the metastasis of
GBM cells, shRNA was used to knock down expression of PARK2 in A172
cells. Knockdown efficiency was verified by RT-qPCR (Fig. 2A). Knockdown of PARK2 enhanced cell
migration in A172 cells (Fig. 2B).
Similar results were obtained in terms of cell invasion, with
invasion being facilitated by silencing the expression of PARK2
(Fig. 2C). These results indicated
that knockdown of PARK2 promoted cell metastasis and the
progression of GBM.
Promotive effects of PARK2 knockdown
on metastasis are reduced by silencing expression of ZEB1
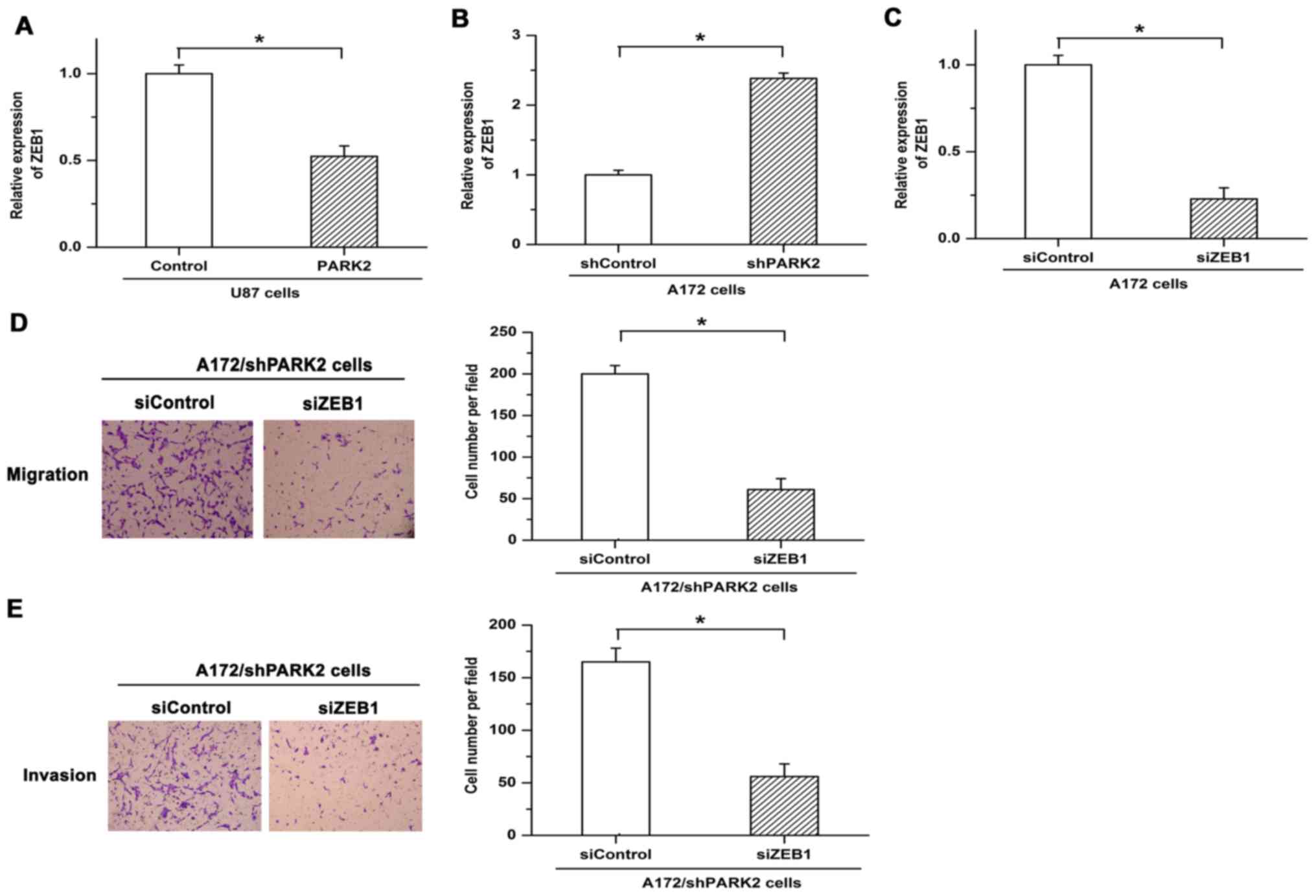
ZEB1 is a key regulator in the metastasis of tumor
cells. Expression of ZEB1 is significantly upregulated in invasive
glioma tissue (13). The present
study assessed whether a regulatory relationship exists between
PARK2 and ZEB1 in GBM cells. Expression of ZEB1 was significantly
decreased following overexpression of PARK2, while inhibiting PARK2
expression via shRNA led to increased expression of ZEB1 (Fig. 3A and B). To verify the functions of
ZEB1 in PARK2 knockdown-promoted metastasis, siRNA was used to
repress the expression of ZEB1. Knockdown efficiency of siZEB1 was
demonstrated by RT-qPCR (Fig. 3C).
Knockdown of PARK2-facilitated cell migration and invasion was
eliminated by siZEB1 (Fig. 3D and E).
These results demonstrated that ZEB1 serves as an important
mediator in PARK2-regulated GBM cell metastasis.
PARK2-regulated EMT is mediated by
ZEB1 in GBM cells
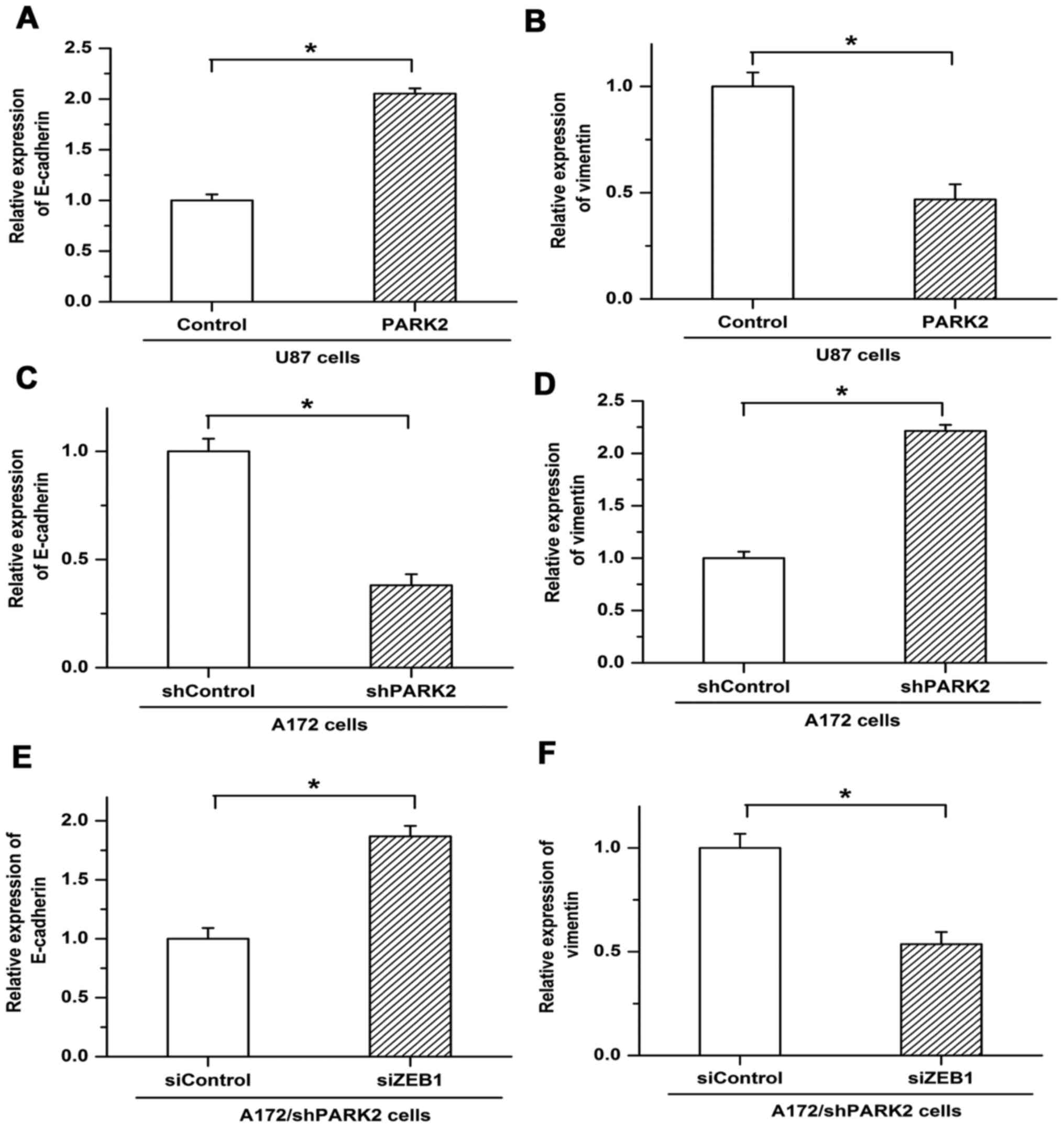
EMT is associated with tumor metastasis, and
chemotherapy resistance is more frequently observed in cancer cells
undergoing EMT (14). The present
study revealed the involvement of PARK2 in EMT. Expression of the
EMT markers epithelial cadherin (E-cadherin) and vimentin was
examined by RT-qPCR. PARK2 overexpression increased the expression
of E-cadherin in U87 cells but was associated with decreased
expression of vimentin (Fig. 4A and
B). Knockdown of PARK2 in A172 cells repressed the expression
of E-cadherin and induced the expression of vimentin (Fig. 4C and D). Furthermore, decreased
expression of E-cadherin and increased expression of vimentin, as
triggered by PARK2 knockdown, were reversed by ZEB1 siRNA, with
E-cadherin expression increasing and vimentin expression decreasing
compared with siControl (Fig. 4E and
F). These results suggested that PARK2 negatively regulated EMT
by depressing ZEB1 expression in glioma cells.
Discussion
GBM is one of the most aggressive human malignancies
(1,2).
However, current treatment strategies are ineffective and the
pathogenesis of GBM and the corresponding molecular mechanisms are
not yet fully understood. Therefore, results potentially leading to
novel therapeutic targets of GBM are vital. The results of the
present study suggested that PARK2 repressed the metastasis and
invasion of GBM cells and that PARK2 negatively regulated EMT by
depressing ZEB1 expression. These results demonstrated a the
involvement of PARK2 in suppressing the metastasis and invasion of
GBM cells.
Crucially, the present study identified PARK2 as a
tumor suppressor in GBM cells. Previous studies have demonstrated
that somatic alterations to PARK2 are frequently observed in
numerous types of human tumor. Deficiency of PARK2 in transgenic
mice results in colorectal adenoma and hepatocellular carcinoma
occurring more frequently (6,7). Furthermore, restoring PARK2 expression
depresses the proliferation of cancer cells derived from brain,
breast, and lung tissue (12,15). However, the function of PARK2 in the
metastasis of GBM and the associated molecular mechanisms are not
yet fully understood. The present study demonstrated that PARK2
overexpression mitigated the metastasis and invasion of GBM cells.
Conversely, migration and invasion of cancer cells were facilitated
by knockdown of PARK2. These results suggested that PARK2
functioned as a tumor suppressor during the metastasis of GBM
cells.
EMT is key in the initiation of metastasis in cancer
cells (16). Cancer cells undergoing
EMT are more resistant to radiotherapy and are able to acquire stem
cell traits (17,18). EMT, a reversible process, is
characterized by the loss of polarized features, the movement away
from neighboring cells and increased motility and invasion,
contributing to a disassembly of cell-cell junctions. EMT is also
characterized by decreased expression of E-cadherin and increased
expression of mesenchymal molecular markers, including vimentin
(19–21). ZEB1 induces EMT, and ZEB1 expression
is associated with the survival and therapy response of patients
with tumors (22). Previous studies
have demonstrated that silencing ZEB1 expression hampers metastasis
and invasion in diverse types of human cancer; ZEB1 is therefore a
potential therapeutic target for repressing the development of
tumors (23,24). The present study suggested that PARK2
has regulatory effects on the expression of ZEB1 and EMT. The
present study demonstrated that the overexpression of PARK2
significantly repressed the expression of ZEB1, while PARK2
knockdown resulted in increased expression of ZEB1. The promotive
effects of PARK2 knockdown on metastasis were reduced by silencing
expression of ZEB1. Furthermore, PARK2 overexpression blocked the
process of EMT, which was represented as the upregulation of
E-cadherin and downregulation of vimentin. Conversely, knockdown of
PARK2 induced the expression of vimentin and repressed E-cadherin
expression in A172 cells, and the effects of PARK2 knockdown on EMT
were attenuated by siZEB1. These results suggested that PARK2
negatively regulated EMT by depressing ZEB1 expression in GBM
cells.
In conclusion, the results of the present study
suggested that PARK2 mitigated the metastasis and invasion of GBM
cells and inhibited the progression of GBM by functioning as a
tumor suppressor. Furthermore, ZEB1 was an important mediator in
PARK2-suppressed the metastasis and EMT in GBM. The present study
elucidated an important underlying mechanism regulating the
metastasis and invasion of GBM cells, and provided a potential
therapeutic approach for GBM.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Heilongjiang Province (grant no. H201434),
the Postdoctoral Foundation of Heilongjiang Province (grant no.
LBH-Z11096) and the National International Science and Technology
Cooperation Foundation of China (grant no. 2014DFA31630).
References
|
1
|
Holdhoff M and Grossman SA: Controversies
in the adjuvant therapy of high-grade gliomas. Oncologist.
16:351–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vehlow A and Cordes N: Invasion as target
for therapy of glioblastoma multiforme. Biochim Biophys Acta.
1836:236–244. 2013.PubMed/NCBI
|
|
3
|
Montana V and Sontheimer H: Bradykinin
promotes the chemotactic invasion of primary brain tumors. J
Neurosci. 31:4858–4867. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stupp R, Hegi ME, Gilbert MR and
Chakravarti A: Chemoradiotherapy in malignant glioma: Standard of
care and future directions. J Clin Oncol. 25:4127–4136. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu L, Lin DC, Yin D and Koeffler HP: An
emerging role of PARK2 in cancer. J Mol Med (Berl). 92:31–42. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Poulogiannis G, McIntyre RE, Dimitriadi M,
Apps JR, Wilson CH, Ichimura K, Luo F, Cantley LC, Wyllie AH, Adams
DJ and Arends MJ: PARK2 deletions occur frequently in sporadic
colorectal cancer and accelerate adenoma development in Apc mutant
mice. Proc Natl Acad Sci USA. 107:pp. 15145–15150. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujiwara M, Marusawa H, Wang HQ, Iwai A,
Ikeuchi K, Imai Y, Kataoka A, Nukina N, Takahashi R and Chiba T:
Parkin as a tumor suppressor gene for hepatocellular carcinoma.
Oncogene. 27:6002–6011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tay SP, Yeo CW, Chai C, Chua PJ, Tan HM,
Ang AX, Yip DL, Sung JX, Tan PH, Bay BH, et al: Parkin enhances the
expression of cyclin-dependent kinase 6 and negatively regulates
the proliferation of breast cancer cells. J Biol Chem.
285:29231–29238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Picchio MC, Martin ES, Cesari R, Calin GA,
Yendamuri S, Kuroki T, Pentimalli F, Sarti M, Yoder K, Kaiser LR,
et al: Alterations of the tumor suppressor gene Parkin in non-small
cell lung cancer. Clin Cancer Res. 10:2720–2724. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Veeriah S, Taylor BS, Meng S, Fang F,
Yilmaz E, Vivanco I, Janakiraman M, Schultz N, Hanrahan AJ, Pao W,
et al: Somatic mutations of the Parkinson's disease-associated gene
PARK2 in glioblastoma and other human malignancies. Nat Genet.
42:77–82. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pardo A, Gibson K, Cisneros J, Richards
TJ, Yang Y, Becerril C, Yousem S, Herrera I, Ruiz V, Selman M and
Kaminski N: Up-regulation and profibrotic role of osteopontin in
human idiopathic pulmonary fibrosis. PLoS Med. 2:e2512005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Zhang W, Li Y, Alvarez A, Li Z,
Wang Y, Song L, Lv D, Nakano I, Hu B, et al: SHP-2-upregulated ZEB1
is important for PDGFRα-driven glioma epithelial-mesenchymal
transition and invasion in mice and humans. Oncogene. 35:5641–5652.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siebzehnrubl FA, Silver DJ, Tugertimur B,
Deleyrolle LP, Siebzehnrubl D, Sarkisian MR, Devers KG, Yachnis AT,
Kupper MD, Neal D, et al: The ZEB1 pathway links glioblastoma
initiation, invasion and chemoresistance. EMBO Mol Med.
5:1196–1212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yeo CW, Ng FS, Chai C, Tan JM, Koh GR,
Chong YK, Koh LW, Foong CS, Sandanaraj E, Holbrook JD, et al:
Parkin pathway activation mitigates glioma cell proliferation and
predicts patient survival. Cancer Res. 72:2543–2553. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun X, Liu M, Hao J, Li D, Luo Y, Wang X,
Yang Y, Li F, Shui W, Chen Q and Zhou J: Parkin deficiency
contributes to pancreatic tumorigenesis by inducing spindle
multipolarity and misorientation. Cell Cycle. 12:1133–1141. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu KJ and Yang MH: Epithelial-mesenchymal
transition and cancer stemness: The Twist1-Bmi1 connection. Biosci
Rep. 31:449–455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo Z, Hardin H and Lloyd RV: Cancer
stem-like cells and thyroid cancer. Endocr Relat Cancer.
21:T285–T300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao D, Dai C and Peng S: Mechanism of the
mesenchymal-epithelial transition and its relationship with
metastatic tumor formation. Mol Cancer Res. 9:1608–1620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
von Gise A and Pu WT: Endocardial and
epicardial epithelial to mesenchymal transitions in heart
development and disease. Circ Res. 110:1628–1645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamada S, Okumura N, Wei L, Fuchs BC,
Fujii T, Sugimoto H, Nomoto S, Takeda S, Tanabe KK and Kodera Y:
Epithelial to mesenchymal transition is associated with shorter
disease-free survival in hepatocellular carcinoma. Ann Surg Oncol.
21:3882–3890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gibbons DL, Lin W, Creighton CJ, Rizvi ZH,
Gregory PA, Goodall GJ, Thilaganathan N, Du L, Zhang Y,
Pertsemlidis A and Kurie JM: Contextual extracellular cues promote
tumor cell EMT and metastasis by regulating miR-200 family
expression. Genes Dev. 23:2140–2151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chu K, Boley KM, Moraes R, Barsky SH and
Robertson FM: The paradox of E-cadherin: Role in response to
hypoxia in the tumor microenvironment and regulation of energy
metabolism. Oncotarget. 4:446–462. 2013. View Article : Google Scholar : PubMed/NCBI
|