Introduction
As a major malignant tumor of the digestive system,
the morbidity and mortality rates of colorectal cancer (CRC) are
increasing globally (1). Similar to
other types of solid tumors, the high mortality rate of CRC is due
to metastasis, a complicated process that is often associated with
alterations in the extracellular matrix to enhance cell motility
and the ability of cells to grow at a remote location (2). The molecular mechanisms underlying this
process remain to be elucidated (3).
Despite progress in improving the early diagnosis and treatment of
CRC, patients with advanced cancer have a poor prognosis, and their
5-year survival rate is only 45% (4,5). Patients
with CRC do not exhibit many early symptoms and a large portion are
diagnosed at the mid-late stage, thus the 5-year survival rate is
low (6,7). Currently, the pathogenesis of CRC
remains ill-defined, thus there is an urgent requirement to
investigate novel treatments.
The growth of CRC involves various genes. In a
previous study, a functionally unknown gene, family with sequence
similarity 172, member A (FAM172A), was identified (8). The association between the presence of
tumors and FAM172A has been investigated in our previous studies.
It was revealed that overexpressed FAM172A inhibited proliferation
of the HepG2 cell line, as assessed by reverse
transcription-quantitative polymerase chain reaction and western
blotting (9). HepG2 cells
demonstrated cell cycle period arrest in the S phase, and their
proliferation was signally inhibited when transfected with high
concentrations of FAM172A recombinant protein (9). An online software program, CELLO 2.5
(http://cello.life.nctu.edu.tw/), was
used to predict the subcellular localization of FAM172A (10), and the finding revealed that it was
generally positioned in the cytoplasm.
Nucleotide-binding protein 1 (NUBP1) is a protein
coding gene and a member of the NUBP/multidrug
resistance-associated protein subfamily of adenosine
triphosphate-binding proteins (11,12). Among
its associated pathways are metabolism and cytosolic iron-sulfur
cluster assembly, and Gene Ontology annotations regarding this gene
include nucleotide-binding and 4 iron, 4 sulfur cluster-binding.
NUBP1 is involved in the regulation of centrosome duplication and
the assembly of cytosolic protein (13,14). NUBP1
is a component of an iron-sulfur scaffold complex, which mediates
the assembly of an iron-sulfur cluster (15).
Taken together, these two proteins may be associated
with regulating the cell cycle. As the association between the
clinical activity and the combined expression levels of NUBP1 and
FAM172A in CRC have not yet been investigated, the present study
examined the association between the expression level and
prognostic impact of NUBP1 and FAM172A in patients with CRC, as
assessed by immunohistochemical staining.
Materials and methods
Samples and patients
A total of 180 patients who underwent surgery
between October 2012 and October 2014 at the Department of
Pathology, Nanfang Hospital and Zhujiang Hospital, which are
affiliated with Southern Medical University (Guangzhou, China) were
enrolled. The study consisted of 83 males and 97 females, with a
median age of 57 years (range, 38–77 years). A total of 180
paraffin-embedded specimens of CRC were included in the present
study. In accordance with the World Health Organization criteria,
the data of all patients were examined and regraded (16), and pathological stage was
distinguished in the light of the Tumor-Node-Metastasis (TNM) stage
system (17). Furthermore, 60 cases
of normal colorectal tissues were also included for the evaluation
of the FAM172A and NUBP1 expression levels in the non-cancerous
colorectal mucosa. The patients did not receive biotherapy,
chemotherapy, radiotherapy or any other treatment strategies
pre-operatively. The pathological diagnosis was formed by three
blinded experts from the Department of Pathology of Nanfang
Hospital and Zhujiang Hospital. The 180 patients were grouped
according to patient age and sex, serum levels of carcinoembryonic
antigen (CEA) and carbohydrate antigen 19–9 (CA19-9), tumor
staging, the identification of lymph node (LN) metastasis, presence
of distant metastasis and type of histology according to the World
Health Organization classification. Written informed consent was
obtained from all patients prior to enrollment in the present
study. The study was approved by the Ethics Committee of Nanfang
Hospital. According to the Declaration of Helsinki, written
informed consent was obtained from all patients prior to enrollment
in the present study.
Main reagents
Rabbit anti-human FAM172A monoclonal antibody (cat.
no. ab121364; Abcam Biotechnology, Inc., Cambridge, UK), mouse
anti-human NUBP1 polyclonal antibody (cat. no. ab88622; Abcam
Biotechnology, Inc., Abcam, Cambridge, UK), SP immunohistochemical
kit (cat. no. SP-9000; Beijing Zhongshan Golden Bridge
Biotechnology, Inc., Beijing, China) and DAB color-developing
reagent (catalog no. ZLI-9017; Beijing Zhongshan Golden Bridge
Biotechnology, Inc.) were used in the present study.
Immunohistochemical staining and
scoring
Firstly, tissue samples were cut into 4-µm thick
slices. The antibodies that were used for immunohistochemistry were
the FAM172A monoclonal antibody clone and NUBP1 monoclonal antibody
clone. The tissue sections were stained according to the
manufacturer's instructions from the SP immunohistochemical kit and
DAB color-developing reagent.
Briefly, the tissues were deparaffinized in xylene
at room temperature for 10 min and rehydrated in an ethanol
gradient. The antigen of paraffin slices was retrieved by citric
acid buffer for 10 min at 95°C (pH=6.0). Hydrogen peroxide solution
(0.3%) was used to block the activation of endogenous peroxidase
for 18 min at room temperature. Incubation was performed overnight
at 4°C and the primary antibodies for FAM172A and NUBP1 were
applied at a dilution of 1:200. At room temperature, the tissue
samples were washed with PBS and incubated with the enzyme
horseradish peroxidase-labeled polymer rabbit or mouse antibodies
for 16 min (cat. no. SP-9000; Beijing Zhongshan Golden Bridge
Biotechnology, Inc.). Subsequently, the tissue samples were
incubated with DAB at room temperature for 5 min in order to
visualize the expression levels. Counterstaining was performed
using hematoxylin and the tissues were dehydrated using a series of
ethanol and xylene.
The scoring of the immunohistochemical staining was
performed independently by two researchers from the Department of
Vascular Surgery, Nanfang Hospital, Southern Medical University,
who were not familiar with the clinicopathological data. PBS, which
was used as the negative control, was a substitute for the primary
antibody. Observations were conducted in 10 random high-power
fields under a light microscope (magnification, ×400) according to
the count and staining intensity of positive cells (CX31-LV320;
Olympus, Inc., Tokyo, Japan).
The H-score method was used in the present study.
The staining intensity of positive cells was scored as follows: 0,
no color; 1, light yellow; 2, light brown; or 3, brown. The
quantity of positive cells was scored as follows: 0, 0%; 1,
<10%; 2, 10–35%; 3, 35–75%; or 4, >75%. The percentage score
was multiplied with the staining intensity score. The score of each
tissue sample was classified into one of four grades: 0–2 (−), 2–4
(+), 4–6 (++) and >6 (+++). When the score was ≥4 for a tissue
sample, the protein expression level was considered to be
positive.
Statistical analysis
Overall survival (OS) and relapse-free survival
(RFS) were the endpoints of interest, and the endpoint of follow-up
was the date of the last contact or mortality between 2000 and
2013. The paired-samples t-test was designed to compare expression
levels in CRC tissues with normal tissues. The period of time from
surgery to mortality or last contact was used as the OS time, and
patients that survived to the last contact period were considered
as censored. The period of time from surgery to recurrence, last
contact or mortality was defined as RFS time, and patients were
regarded as censored if they survived and experienced no relapse.
For prognosis, Pearson's χ2 test was used for analyzing
correlations between overall scores and potentially meaningful
categorical variables. For clinicopathological factors and FAM172A
and NUBP1, the present study applied univariate and multivariate
cox proportional hazards regression analysis to evaluate their
effect on OS and RFS. Kaplan-Meier survival curves were constructed
to estimate the OS of patients and further analyze the impact of
FAM172A and NUBP1 expression using the log-rank test. SPSS software
(version 20.0; IBM Corp., Armonk, NY, USA) was used for statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of FAM172A and NUBP1
are associated with certain clinicopathological features of
patients with CRC
The clinicopathological characteristics are outlined
in Table I. Associations between
FAM172A and NUBP1 expression levels in normal colorectal mucosa and
CRC tissues are presented in Table
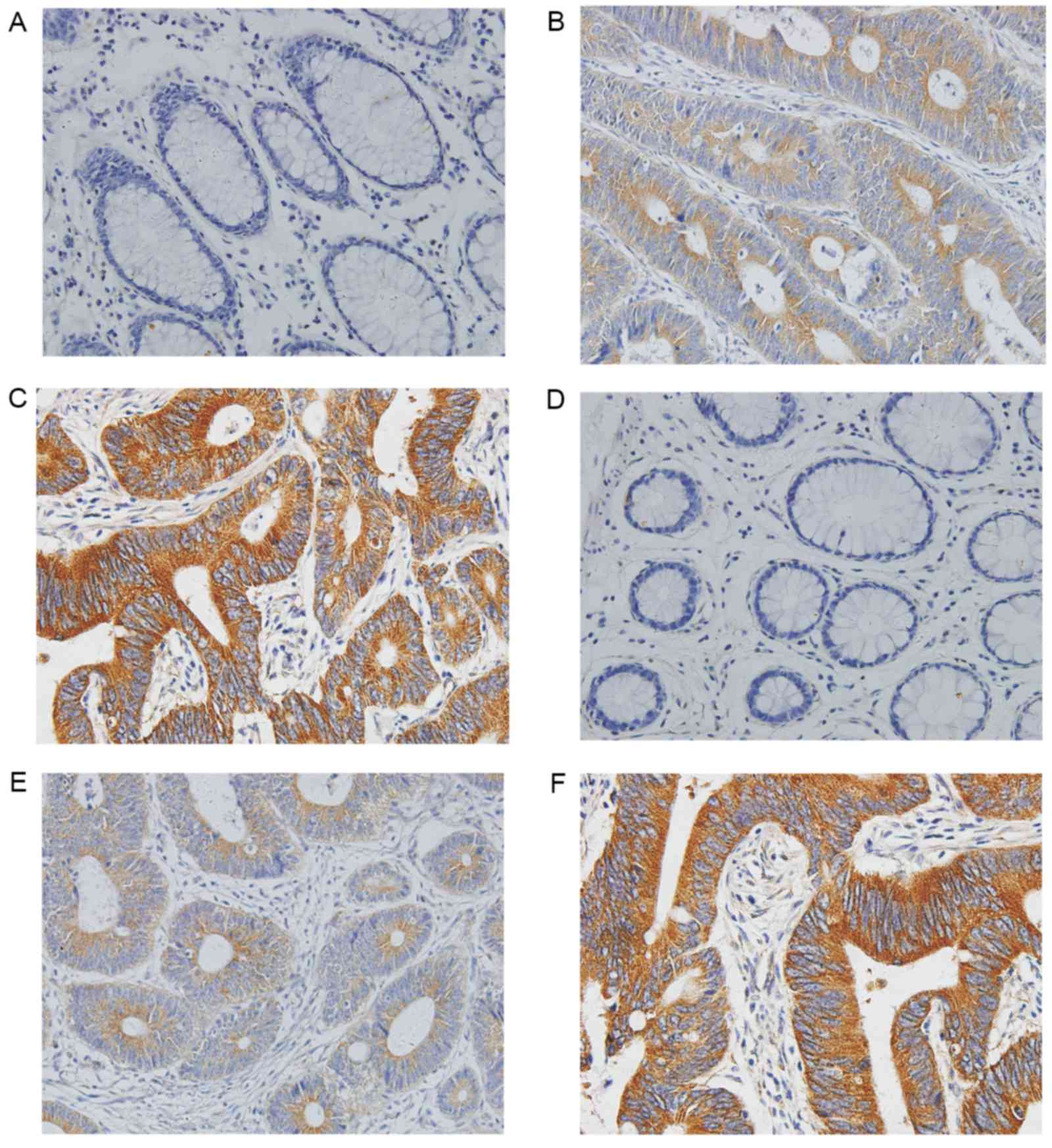
II. The present study revealed that immunoreactivity of FAM172A
and NUBP1 existed primarily in the cytoplasm (Fig. 1). Weak nuclear staining was identified
in 11 cases for FAM172A and 10 cases for NUBP1, and positive
expression levels of FAM172A and NUBP1 were absent in 60 samples of
normal colorectal mucosa. However, positive expression levels of
FAM172A and NUBP1 were observed in 85% (153/180) and 83% (149/180)
of patients with CRC, respectively.
 | Table I.Association between expression levels
of FAM172A and NUBP1 and various clinicopathological
parameters. |
Table I.
Association between expression levels
of FAM172A and NUBP1 and various clinicopathological
parameters.
|
|
| FAM172A | NUBP1 |
|---|
|
|
|
|
|
|---|
| Characteristics | No. of patients | Positive, n (%) | P-value | Positive, n (%) | P-value |
|---|
| Patient age,
years |
|
| 0.084 |
| 0.944 |
|
<40 | 60 | 42 (70) |
| 39 (65) |
|
|
40–60 | 58 | 34 (59) |
| 38 (66) |
|
|
>60 | 62 | 48 (77) |
| 42 (68) |
|
| Sex |
|
| 0.851 |
| 0.924 |
|
Female | 97 | 82 (85) |
| 80 (82) |
|
| Male | 83 | 71 (86) |
| 68 (82) |
|
| CEA |
|
| 0.023 |
| 0.006 |
|
Normal | 66 | 26 (39) |
| 23 (35) |
|
|
Elevated | 114 | 65 (57) |
| 64 (56) |
|
| CA19-9 |
|
| 0.016 |
| 0.001 |
|
Normal | 71 | 32 (45) |
| 37 (52) |
|
|
Elevated | 109 | 66 (61) |
| 75 (69) |
|
| TNM stage |
|
| <0.001 |
| <0.001 |
| I and
II | 101 | 50 (50) |
| 47 (47) |
|
| III and
IV | 79 | 72 (91) |
| 75 (95) |
|
| LN metastasis |
|
| 0.004 |
| 0.005 |
|
Absence | 109 | 83 (76) |
| 81 (74) |
|
|
Presence | 71 | 66 (93) |
| 65 (92) |
|
| Distant
metastasis |
|
| 0.071 |
| 0.079 |
|
Absence | 138 | 77 (56) |
| 81 (59) |
|
|
Presence | 42 | 30 (72) |
| 31 (74) |
|
| Differentiation |
|
| 0.016 |
| 0.024 |
| WD | 44 | 20 (45) |
| 22 (50) |
|
| MD | 121 | 85 (70) |
| 88 (73) |
|
| PD | 15 | 11 (73) |
| 10 (67) |
|
| Tumor magnitude,
cm |
|
| 0.068 |
| 0.064 |
|
<2 | 26 | 10 (38) |
| 12 (46) |
|
| 2–4 | 109 | 59 (54) |
| 64 (59) |
|
|
>4 | 45 | 30 (67) |
| 33 (73) |
|
| Dukes' stage |
|
| <0.001 |
| <0.001 |
| A and
B | 102 | 50 (49) |
| 48 (39) |
|
| C and
D | 78 | 74 (95) |
| 71 (73) |
|
| NUBP1 |
|
| 0.026 |
|
Positive | 149 | 144 (92) |
|
|
|
|
Negative | 31 | 27 (16) |
|
|
|
 | Table II.Comparison of FAM172A and NUBP1
positive expression levels with colorectal cancer and normal
colorectal tissues. |
Table II.
Comparison of FAM172A and NUBP1
positive expression levels with colorectal cancer and normal
colorectal tissues.
| Colorectal
tissue | n | FAM172A-positive, n
(%) | P-value | NUBP1-positive, n
(%) | P-value |
|---|
| Cancer | 60 | 58 (85.0) | 0.022 | 59 (98.3) | 0.012 |
| Normal | 60 | 11 (18.3) |
| 10 (20.0) |
|
The expression level of FAM172A was markedly
associated with the expression levels of serum CEA (P=0.023) and
CA19-9 (P=0.016), TNM staging (P<0.001), LN metastasis
(P=0.004), histological grade (P=0.01), Dukes' staging (P<0.001)
and NUBP1 expression level (P=0.026). Furthermore, the expression
level of NUBP1 was markedly associated with the serum CEA (P=0.006)
and CA19-9 (P=0.001) expression levels, TNM staging (P<0.001),
LN metastasis (P=0.005), histological type (P=0.024) and Dukes'
staging (P<0.001), as presented in Table I.
The present study used univariate cox regression
analysis to investigate the association between clinicopathological
characteristics plus the expression levels of the two proteins and
RFS and OS (Table III). Results of
univariate analysis revealed lower TNM staging and the existence of
LN metastasis were associated with longer OS and RFS times, and
that there were negative associations between the expression level
of FAM172A and OS and RFS (P=0.013 and P=0.012, respectively) and
there was also a negative correlation between NUBP1 expression
level and OS and RFS (P<0.001 and P<0.001, respectively).
 | Table III.Univariate cox proportional hazards
regression analysis of clinicopathological characteristics and
their effect on RFS and OS. |
Table III.
Univariate cox proportional hazards
regression analysis of clinicopathological characteristics and
their effect on RFS and OS.
|
|
| OS | RFS |
|---|
|
|
|
|
|
|---|
|
Characteristics | No. of
patients | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| CEA |
|
|
|
|
|
|
Normal | 66 | 1.000 |
| 1.000 |
|
|
Elevated | 114 | 1.499
(1.703–5.008) | 0.217 | 1.473
(0.775–2.802) | 0.237 |
| TNM stage |
|
|
|
|
|
| I and
II | 101 | 1.000 |
| 1.000 |
|
| III and
IV | 79 | 6.391
(3.790–10.744) | <0.001 | 5.940
(3.551–9.937) | <0.001 |
| LN metastasis |
|
|
|
|
|
|
Absence | 109 | 1.000 |
| 1.000 |
|
|
Presence | 71 | 2.816
(1.641–4.831) | <0.001 | 2.758
(1.609–4.726) | <0.001 |
| NUBP1 |
|
|
|
|
|
|
Negative | 31 | 1.000 |
| 1.000 |
|
|
Positive | 149 | 4.293
(1.967–9.373) | <0.001 | 1.612
(1.946–9.268) | <0.001 |
| FAM172A |
|
|
|
|
|
|
Negative | 29 | 1.000 |
| 1.000 |
|
|
Positive | 145 | 4.200
(1.816–9.714) | 0.01 | 4.018
(1.740–9.275) | 0.012 |
Multivariate analysis was performed according to the
total data of 180 patients for the considered variables, including
the serum expression levels of CEA and CA19-9, TNM staging and
Dukes' staging, the presence of LN metastasis and distant
metastasis, and FAM172A and NUBP1 expression levels. The results of
the present study suggested that only the expression levels of
FAM172A and NUBP1 and tumor staging may be independent prognostic
makers for OS and RFS of patients with CRC (Table IV). Patients with positive FAM172A
expression had a 2.726-fold [95% confidence interval (95% CI),
1.121–6.630] increase in the mortality risk and a 2.478-fold (95%
CI, 1.027–5.982) increase in the risk of disease. Patients with
positive expression of NUBP1 had a 3.029-fold (95% CI, 1.286–7.130)
higher risk of mortality and a 3.101-fold (95% CI, 1.318–7.296)
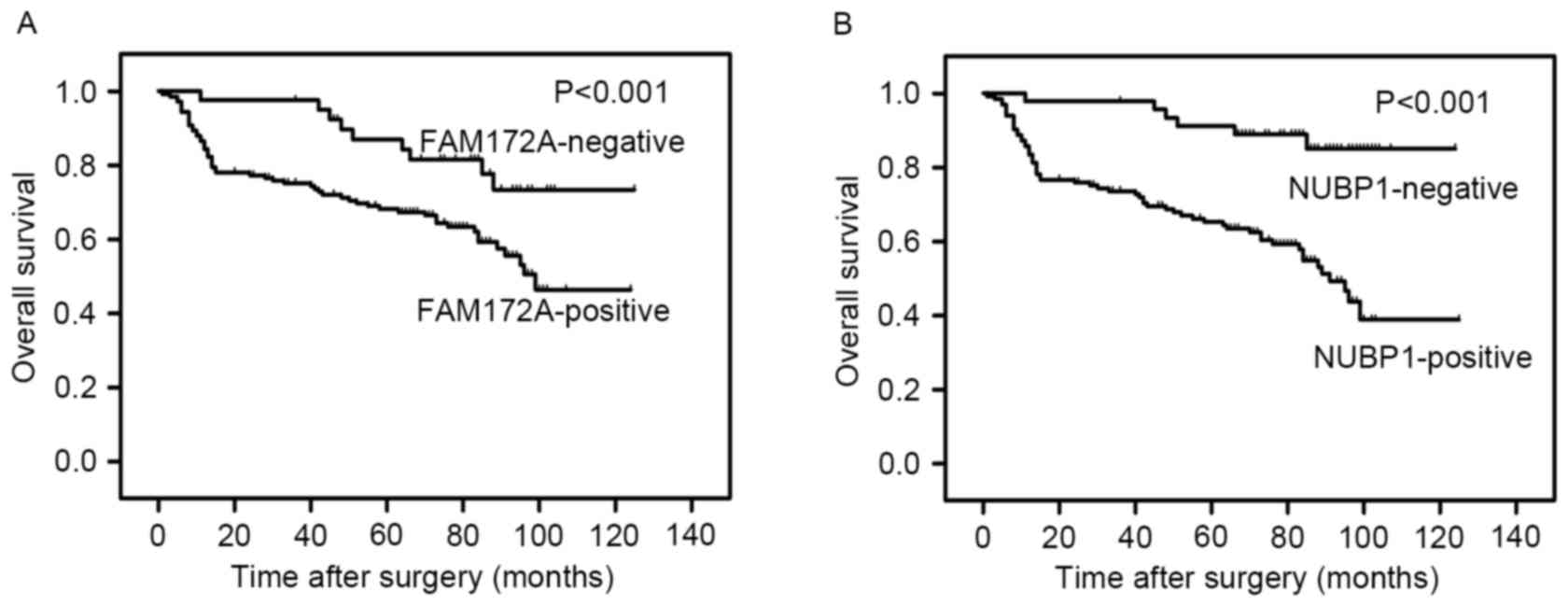
higher risk for recurrence of disease. Kaplan-Meier survival curves
were constructed to estimate the OS of patients and further analyze
the impact of FAM172A and NUBP1 expression using the log-rank test
(Fig. 2). Higher expression levels of
FAM172A and NUBP1 were associated with a significantly shorter
survival and higher relapse rate in colorectal carcinoma patients
(P<0.001 and P<0.001, respectively).
 | Table IV.Multivariate Cox regression analysis
for RFS and OS. |
Table IV.
Multivariate Cox regression analysis
for RFS and OS.
|
| OS | RFS |
|---|
|
|
|
|
|---|
| Characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| FAM172A |
|
|
|
|
|
Negative | 1.000 |
| 1.000 |
|
|
Positive | 2.726
(1.121–6.630) | 0.027 | 2.478
(1.027–5.982) | 0.03 |
| NUBP1 |
|
|
|
|
|
Negative | 1.000 |
| 1.000 |
|
|
Positive | 3.029
(1.286–7.130) | 0.011 | 3.101
(1.318–7.296) | 0.01 |
| TNM stage |
|
|
|
|
| I and
II | 1.000 |
| 1.000 |
|
| III and
IV | 4.472
(2.403–8.323) | <0.001 | 4.083
(2.216–7.523) | <0.001 |
Discussion
The development of novel tumor markers may
contribute to improving the early diagnosis of CRC and the 5-year
patient survival rate. Firstly, the present study performed
immunohistochemical staining to investigate the expression levels
of FAM172A and NUBP1 in human CRC tissues. Secondly, analysis of
the association between the expression levels of FAM172A and NUBP1
and the corresponding prognostic significance was performed. To the
best of our knowledge, the present study demonstrated the following
for the first time: i) A high proportion of CRC tissue samples
expressed FAM172A and NUBP1; ii) expression levels of FAM172A and
NUBP1 were associated with unfavorable clinicopathological
variables, including high serum level of CEA, high clinical stage
(stage III and IV) and the existence of LN metastasis; and iii)
higher expression levels of FAM172A and NUBP1 were associated with
a significantly shorter survival time and higher relapse rate in
patients with CRC. Additionally, NUBP1 and FAM172A were revealed to
be independent prognostic factors of CRC by multivariate analysis.
Taken together, the results of the present study demonstrated that
FAM172A and NUBP1 may participate in the progression of CRC and be
independent indicators of poor prognoses for patients with CRC.
Numerous previous studies have revealed that NUBP1
was involved in regulating centrosome duplication in mammalian
cells (14,18). Other previous studies have suggested
that NUBP1 protein may be involved in chaperonin-containing
TCP-1/TCP-1 ring complex chaperone activity in ciliogenesis
(12,19) and that the C-terminal region of NUBP1
may be crucial for nuclear transfer (18). NUBP1 was primarily believed to be a
nuclear protein (20–22); however, little is known about the
association between NUBP1 and CRC.
Cytoplasmic localization of FAM172A was observed by
the present study. Our previous study demonstrated that complete
cell cycle arrest was exhibited in the S phase of HepG2 cells at a
high concentration when HepG2 cells were co-cultured with the
FAM172A recombinant protein (9).
Evaluation of cytoplasmic FAM172A expression levels revealed that
144/180 cases in the present study exhibited cytoplasmic FAM172A
expression. However, the association between CRC and the expression
of FAM172A remains unknown.
In the present study, FAM172A and NUBP1 were
demonstrated to be expressed in normal colorectal mucosa and CRC
tissues, and CRC tissues exhibited higher expression levels. Thus,
the results revealed that FAM172A and NUBP1 genes may act as
important and novel cancer-associated genes, and the deficiency or
variation of these two genes may result in the development of
CRC.
However, when considering the anti-oncogenic role of
FAM172A in hepatocellular carcinoma development and progression in
our previous study (9), FAM172A may
also serve an anti-oncogenic role in CRC. Therefore, it has been
suggested that loss of FAM172A may result in the inhibition of cell
death and promote tumorigenesis. In addition, the present study
indicated that there was a positive association between the
expression levels of FAM172A and NUBP1, and that the FAM172A and
NUBP1 expression levels were significantly associated with advanced
clinicopathological factors and a poor prognosis for patients with
CRC.
Although our previous study demonstrated that
FAM172A was essential to the cell cycle regulation and
proliferation of HepG2 cells (9), the
reason the high expression level of FAM172A is indicated as a tumor
suppressor and is associated with advanced cancer characteristics
remains unknown. This may be due to an accumulation of
immunohistochemically detectable mutant FAM172A or due to
downstream functional defects, despite the presence of normal
FAM172A protein expression. The results of the present study
indicated that FAM172A expression was significantly associated with
NUBP1 expression level, which supports the contention that NUBP1 is
upregulated to modulate FAM172A activity. Therefore, the present
study assumed that the expression levels of FAM172A and NUBP1 serve
important roles. They may make a difference in regulating cell
survival in CRC, and the poor prognosis of patients with CRC may
result from decreased or low expression levels of FAM172A. However,
studies investigating the exact role of FAM172A and NUBP1 have
previously been limited. Therefore, further analysis of NUBP1 and
FAM172A expression levels in CRC is required in order to identify
their mechanism of action and to determine the role of NUBP1 and
FAM172A in carcinogenesis.
In conclusion, a high proportion of CRC tissues
demonstrated expression of FAM172A and NUBP1, and the expression
was associated with unfavorable CRC characteristics and a poor
outcome for patients with CRC. Although the role of FAM172A and
NUBP1 in tumorigenesis remains unclear, the results of the present
study suggested that their expression levels may be a diagnostic
and prognostic index with prospective clinical function for
patients with CRC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172383), the
Guangdong Natural Science Foundation (grant no. 2014A030313324) and
the Key Clinical Specialty Discipline Construction Program.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steeg PS: Metastasis suppressors alter the
signal transduction of cancer cells. Nat Rev Cancer. 3:55–63. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fidler IJ: Critical determinants of
metastasis. Semin Cancer Biol. 12:89–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin L, Piao J, Gao W, Piao Y, Jin G, Ma Y,
Li J and Lin Z: DEK over expression as an independent biomarker for
poor prognosis in colorectal cancer. BMC Cancer. 13:3662013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vodicka J, Spidlen V, Treska V, Fichtl J,
Simanek V, Safranek J, Vejvodova S, Mukensnabl P and Topolcan O:
Surgical treatment of colorectal cancer pulmonary metastases:
12-year results. Anticancer Res. 34:4239–4245. 2014.PubMed/NCBI
|
|
6
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cortéa H, Manceau G, Blons H and
Laurent-Puiga P: MicroRNA and colorectal cancer. Dig Liver Dis.
44:195–200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li LX, Tao Z, Dong XH, Liang WC, Yang ZH,
Mou B, Bao YQ, Wang C, Jia WP and Hu RM: Molecular cloning of a
novel gene, C5orf21 gene and its roles in diabetic macroangiopathy.
Zhonghua Yi Xue Za Zhi. 89:2574–2577. 2009.(In Chinese). PubMed/NCBI
|
|
9
|
Feng Z, Li H, Liu S, Cheng J, Xiang G and
Zhang J: FAM172A induces S phase arrest of HepG2 cells via Notch 3.
Oncol Rep. 29:1154–1160. 2013.PubMed/NCBI
|
|
10
|
Yu CS, Chen YC, Lu CH and Hwang JK:
Prediction of protein subcellular localization. Proteins.
64:643–651. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakashima H, Grahovac MJ, Mazzarella R,
Fujiwara H, Kitchen JR, Threat TA and Ko MS: Two novel mouse
genes-Nubp2, mapped to the t-complex on chromosome 17, and Nubp1,
mapped to chromosome 16-establish a new gene family of
nucleotide-binding proteins in eukaryotes. Genomics. 60:152–160.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kypri E, Christodoulou A, Maimaris G,
Lethan M, Markaki M, Lysandrou C, Lederer CW, Tavernarakis N,
Geimer S, Pedersen LB and Santama N: The nucleotide-binding
proteins Nubp1 and Nubp2 are negative regulators of ciliogenesis.
Cell Mol Life Sci. 71:517–538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kupke T, Di Cecco L, Müller HM, Neuner A,
Adolf F, Wieland F, Nickel W and Schiebel E: Targeting of Nbp1 to
the inner nuclear membrane is essential for spindle pole body
duplication. EMBO J. 30:3337–3352. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Christodoulou A, Lederer CW, Surrey T,
Vernos I and Santama N: Motor protein KIFC5A interacts with Nubp1
and Nubp2, and is implicated in the regulation of centrosome
duplication. J Cell Sci. 119:2035–2047. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stehling O, Netz DJ, Niggemeyer B, Rösser
R, Eisenstein RS, Puccio H, Pierik AJ and Lill R: Human Nbp35 is
essential for both cytosolic iron-sulfur protein assembly and iron
homeostasis. Mol Cell Biol. 28:5517–5528. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hamilton SR and Aaltonen LA: World Health
Organization classification of tumoursPathology and Genetics of
Tumours of the Digestive System. IARC Press; Lyon: 2000
|
|
17
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ververis A, Christodoulou A, Christoforou
M, Kamilari C, Lederer CW and Santama N: A novel family of
katanin-like 2 protein isoforms (KATNAL2), interacting with
nucleotide-binding proteins Nubp1 and Nubp2, are key regulators of
different MT-based processes in mammalian cells. Cell Mol Life Sci.
73:163–184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kupke T, Di Cecco L, Müller HM, Neuner A,
Adolf F, Wieland F, Nickel W and Schiebel E: Targeting of Nbp1 to
the inner nuclear membrane is essential for spindle pole body
duplication. EMBO J. 30:3337–3352. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okuno T, Yamabayashi H and Kogure K:
Comparison of intracellular localization of Nubp1 and Nubp2 using
GFP fusion proteins. Mol Biol Rep. 37:1165–1168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Araki Y, Lau CK, Maekawa H, Jaspersen SL,
Giddings TH Jr, Schiebel E and Winey M: The Saccharomyces
cerevisiae spindle pole body (SPB) component Nbp1p is required
for SPB membrane insertion and interacts with the integral membrane
proteins Ndc1p and Mps2p. Mol Biol Cell. 17:1959–1970. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimizu Y, Akashi T, Okuda A, Kikuchi A
and Fukui K: NBP1 (Nap1 binding protein 1), an essential gene for
G2/M transition of Saccharomyces cerevisiae, encodes a
protein of distinct sub-nuclear localization. Gene. 246:395–404.
2000. View Article : Google Scholar : PubMed/NCBI
|