Introduction
Acute lymphoblastic leukemia (ALL) is a malignant
disease of the hematopoietic system that arises in the bone marrow
(BM) (1). ALL is the most common type
of cancer among children, but is also one of the most treatable.
The majority of children with ALL either receive successful therapy
or have positive outcomes, however children may relapse with poor
outcomes subsequent to systemic treatments (2). These outcomes may be attributed to drug
resistance of leukemia cells to chemotherapeutic reagents. Leukemia
cells depend on the BM microenvironment for their growth and
survival by interacting with BM-SCs.
BM-SCs are important components of the BM
hematopoietic microenvironment because of their ability to
self-renew and differentiate into a variety of mesodermal lineages,
such as osteoblasts, chondrocytes and adipocytes (3). BM-SCs support the hematopoietic process,
survival, differentiation and proliferation of hematopoietic cells,
in vivo by secreting soluble cytokines, growth factors and
small molecular mediators (4).
Previous studies showed that BM-SCs play an important role in the
initiation, development, progression and drug resistance of
leukemia (5). In addition, BM-SCs
affect the chemoresistance of leukemia cells during chemotherapy.
Mudry et al (6) found that
BM-SCs regulated the reaction of leukemia cells to chemotherapeutic
drugs by protecting them against cell apoptosis induced by
chemotherapeutic regimens. Although previous studies reported
possible mechanisms by which BM-SCs protect leukemia cells against
chemotherapeutic regimens, little is known about the exact
mechanisms of chemoresistance of leukemia cells.
A series of cytokines secreted by BM-SCs can be
involved in the leukemia chemoresistance process (7,8). Aside
from soluble factors, exosomes are an emerging tool for mediating
cell-cell communications and contain enriched proteins, mRNA, and
miRNAs. Exosomes are 40–100 nm-diameter nanovesicles and are
secreted by various cell types upon fusion of multivesicular
endosomes with plasma membranes. Exosomes mediate intercellular
communications through transferring bioactive factors to other
cells (9,10). BM-SC-derived exosomes have been
reported as a new mechanism for the paracrine action of BM-SCs
(11). However, little is known about
the role of BM-SC exosomes in the chemoresistance of leukemia
cells.
In the present study, exosomes were isolated from
BM-SCs culture medium by serial centrifugation and the effects of
co-cultured BM-SCs-exosomes on the cell viability, apoptosis, and
drug resistance of leukemia cells in vitro were
investigated. The possible mechanism involving exosomes was also
explored to provide additional insight into the biological role of
BM-SCs-exosomes and their potential clinical applications. The
present study found that BM-SCs-derived exosomes protected leukemia
cells against chemotherapeutic drugs by decreasing the sensitivity
of leukemia cells to chemotherapy. The current findings provide new
evidence about the mechanism by which leukemia cells produce
chemotherapeutic resistance.
Materials and methods
Cell culture
The human B-cell ALL nalm-6 cell line was cultured
in suspension with RPMI-1640 medium (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) that contained 10% fetal bovine serum
(FBS) (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
The cells were incubated at 37°C in a humidified atmosphere of 5%
CO2 and passaged every 3 days to maintain a cell density
of ≤1×106 cells/ml. The BM-SCs HS-5 cell line (American
Type Culture Collection, Manassas, VA, USA) was cultured in high
glucose Dulbecco's modified Eagle's medium (Hyclone; GE Healthcare
Life Sciences) with 10% FBS and 1% penicillin/streptomycin at 37°C
with 5% CO2. The cells were then trypsinized and
passaged at 1×104 cells/cm2 in the medium as
aforementioned. The cells were used subsequent to between 4 and 7
passages.
Isolation and identification of
exosomes
BM-SCs were grown to 50% confluence in a
225-cm2 flask (Corning Incorporated, Corning, NY, USA),
and the culture medium was removed. The cells were washed with PBS
twice and cultured in UltraCULTURE Serum-free medium (Lonza Group
Ltd., Basel, Switzerland) for 48 h. Subsequently, exosomes were
collected from the BM-SCs cultures following 48 h through standard
differential centrifugation steps using a LYNX 6000 centrifuge
(Thermo Fisher Scientific, Inc.). The supernatants were centrifuged
at 300 × g for 10 min and 2,000 × g for 10 min to remove residual
cells and debris, then 10,000 × g for 30 min to remove
microparticles, and 100,000 × g for 12 h to obtain the exosome
pellets. Finally, 70 kilodalton heat shock protein (HSP70;
dilution, 1:2,000; catalog no., EXOAB-Hsp70A-1; System Biosciences,
Inc., Palo Alto, CA, USA) and lysosomal-associated membrane protein
3 (CD63; dilution, 1:1,000; catalog no., ab134045; Abcam,
Cambridge, MA, USA) were determined by western blot analysis for
the identification of exosomes. The aliquots were stored at −80°C
for later use, and the exosomes isolated from 5 ml of the
conditioned medium were used as a unit.
Transmission electron microscopy
Purified exosomes were fixed with 3% glutaraldehyde
for >4 h at 4°C. Subsequently, the samples were washed with PBS
3 times and post-fixed with 1% osmic acid for 2 h at room
temperature. Subsequent to a series of ethanol and acetone
dehydration, penetration, embedding, and polymerization, the
samples were cut into slices (thickness, 70 nm) and stained with
uranyl acetate and citric acid. Samples were dried and visualized
under a JEOL-1200EX transmission electron microscope (JEOL, Ltd.,
Tokyo, Japan) and recorded using MORADA-2 software (Olympus
Corporation, Tokyo, Japan).
Cell viability assay
Chemoresistant characteristics were detected by the
Cell Counting Kit-8 assay (Beyotime Institute of Biotechnology,
Shanghai, China) according to the manufacturer's protocol. Nalm-6
cells were seeded at 4×104 cells per well in 96-well
plates and co-cultured with or without 1 U/ml exosomes in the
presence or absence of 0.3 µg/ml etoposide (VP16). A group without
cells served as blank. Cell viability was examined subsequent to
treatment with exosomes for 48 h. Optical density was determined at
450 nm using a microplate reader. Each group was completed in
triplicate, and the experiment was repeated 3 times to ensure
accuracy.
Cell apoptosis analysis
Nalm-6 cells were seeded at 4×105/ml per
well in serum-free medium in 12-well plates and were treated with
or without 1 U/ml BM-SCs-exosomes in the presence of 0.3 µg/ml VP16
for 48 h. A group with nalm-6 cells alone served as the control.
Collected cells were washed three times with PBS, resuspended in
100 µl of binding buffer, and incubated with 5 µl Annexin V and 10
µl propidium iodide (Beyotime Institute of Biotechnology) for 15
min at room temperature according to the manufacturer's protocol.
Subsequently, the apoptosis of nalm-6 cells was analyzed by flow
cytometry using a Guava easyCyte 8HT flow cytometer (EMD Millipore,
Billerica, MA, USA) and Guava Incyte (version 3.1.1; EMD
Millipore).
Western blot analysis
Nalm-6 cells were seeded at 4×105/ml in
serum-free medium in a 25-cm2 flask and treated with or
without 1 U/ml BM-SCs-exosomes in the presence of 0.3 µg/ml VP16
for 48 h. A group with nalm-6 cells alone served as control. Whole
cell lysates were then extracted from cells suspended in
radioimmunoprecipitation assay lysis buffer (catalog no., P0013B;
Beyotime Institute of Biotechnology), which contained protease and
phosphatase inhibitors. Lysates were separated by 12% SDS-PAGE
(marker ladder, 10–170 kDa; catalog no., SM0671/26616; Fermentas;
Thermo Fisher Scientific, Inc.) and transferred to polyvinylidene
difluoride membranes (EMD Millipore). The membranes were blocked in
a blocking buffer of 20% skimmed-milk in TBS-Tween 20 for 1 h at
room temperature, and incubated with primary antibody from rabbit
against human β-actin (dilution, 1:1,000; catalog no., 20536–1-AP;
ProteinTech Group, Inc., Wuhan, China), B-cell lymphoma 2 (BCL-2;
dilution, 1:100; catalog no., ab7973; Abcam), BCL-2-like protein 4
(BAX; dilution, 1:2,000; catalog no., ab32503; Abcam), caspase-3
(dilution, 1:500; catalog no., 19677-1-AP; ProteinTech Group,
Inc.), cleaved caspase-3 (dilution, 1:500; catalog no., 19677-1-AP;
ProteinTech Group, Inc.), poly ADP-ribose polymerase (PARP;
dilution, 1:400; catalog no., ab6079; Abcam), and cleaved PARP
(dilution, 1:250; catalog no., ab6079; Abcam) overnight at 4°C. The
membranes were then exposed to goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (dilution, 1:20,000;
catalog no., ZB-2301; OriGene Technologies, Inc., Beijing, China)
for 1 h at room temperature. The bands were visualized and captured
using ECL Western Blotting Substrate (EMD, Millipore) and C-Digit
Image Studio (LI-COR, Nebraska, NE, USA) 47 software.
Results
Isolation and identification of
BM-SCs-exosomes
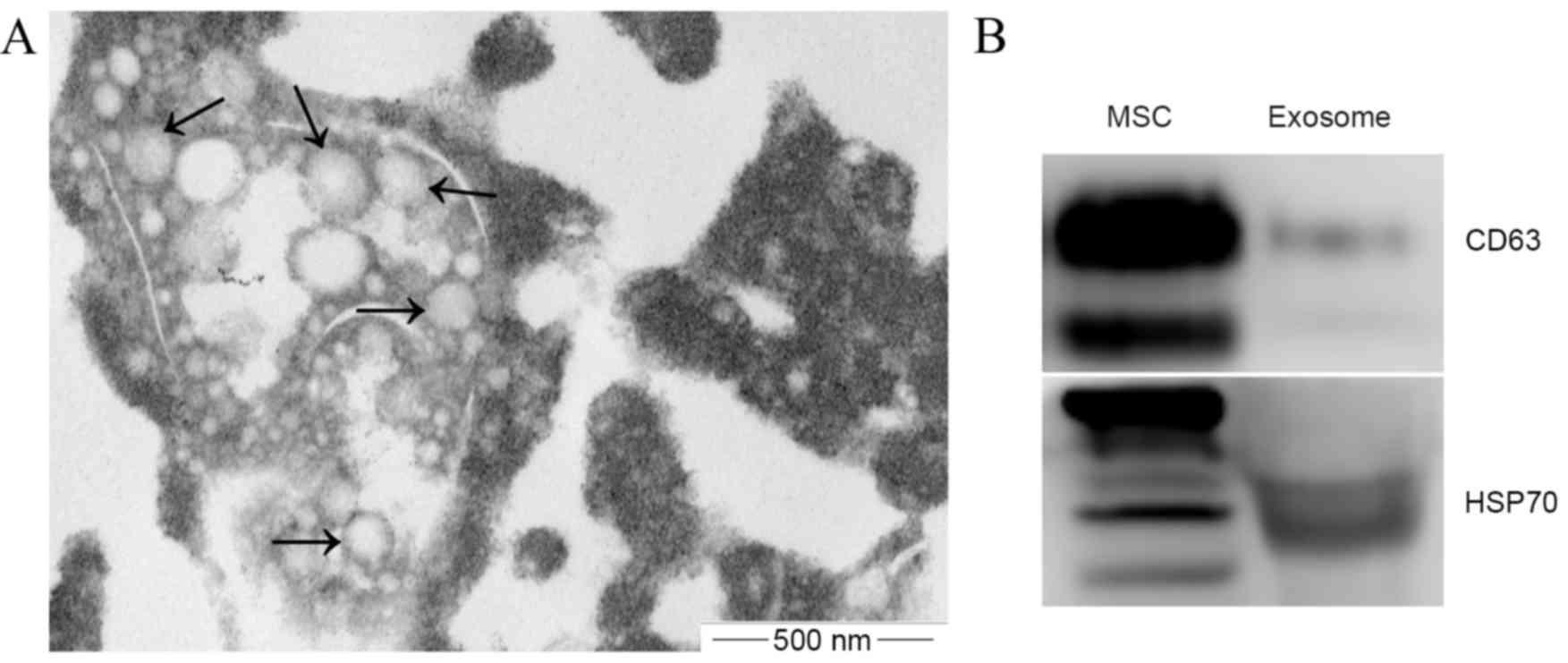
Exosomes were successfully isolated from the
conditioned medium of BM-SCs. The size and shape of the isolated
exosomes were confirmed using transmission electron microscopy, and
typical cup-shaped membrane particles with a diameter of 50–150 nm
were observed (Fig. 1A). Western blot
analysis showed that exosomal markers CD63 and HSP70 were detected
in these exosomes (Fig. 1B). These
results confirmed that the present study successfully isolated
exosomes from the BM-SCs-conditioned medium.
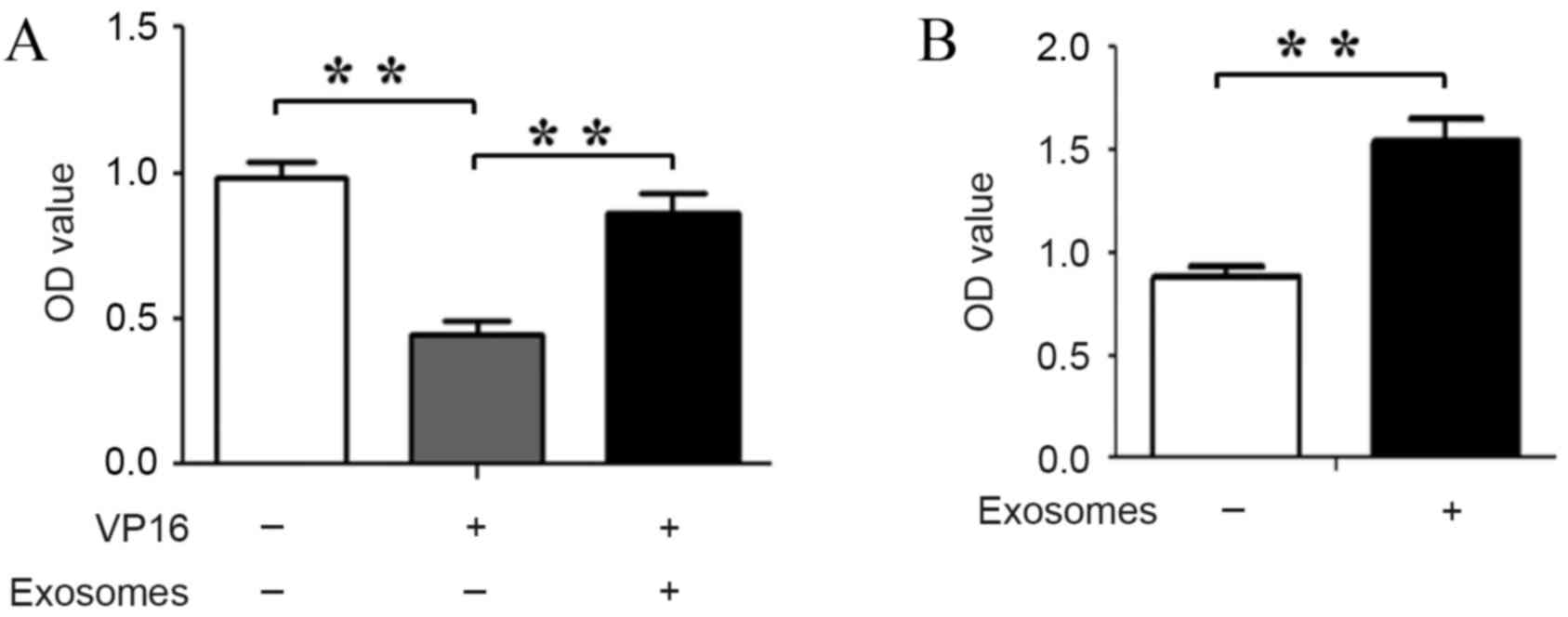
BM-SCs-exosomes promote the viability
of leukemia cells
BM-SCs are known to increase the viability of
leukemia cells, but the role of exosomes in these actions is not
clearly elucidated. In the present study, the effects of
BM-SCs-derived exosomes on the cell viability of nalm-6 cells were
investigated in the presence or absence of VP16. As expected, the
viability of nalm-6 cells was increased subsequent to the cells
being co-cultured with BM-SCs-derived exosomes in the presence or
absence of VP16 compared with the untreated cells (P=0.00081 and
P=0.00073, respectively; Fig. 2A and
B). These results demonstrated that BM-SCs-derived exosomes
maintain the survival of nalm-6 cells; thereby decreasing the
latter's sensitivity to VP16.
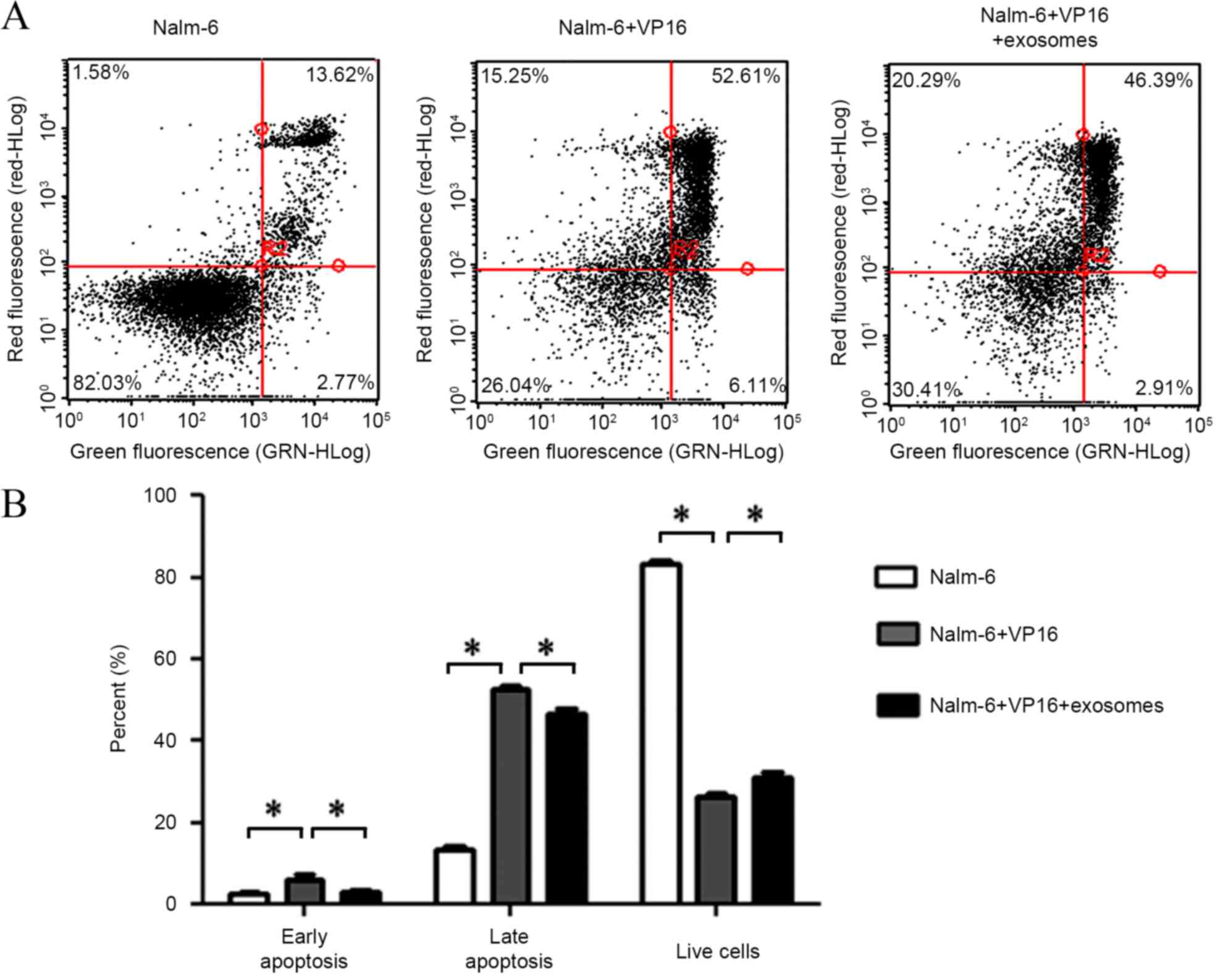
BM-SCs-derived exosomes decrease the
apoptosis of leukemia cells
To additionally explore the effects of exosomes on
nalm-6 cells, the present study evaluated the apoptosis of nalm-6
cells subsequent to co-culturing with BM-SCs-derived exosomes.
Fluorescence-activated cell sorting results showed that
BM-SCs-derived exosomes decreased the apoptotic percentage of
nalm-6 cells (nalm-6 and VP16 vs. nalm-6, VP16 and exosomes;
P=0.00816 early apoptosis and P=0.00415 late apoptosis) induced by
VP16 while increasing the percentage of live cells (P=0.00727)
compared with the untreated cells (Fig.
3A and B). These data indicate that BM-SCs-derived exosomes
protect nalm-6 cells from VP16-induced apoptosis to maintain its
survival.
BM-SCs-derived exosomes regulate the
apoptotic-associated protein levels in leukemia cells
In the present study, the expression levels of
apoptotic-associated proteins BAX, BCL-2, caspase-3, cleaved
caspase-3, PARP, and cleaved PARP were evaluated by western blot
analysis. The results showed that the expression levels of BAX,
cleaved caspase-3, and cleaved PARP were significantly upregulated
in the VP16 group (BAX, P=0.00011; cleaved caspase-3, P=0.00012;
cleaved PARP, P=0.00015), but were low in the control group.
However, their expressions were downregulated subsequent to
treatment with exosomes for 48 h (BAX, P=0.00287; cleaved
caspase-3, P=0.00606; cleaved PARP, P=0.00712). The expression
levels of BCL-2, caspase-3, and PARP were significantly
downregulated in the VP16 group compared with those in the control
group (BCL-2, P=0.00024; caspase-3, P=0.00488; PARP, P=0.00083),
whereas they were significantly increased in the exosome-treated
group compared with the VP16 group (BCL-2, P=0.00601; caspase-3,
P=0.00221; PARP, P=0.00671) (Fig. 4A and
B). These data indicate that apoptosis-associated proteins may
be involved in the drug resistance of leukemia cells induced by
BM-SCs-derived exosomes.
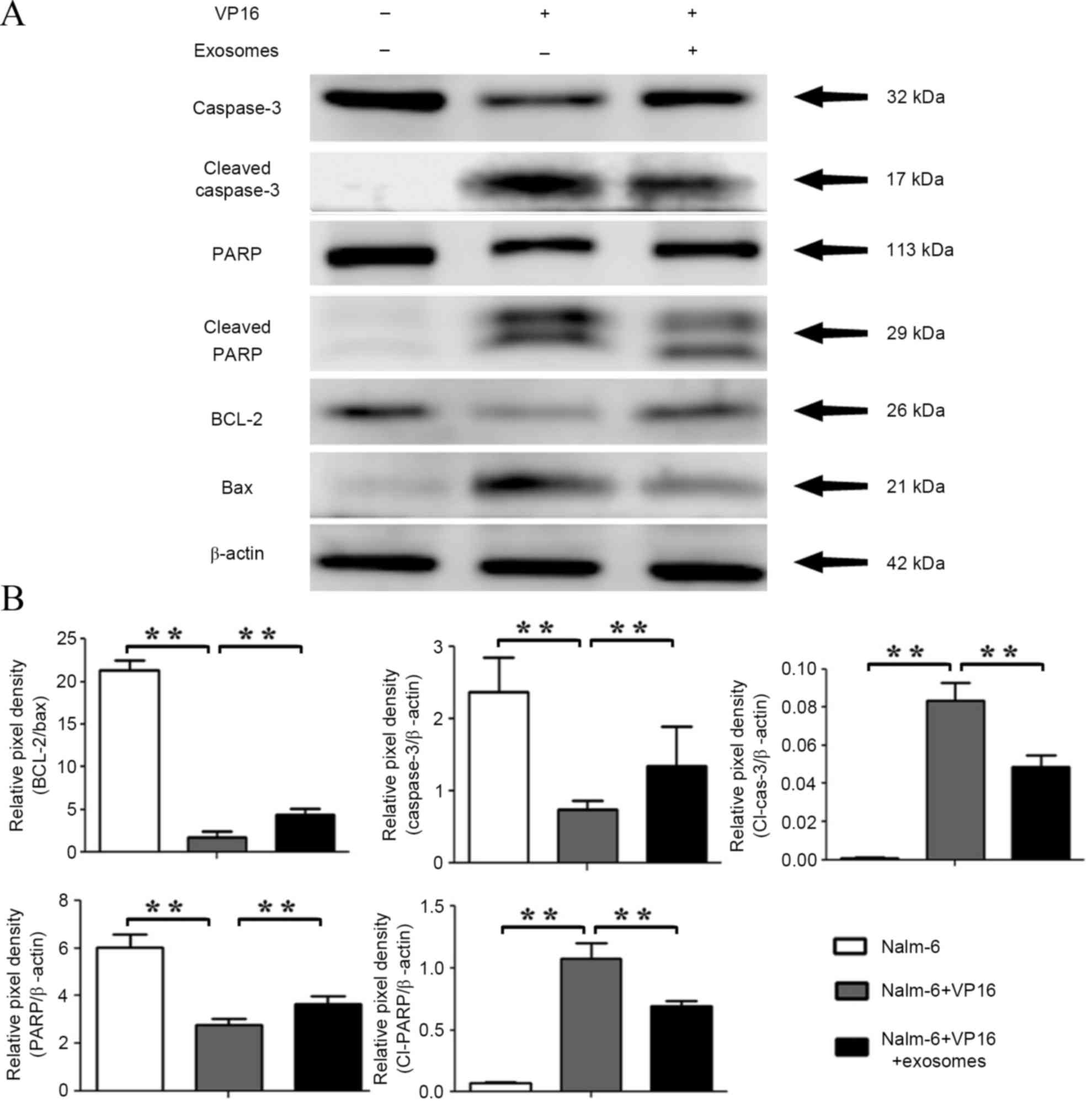 | Figure 4.Effects of BM-SC-exosomes on the
expression levels of apoptosis-associated proteins. (A) Nalm-6
cells in serum medium were treated with BM-SC-derived exosomes in
the presence of 0.3 µg/ml VP16, and apoptosis-associated proteins
BCL-2, BAX, caspase-3, and PARP were detected using western blot
analysis. (B) Pixel densities of the proteins were quantified from
3 independent experiments and presented by histograms. The mean
values ± standard deviation for three independent experiments are
shown. *P<0.05, **P<0.01. BM-SC, bone marrow stromal cells;
VP16, etoposide; BCL-2, B-cell lymphoma 2; BAX, BCL-2-like protein
4; PARP, poly ADP-ribose polymerase; Cl-cas-3, cleaved caspase-3;
Cl-PARP, cleaved PARP. |
Discussion
Previous studies have reported that exosomes may
mediate cell-to-cell communication (12,13) and
possess crucial roles in hematological malignancies. These studies
have demonstrated the functions of exosomes in cell apoptosis
(14), cell proliferation (15,16),
differentiation (15), angiogenesis
(17,18), natural killer cell cytotoxicity
(19) and induction of antitumor
T-cell immunity (20). However, the
role of exosomes in the pathogenesis and progression of leukemia
has not yet been thoroughly evaluated. BM-SCs directly interact
with leukemia cells and secrete soluble factors and other
functional components to support the growth of these cells.
However, the role of exosomes in these actions remains largely
unclear. The present study provides novel evidence on the role of
BM-SCs-derived exosomes in BM-SCs-induced leukemia cell growth,
survival and drug resistance, leading to a better understanding of
the interactions between exosomes in the BM microenvironment and
the leukemia cells. In the present study, exosomes were isolated by
ultracentrifugation, and the exosomal morphology and markers were
confirmed using electron microscopy and western blot analysis. The
results of the present study demonstrated that exosomes are
nanoparticles of ~100 nm in size and exhibit a membrane-like
bilayer with a typical cup-like shape. They commonly expressed CD63
and HSP70 markers.
BM-SCs affect the survival and proliferation of
leukemia cells by activating anti-apoptotic and growth signaling
pathways to secrete growth factors (21,22). In
the current study, the results demonstrate that BM-SCs-exosomes
increased the viability and survival of leukemia cells, and
different pathways, such as BCL-2/BAX, caspase-3, and PARP are
involved in these actions. BM-SCs-exosomes may maintain cell
survival through the following ways: Stimulating target cells
through surface-expressed receptors to induce signal transduction
and activation of anti-apoptotic pathways in leukemic cells;
horizontally transferring transcription factors and miRNAs to
leukemic cells to activate relevant pathways; and transferring
receptors to target cells to induce more signaling pathways
(23). The current results show that
BM-SC-exosomes blocked the reduction of protein expression levels
of BCL-2, full-length caspase-3, and PARP induced by VP16; and
inhibited the expression levels of BAX, cleaved caspase-3, and
cleaved PARP. These results indicate that BM-SC-exosomes may
protect nalm-6 cells from VP16-induced cell apoptosis by regulating
apoptosis-associated signal molecules to induce drug
resistance.
Drug resistance is a major problem in clinical
cancer treatment and a consequence of multiple factors, including
inhibition of apoptosis, enhanced drug export, reduced drug uptake,
enhanced DNA repair, and enhanced drug inactivation (24). Evasion of apoptosis is typical of
numerous cancers and a frequent cause of therapeutic
resistance.
Cell apoptosis is a type of programmed cell death
that plays a critical role in normal tissue development and removal
of damaged, old or infected cells. During the course of apoptosis,
cells and nucleosomal DNA fragments undergo structural changes,
including condensation of nucleus and cytoplasm and formation of
small membrane-bound structures. In malignancies, the apoptosis
program is inhibited in cancer cells, particularly in hematologic
malignancies (25). Cell apoptosis is
initiated by 2 signaling pathways, the intrinsic and extrinsic
pathways. The intrinsic pathway is more commonly perturbed in
lymphoid malignancies. The intrinsic pathway, also termed stress or
mitochondrial pathway, is evolutionarily highly conserved and
mainly controlled by the BCL-2 protein family (26). This protein family has two subgroups
based on their pro-apoptotic or anti-apoptotic activity, namely,
pro-apoptotic protein members [BAX, BCL-2 homologous antagonist
killer (BAK), BCL-2-like protein 11 (BIM), BH3 interacting-domain
death agonist, and BCL-2-associated death promoter] and
pro-survival protein members (BCL-2, BCL-extra large, BCL like 2,
myeloid cell leukemia 1, BCL-like protein, and BCL2 related protein
A1) (27). Under normal conditions in
healthy lymphoid cells, the pro-survival members of the BCL2 family
constrain the essential cell death mediators BAX and BAK, thereby
maintaining cell viability. Stress signals, such as DNA damage
induced by chemotherapy, can trigger the activation of BH3-only
proteins, such as BIM. These proteins can bind to and inactivate
the pro-survival protein families, such as BCL-2, and allow the
activation of pro-apoptotic protein families such as BAX and BAK.
Once activated, BAX and BAK permeabilize the outer mitochondrial
membrane and trigger the release of factors, such as cytochrome c,
which functions as a co-factor for the activation of caspases,
including caspase-3, caspase-8 and caspase-9; as well as damage the
mitochondria which is the cell's major energy source (28). Caspases (cysteine-aspartic proteases)
are proteolytic enzymes that play an important role in controlling
cell death and inflammation. Caspase family members are classified
into two subgroups, namely, upstream or initiator (caspase-1, −2,
−4, −5, −8, −9, −10, −11 and 12) and downstream or effector
(caspase-3, −6, −7 and −14). Caspase-3 plays an important role in
cell apoptosis (29). PARP
participates in DNA damage by attaching to DNA repair proteins.
Adequate PARP expression is able to facilitate the DNA repair
process; however, activation of PARP is also able to induce cell
apoptosis (30). A recent study has
shown that BM-SCs-exosomes can protect multiple myeloma cells from
bortezomib-induced apoptosis to maintain their survival and induce
drug resistance by blocking the significant reduction of BCL-2 and
by reducing the expression levels of cleaved caspase-3 and PARP
(31).
The present results show that VP16 can inhibit the
expression of BCL-2 to allow the activation of BAX, which in turn
activates the executioner caspase-3 to increase the expression
level of the cleaved form of PARP and induce the apoptosis of
nalm-6 cells. However, exosomes derived from BM-SCs significantly
increased BCL-2, full-length caspase-3, and PARP, and decrease the
protein levels of BAX, cleaved caspase-3, and cleaved PARP to
reduce the apoptosis of nalm-6 cells. These results indicate that
BM-SCs-exosomes may be involved in the drug resistance of nalm-6
cells to VP16 by regulating cell apoptosis. This provides new ideas
and strategies for the clinical treatment of leukemia. However,
additional research is required to determine the potential effects
of these exosomes on the development, progression, and prognosis of
leukemia.
Acknowledgements
Shandong Province Natural Science Foundation (grant
nos., 2014GSF118131 and 201403010), The Major State Basic Research
Development Program (grant no., 2012CB966504), Shenzhen Science
Development Fund (grant no., JCYJ20140418115449178), Basic
Scientific Fund of Shandong University (grant no.,
2014QLKY02&2014QY003-11) and Science Foundation of Qilu
Hospital of Shandong University (grant no., 2016QLQN23) all
supported this study.
References
|
1
|
Wong RS and Cheong SK: Leukaemic stem
cells: Drug resistance, metastasis and therapeutic implications.
Malays J Pathol. 34:77–88. 2012.PubMed/NCBI
|
|
2
|
Raetz EA and Bhatla T: Where do we stand
in the treatment of relapsed acute lymphoblastic leukemia?
Hematology Am Soc Hematol Educ Program. 2012:129–136.
2012.PubMed/NCBI
|
|
3
|
Bianco P, Cao X, Frenette PS, Mao JJ,
Robey PG, Simmons PJ and Wang CY: The meaning, the sense and the
significance: Translating the science of mesenchymal stem cells
into medicine. Nat Med. 19:35–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meads MB, Hazlehurst LA and Dalton WS: The
bone marrow microenvironment as a tumor sanctuary and contributor
to drug resistance. Clin Cancer Res. 14:2519–2526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong RY and Cheong SK: Role of mesenchymal
stem cells in leukaemia: Dr. Jekyll or Mr. Hyde? Clin Exp Med.
14:235–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mudry RE, Fortney JE, York T, Hall BM and
Gibson LF: Stromal cells regulate survival of B-lineage leukemic
cells during chemotherapy. Blood. 96:1926–1932. 2000.PubMed/NCBI
|
|
7
|
Dias S, Choy M, Alitalo K and Rafii S:
Vascular endothelial growth factor (VEGF)-C signaling through FLT-4
(VEGFR-3) mediates leukemic cell proliferation, survival, and
resistance to chemotherapy. Blood. 99:2179–2184. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Benabbou N, Mirshahi P, Bordu C, Faussat
AM, Tang R, Therwath A, Soria J, Marie JP and Mirshahi M: A subset
of bone marrow stromal cells regulate ATP-binding cassette gene
expression via insulin-like growth factor-I in a leukemia cell
line. Int J Oncol. 45:1372–1380. 2014.PubMed/NCBI
|
|
9
|
Record M, Subra C, Silvente-Poirot S and
Poirot M: Exosomes as intercellular signalosomes and
pharmacological effectors. Biochem Pharmacol. 81:1171–1182. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vlassov AV, Magdaleno S, Setterquist R and
Conrad R: Exosomes: Current knowledge of their composition,
biological functions, and diagnostic and therapeutic potentials.
Biochim Biophys Acta. 1820:940–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lai RC, Arslan F, Lee MM, Sze NS, Choo A,
Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, et al:
Exosome secreted by MSC reduces myocardial ischemia/reperfusion
injury. Stem Cell Res. 4:214–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huan J, Hornick NI, Shurtleff MJ, Skinner
AM, Goloviznina NA, Roberts CT Jr and Kurre P: RNA trafficking by
acute myelogenous leukemia exosomes. Cancer Res. 73:918–929. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Umezu T, Ohyashiki K, Kuroda M and
Ohyashiki JH: Leukemia cell to endothelial cell communication via
exosomal miRNAs. Oncogene. 32:2747–2755. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raimondo S, Saieva L, Corrado C, Fontana
S, Flugy A, Rizzo A, De Leo G and Alessandro R: Chronic myeloid
leukemia-derived exosomes promote tumor growth through an autocrine
mechanism. Cell Commun Signal. 13:82015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ansa-Addo EA, Lange S, Stratton D,
Antwi-Baffour S, Cestari I, Ramirez MI, McCrossan MV and Inal JM:
Human plasma membrane-derived vesicles halt proliferation and
induce differentiation of THP-1 acute monocytic leukemia cells. J
Immunol. 185:5236–5246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roccaro AM, Sacco A, Maiso P, Azab AK, Tai
YT, Reagan M, Azab F, Flores LM, Campigotto F, Weller E, et al: BM
mesenchymal stromal cell-derived exosomes facilitate multiple
myeloma progression. J Clin Invest. 123:1542–1555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mineo M, Garfield SH, Taverna S, Flugy A,
De Leo G, Alessandro R and Kohn EC: Exosomes released by K562
chronic myeloid leukemia cells promote angiogenesis in a
Src-dependent fashion. Angiogenesis. 15:33–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tadokoro H, Umezu T, Ohyashiki K, Hirano T
and Ohyashiki JH: Exosomes derived from hypoxic leukemia cells
enhance tube formation in endothelial cells. J Biol Chem.
288:34343–34351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reiners KS, Topolar D, Henke A, Simhadri
VR, Kessler J, Sauer M, Bessler M, Hansen HP, Tawadros S, Herling
M, et al: Soluble ligands for NK cell receptors promote evasion of
chronic lymphocytic leukemia cells from NK cell anti-tumor
activity. Blood. 121:3658–3665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen W, Wang J, Shao C, Liu S, Yu Y, Wang
Q and Cao X: Efficient induction of antitumor T cell immunity by
exosomes derived from heat-shocked lymphoma cells. Eur J Immunol.
36:1598–1607. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fortney JE, Zhao W, Wenger SL and Gibson
LF: Bone marrow stromal cells regulate caspase 3 activity in
leukemic cells during chemotherapy. Leuk Res. 25:901–907. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hall BM, Fortney JE, Taylor L, Wood H,
Wang L, Adams S, Davis S and Gibson LF: Stromal cells expressing
elevated VCAM-1 enhance survival of B lineage tumor cells. Cancer
Lett. 207:229–239. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Camussi G, Deregibus MC, Bruno S, Grange
C, Fonsato V and Tetta C: Exosome/microvesicle-mediated epigenetic
reprogramming of cells. Am J Cancer Res. 1:98–110. 2011.PubMed/NCBI
|
|
24
|
Fodale V, Pierobon M, Liotta L and
Petricoin E: Mechanism of cell adaptation: When and how do cancer
cells develop chemoresistance? Cancer J. 17:89–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zaman S, Wang R and Gandhi V: Targeting
the apoptosis pathway in hematologic malignancies. Leuk Lymphoma.
55:1980–1992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Delbridge AR and Strasser A: The BCL-2
protein family, BH3-mimetics and cancer therapy. Cell Death Differ.
22:1071–1080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reed JC: Bcl-2-family proteins and
hematologic malignancies: History and future prospects. Blood.
111:3322–3330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yi CH and Yuan J: The Jekyll and Hyde
functions of caspases. Dev Cell. 16:21–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bai P: Biology of poly(ADP-Ribose)
polymerases: The factotums of cell maintenance. Mol Cell.
58:947–958. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Hendrix A, Hernot S, Lemaire M, De
Bruyne E, Van Valckenborgh E, Lahoutte T, De Wever O, Vanderkerken
K and Menu E: Bone marrow stromal cell-derived exosomes as
communicators in drug resistance in multiple myeloma cells. Blood.
124:555–566. 2014. View Article : Google Scholar : PubMed/NCBI
|