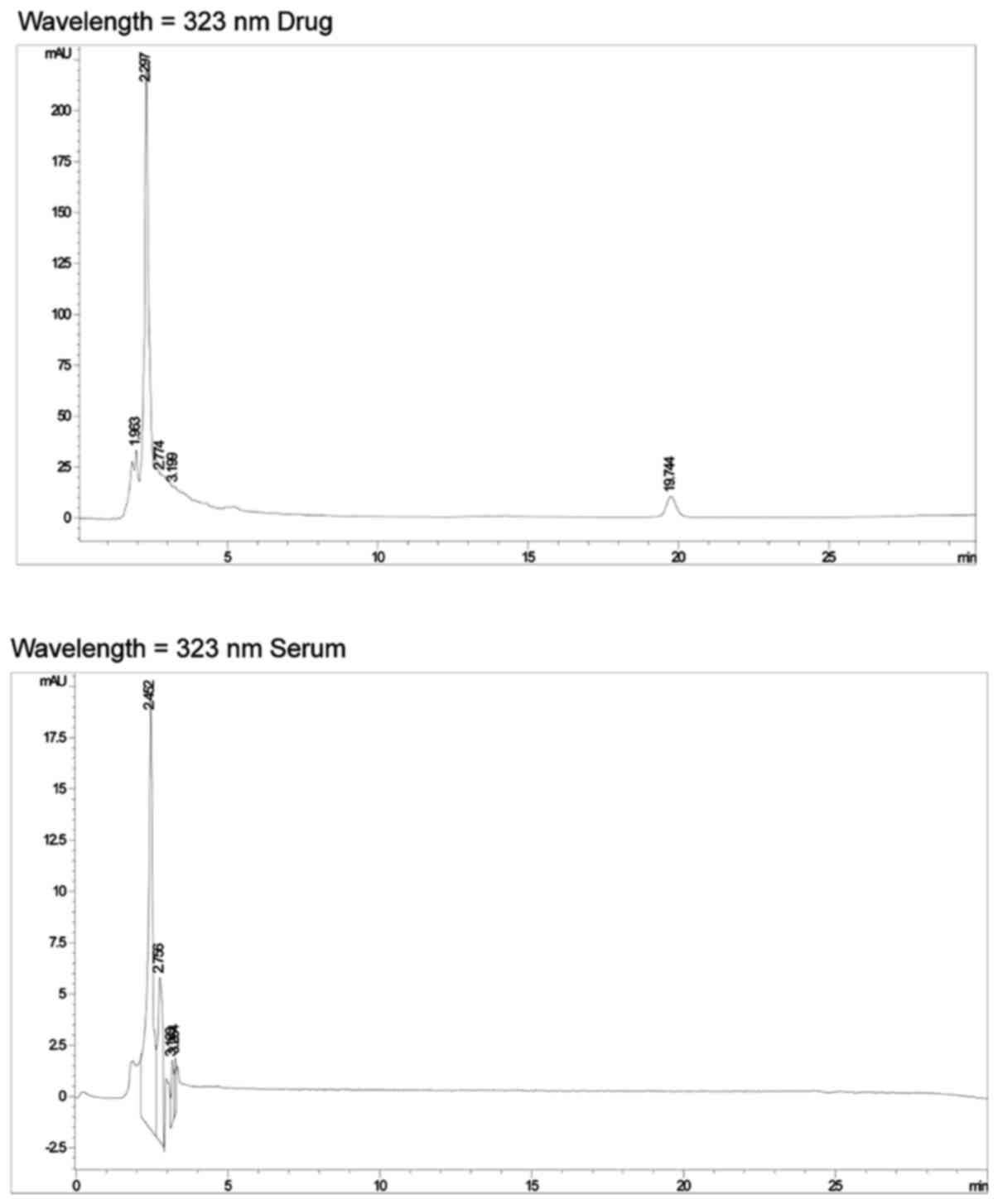
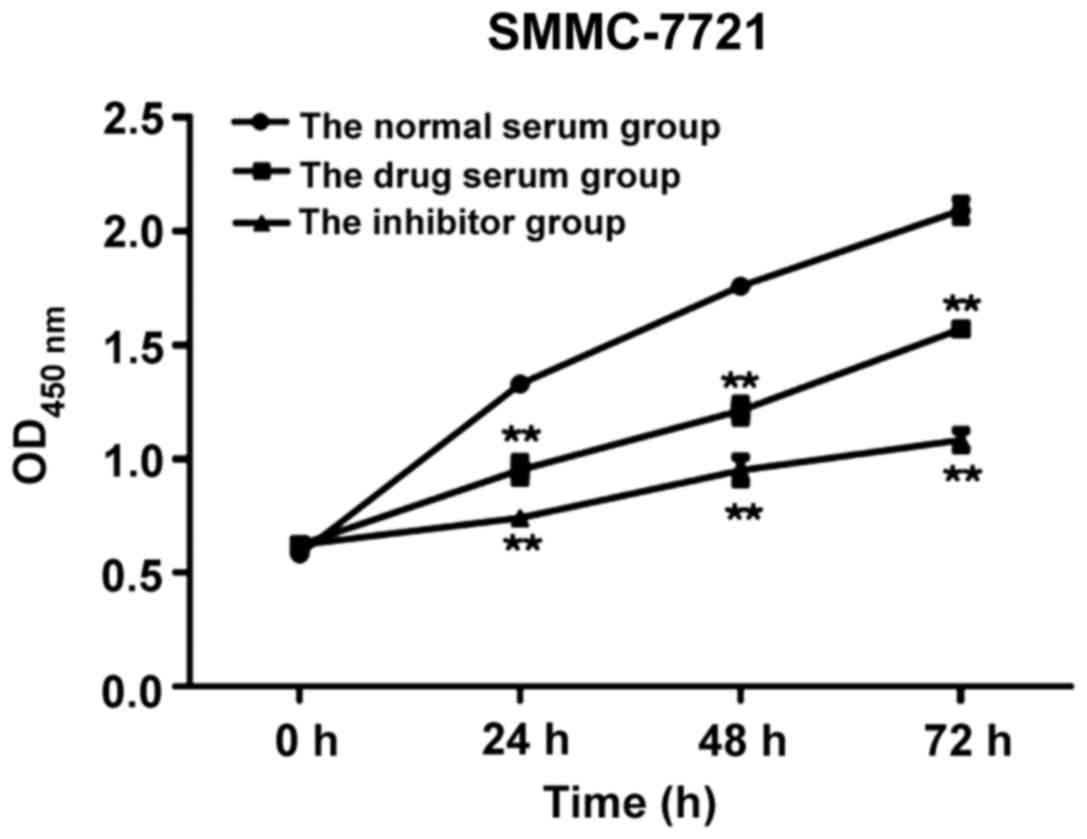
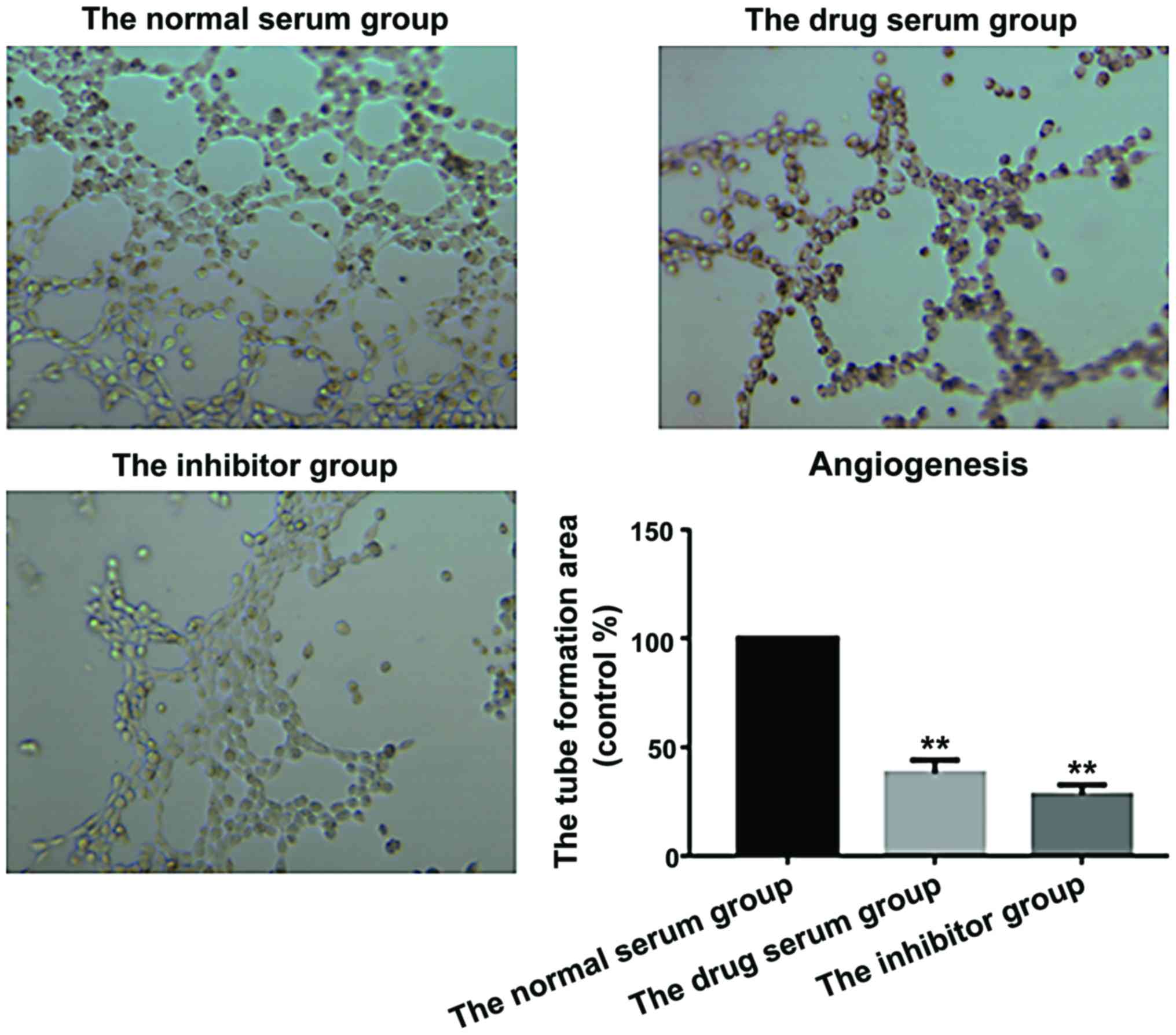
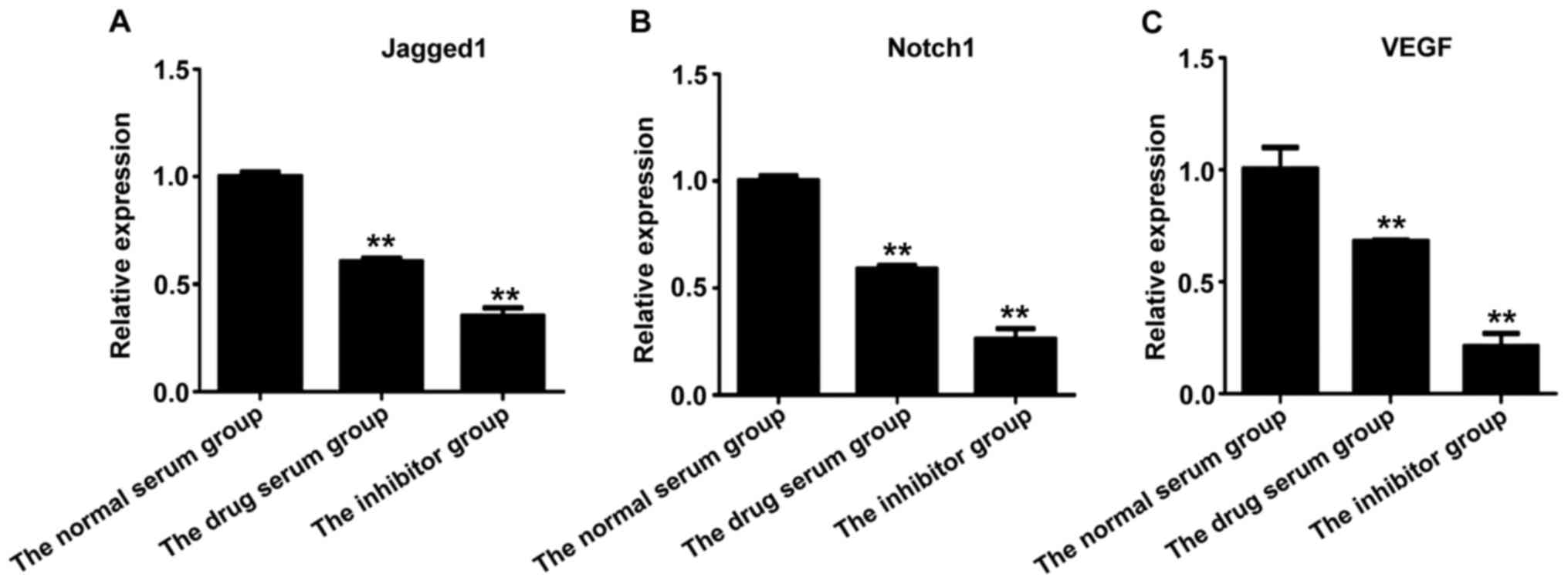
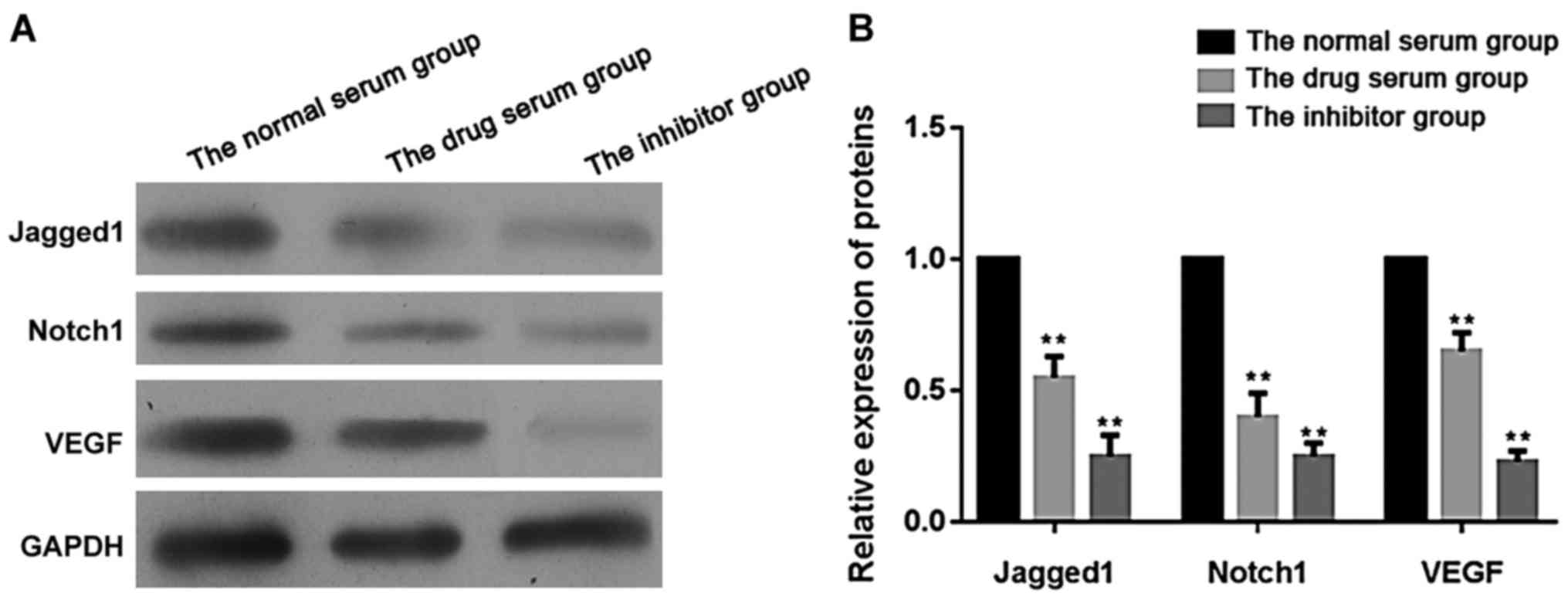
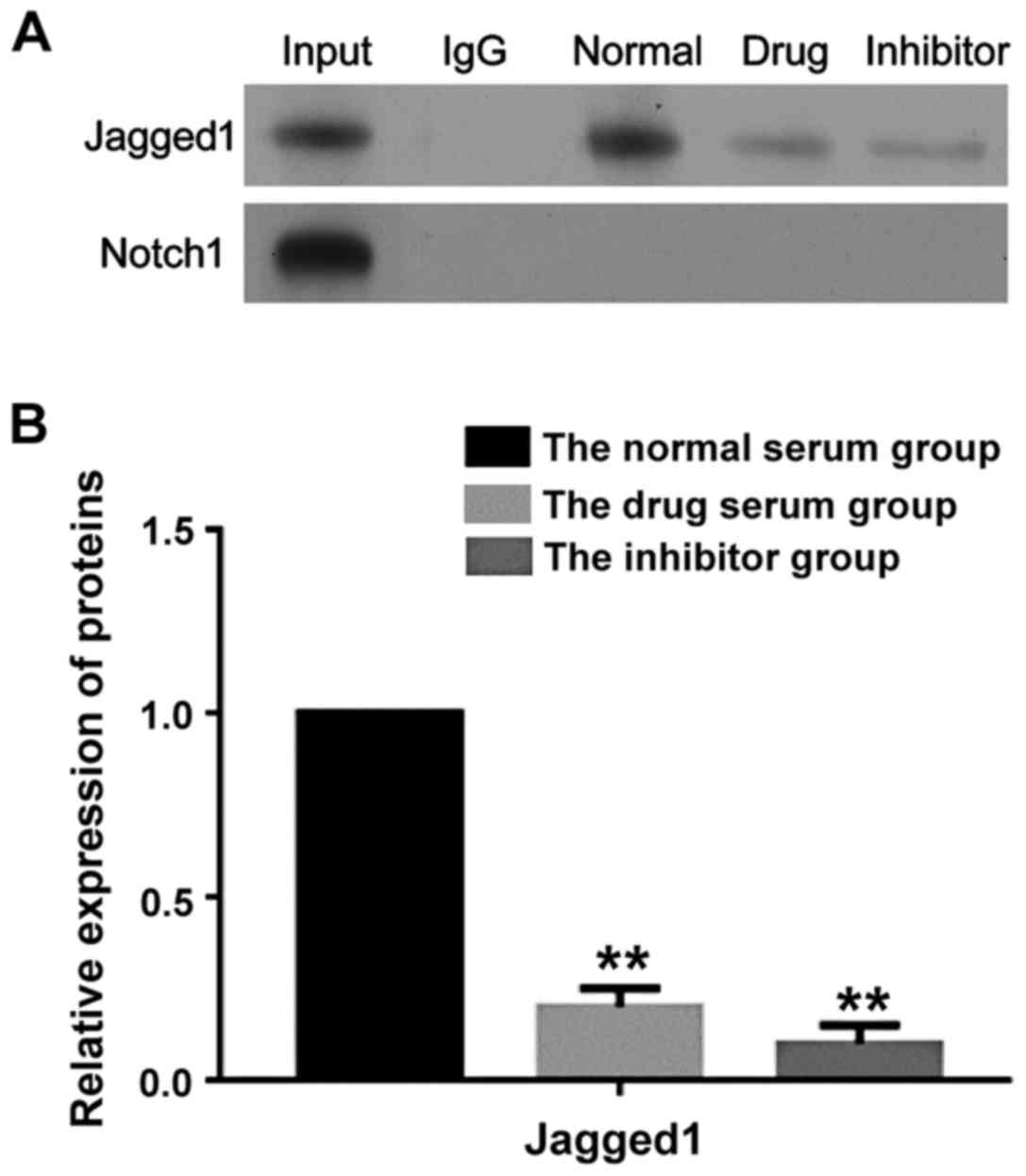
|
1
|
Catto JW, Alcaraz A, Bjartell AS, De Vere
White R, Evans CP, Fussel S, Hamdy FC, Kallioniemi O, Mengual L,
Schlomm T and Visakorpi T: MicroRNA in prostate, bladder, and
kidney cancer: A systematic review. Eur Urol. 59:671–681. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fabregat I, Roncero C and Fernández M:
Survival and apoptosis: A dysregulated balance in liver cancer.
Liver Int. 27:155–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yan SL, Huang CY, Wu ST and Yin MC:
Oleanolic acid and ursolic acid induce apoptosis in four human
liver cancer cell lines. Toxicol In Vitro. 24:842–848. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hainaud P, Contrerès JO, Villemain A, Liu
LX, Plouët J, Tobelem G and Dupuy E: The role of the vascular
endothelial growth factor-Delta-like 4 ligand/Notch4-ephrin B2
cascade in tumor vessel remodeling and endothelial cell functions.
Cancer Res. 66:8501–8510. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi R, An H, Yu Y, Zhang M, Liu S, Xu H,
Guo Z, Cheng T and Cao X: Notch1 signaling inhibits growth of human
hepatocellular carcinoma through induction of cell cycle arrest and
apoptosis. Cancer Res. 63:8323–8329. 2003.PubMed/NCBI
|
|
6
|
Benedito R, Roca C, Sörensen I, Adams S,
Gossler A, Fruttiger M and Adams RH: The notch ligands Dll4 and
Jagged1 have opposing effects on angiogenesis. Cell. 137:1124–1135.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kume T: Novel insights into the
differential functions of Notch ligands in vascular formation. J
Angiogenes Res. 1:82009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di Carlo JV and Alexander SR:
Hemofiltration for cytokine-driven illnesses: The mediator delivery
hypothesis. Int J Artif Organs. 28:777–786. 2005.PubMed/NCBI
|
|
9
|
Li S, Lin H, Tang Y, Li W, Shen J, Kai J,
Yue S, Shang G, Zhu Z, Shang E, et al: Comparative metabolomics
analysis on invigorating blood circulation for herb pair Gui-Hong
by ultra-high-performance liquid chromatography coupled to
quadrupole time-of-flight mass spectrometry and pattern recognition
approach. J Pharm Biomed Anal. 107:456–463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tian S, Tang J, Liu H, Wang L, Shen J, Li
J and Gan Y: Propyl gallate plays a nephroprotective role in early
stage of diabetic nephropathy associated with suppression of
glomerular endothelial cell proliferation and angiogenesis. Exp
Diabetes Res. 2012:2095672012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sherry N, Hagopian W, Ludvigsson J, Jain
SM, Wahlen J, Ferry RJ Jr, Bode B, Aronoff S, Holland C, Carlin D,
et al: Protégé Trial Investigators: Teplizumab for treatment of
type 1 diabetes (Protégé study): 1-year results from a randomised,
placebo-controlled trial. Lancet. 378:487–497. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang HL, Singh SK, Yan JM, Zhang XB and
Xu Q: Liquid-phase chemical hydrogen storage: Catalytic hydrogen
generation under ambient conditions. ChemSusChem. 3:541–549. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang M, Xue L, Cao Q, Lin Y, Ding Y, Yang
P and Che L: Expression of Notch1, Jagged1 and beta-catenin and
their clinicopathological significance in hepatocellular carcinoma.
Neoplasma. 56:533–541. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bray SJ: Notch signalling: A simple
pathway becomes complex. Nat Rev Mol Cell Biol. 7:678–689. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Poon RT, Fan ST and Wong J: Clinical
significance of angiogenesis in gastrointestinal cancers: A target
for novel prognostic and therapeutic approaches. Ann Surg.
238:9–28. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang X and Wu XZ: Main anti-tumor
angiogenesis agents isolated from Chinese herbal medicines. Mini
Rev Med Chem. 15:1011–1023. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tu X, Deng Y, Chen J, Hu Q, He C, Jordan
JB and Zhong S: Screening study on the anti-angiogenic effects of
Traditional Chinese Medicine - Part I: Heat-clearing and
detoxicating TCM. J Ethnopharmacol. 194:280–287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan G, Zong W and Zuo J: Dynamic
observation and clinical significance of integrated traditional
Chinese and Western medicine on interleukin-2 system, T cell and
erythrocyte immune system in patients of lung cancer. Zhongguo
Zhong Xi Yi Jie He Za Zhi. 20:586–588. 2000.(In Chinese).
PubMed/NCBI
|
|
20
|
Meng RD, Shelton CC, Li YM, Qin LX,
Notterman D, Paty PB and Schwartz GK: gamma-Secretase inhibitors
abrogate oxaliplatin-induced activation of the Notch-1 signaling
pathway in colon cancer cells resulting in enhanced
chemosensitivity. Cancer Res. 69:573–582. 2009. View Article : Google Scholar : PubMed/NCBI
|