Introduction
Green tea is one of the main tea species in China.
It contains tea polyphenols, catechins, chlorophyll, caffeine,
amino acids, vitamins and other active ingredients (1). Epigallocatechin-3-gallate (EGCG) content
is very high in green tea (1). EGCG
has anti-aging, sterilization, anti-inflammatory and anticancer
properties, and it also can inhibit the growth of a variety of
malignant cells and induce apoptosis (2–4). It can
also inhibit the angiogenesis, invasion and metastasis of malignant
tumor cells, in various types of cancer (5).
Breast cancer is one of the common malignancies in
women. It is crucial to identify new effective drugs with minimal
side effects to treat breast cancer. Liang et al (6) identified that EGCG inhibited the
proliferation of breast cancer cells by repressing cyclin-dependent
protein activity and also inhibited the growth and metastasis of
breast cancer cells by downregulating the expression of VEGF and
MMP-9 (7–9). However, the mechanism by which EGCG
promoted breast cancer apoptosis has rarely been studied.
The present study provides a theoretical reference
for the development of breast cancer target by investigating the
mechanism of apoptosis of EGCG on breast cancer.
Materials and methods
Materials
EGCG and thiazolyl blue were purchased from Sigma
(St. Louis, MO, USA). RPMI-1640 basal medium and fetal bovine serum
(FBS) was produced by HyClone (Logan, UT, USA). RPMI-1640 basal
medium and EGCG were used to prepare a 10 mmol/l storage solution.
It was sterilized by filtration (0.22 µm) and stored at −80°C in
the dark. MTT was dissolved in phosphate-buffered saline (PBS)
solution at 5 mg/ml, and was stored at −20°C in the dark after
sterilization. Mouse anti-human P53, Bcl-2 and GAPDH were all
purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA)
and the RT-PCR kit was purchased from Takara (Otsu, Shiga, Japan).
P53, Bcl-2 and GAPDH primers were produced by Invitrogen (Carlsbad,
CA, USA) (Table I) and si-P53 was
supplied by Gemma (Shanghai, China).
 | Table I.Primers of P53, Bcl-2 and GAPDH used
in RT-qPCR. |
Table I.
Primers of P53, Bcl-2 and GAPDH used
in RT-qPCR.
| Gene | Forward primer | Reverse primer |
|---|
| P53 |
5′-TTCCCTGGATTGGCCAGACT-3′ |
5′-ACCATCGCTATCTGAGCAGC-3′ |
| Bcl-2 |
5′-CGACTTCGCCGAGATGCCAGCCAG-3′ |
5′-ACTTGTGGCCCAGATAGGCACCCAG-3′ |
| GAPDH |
5′-AGAAGGCTGGGGCTCATTTG-3′ |
5′-AGGGGCCATCCACAGTCTTC-3′ |
Cell line
MCF-7 cells were purchased from the Shanghai ATCC
cell bank. The cells were cultured in RPMI-1640 medium containing
10% FBS (with 100 U/ml green-streptomycin) and incubated under 5%
CO2 at 37°C.
Methods
MTT detection of the sensitivity of MCF-7 cells
to EGCG
The EGCG concentration gradient was 0, 2, 4, 8, 16,
32, 64 and 128 µmol/l. MCF-7 cells (2×103) were seeded
in a 96-well plate with blank control wells. After cells adhered to
the walls, EGCG of the concentration gradient of 0, 2, 4, 8, 16,
32, 64 and 128 µmol/l was added. After incubation for 72 h, the
supernatant was discarded and 20 µl of MTT was added to each well.
After incubation for 4 h, the liquid was discarded and 150 µl of
dimethyl sulfoxide (DMSO) was added to each well of the EGCG. The
optical density (OD) of each well at 490 nm was measured by a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The inhibition rate was calculated as: (control group OD -
experimental group OD/control group OD) × 100%.
Effect of EGCG on MCF-7 cell apoptosis measured
by flow cytometry (FCM)
MCF-7 cells were seeded in a 6-well cell culture
plate. After adherence, 30 µmol/l EGCG was added and the cells were
stained after 48 h with Annexin V-FITC kit. The change in apoptotic
rate was measured, and the experiment was repeated three times.
RT-qPCR and western blot analysis determine how
EGCG treatment on MCF-7 affects the mRNA and protein levels of P53
and Bcl-2
The experiment was conducted on the control and EGCG
groups. EGCG was added to fresh MCF-7 cells in the experimental
group after cell adhesion (EGCG at a final concentration of 30
µmol/l), but the control group was not treated. After 72 h of
incubation, total RNA was extracted using TRIzol reagent. The
RT-qPCR assay was performed using the Takara kit, and the
GAPDH gene was used as an internal control. The conditions
for RT-qPCR were: Preheating at 95°C for 2 min, denaturation at
95°C for 30 sec, annealing at 58°C for 30 sec and extension at 72°C
for 40 sec. The experiment was repeated three times. The results
were expressed as 2−ΔΔCq. MCF-7 cells were treated with
30 µmol/l EGCG for 48 h. The cells were then rinsed three times
with PBS and 100 µl of RIPA lysate was added. The cells were placed
on ice for 30 min and centrifuged at 12,000 × g for 20 min at 4°C.
The supernatant was collected and stored at −80°C. Protein
concentration was determined using the BCA protein method. The
protein extract was subjected to SDS-PAGE (10%) and transferred to
a PVDF membrane. The membrane was blocked with 3% BSA solution for
2 h. Then, primary rabbit monoclonal P53 antibody (dilution, 1:500;
cat. no. ab32049), rabbit polyclonal Bcl-2 antibody (dilution,
1:500; cat. no. ab59348) and rabbit polyclonal GAPDH antiboody
(dilution, 1:500; cat. no. ab37168), purchased from Abcam
(Cambridge, MA, USA), were added at 1:1,000 and incubated at 4°C
overnight. The membrane was washed with PBS three times (5 min each
time). Then secondary goat anti-rabbit (HRP) IgG antibody
(dilution, 1:2,000; cat. no. ab6721) was added followed by 2-h
incubation and washing with PBS three times (5 min each time).
Finally, it was placed on the ECL for exposure and development.
RT-qPCR and western blot analysis of si-P53
interference effect
The cells were divided into the si-P53, si-NC and NC
groups (si-P53 was added with small fragments of interference P53,
with si-NC as a positive control and NC as a negative control).
Cells in the logarithmic growth phase were inoculated into the
culture flask and transiently transfected according to the protocol
of the Lipofectamine™ 2000 kit. After 48 h, the RNA was extracted
and purified according to the kit protocol, and RNA purity and
concentration was determined by a spectrophotometer (Hitachi,
Tokyo, Japan). The P53 mRNA expression was evaluated using RT-qPCR,
and the P53 protein expression level was measured using western
blot analysis. The three groups were compared and the transfection
efficiency was calculated.
Alterations in the expression of P53 and Bcl-2
proteins in MCF-7 cells transfected with si-P53 detected by western
blot analysis
The cells were divided into the si-P53, EGCG and
EGCG + si-P53, (si-P53 plus interference P53 small fragments,
si-P53 for the positive control, NC for the negative control)
groups. The P53 and Bcl-2 protein expression levels in the three
groups were evaluated using western blot analysis using the same
method explained before.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA) and SPSS
20.0 (Chicago, IL, USA) statistical software. The measurement data
were presented as mean ± standard deviation. Comparison between two
groups was conducted using a t-test. The one-way analysis of
variance (ANOVA) test was used for comparison among three groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
MTT detection of the sensitivity of MCF-7 cells
to EGCG
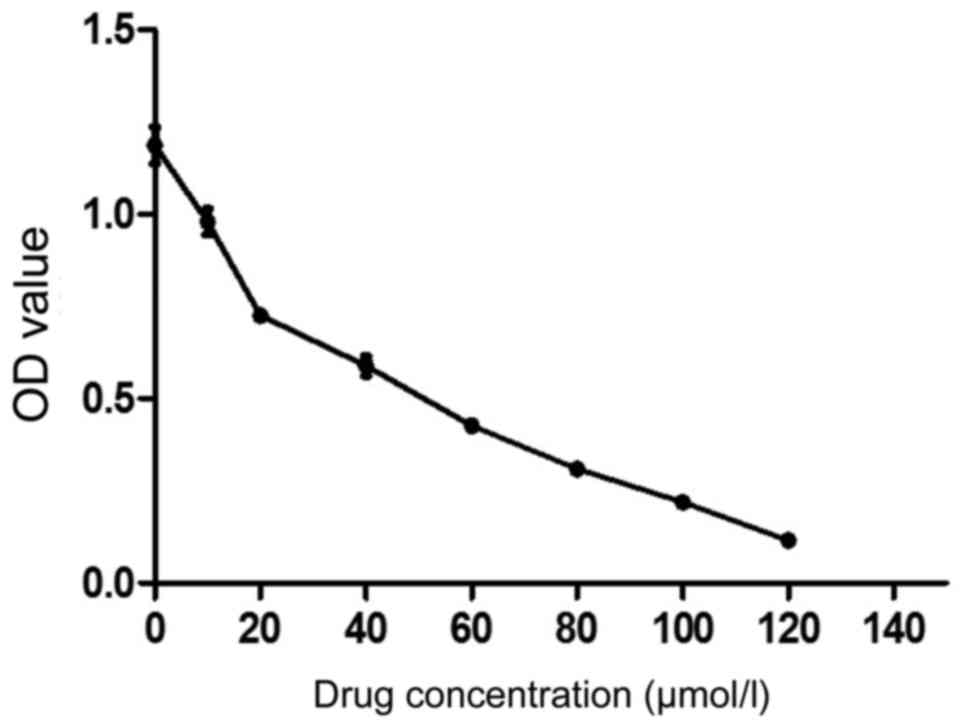
The sensitivity of EGCG to MCF-7 was evaluated using
MTT assay. As shown in Fig. 1, 10
µmol/l EGCG significantly inhibited the proliferation of MCF-7
cells and the inhibition was enhanced with the increase of drug
concentration. The IC50 was 37.684 µmol/l. We selected
30 µmol/l as the follow-up dose (Table
II).
 | Table II.Different concentrations of EGCG and
the inhibitory rate of MCF-7 cells using MTT. |
Table II.
Different concentrations of EGCG and
the inhibitory rate of MCF-7 cells using MTT.
| Concentration | OD (mean ± SD) | Inhibition rate
(%) |
|---|
| 0 | 1.187±0.049 | 0 |
| 10 | 0.979±0.035 | 17.52 |
| 20 | 0.726±0.021 | 38.85 |
| 40 | 0.589±0.027 | 50.39 |
| 60 | 0.427±0.012 | 64 |
| 80 | 0.309±0.017 | 73.92 |
| 100 | 0.219±0.016 | 81.53 |
| 120 | 0.116±0.011 | 90.23 |
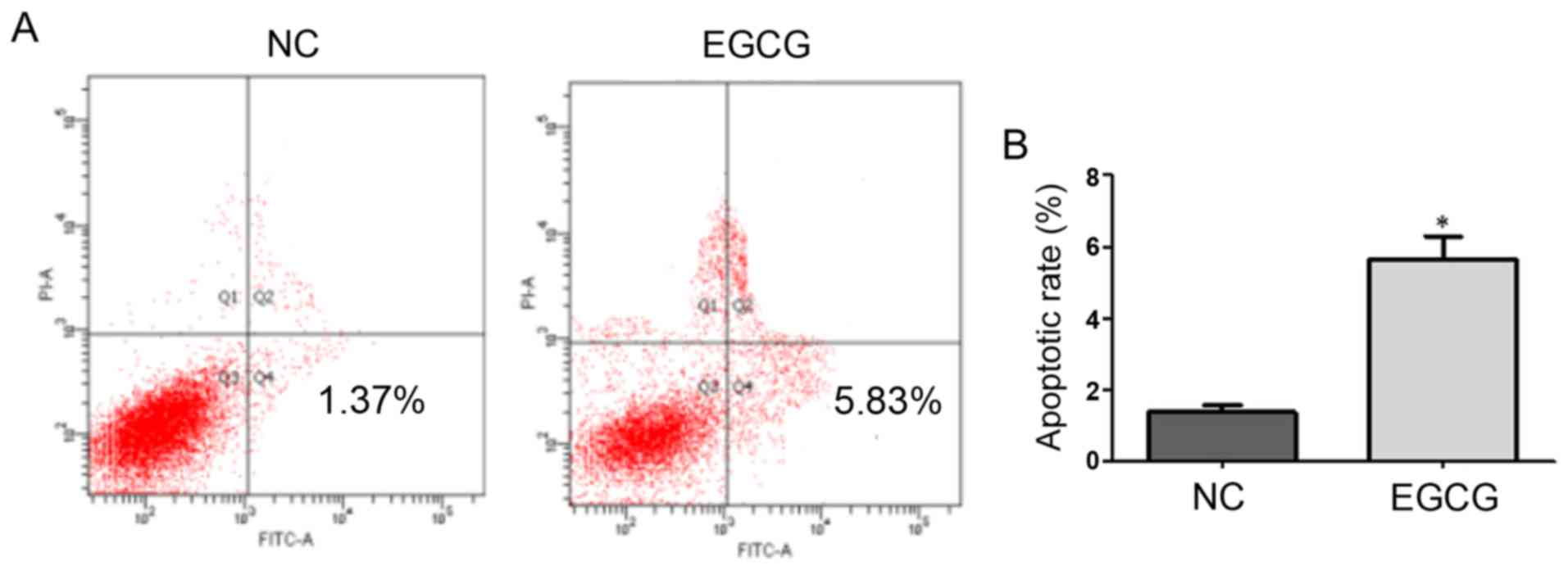
Effect of EGCG on MCF-7 cell apoptosis measured
using FCM
As shown in Fig. 2,
the apoptotic rates of the EGCG and control groups were 1.37 and
5.83%, respectively, with a significant difference (t=8.9,
p=0.0124). These results suggested that EGCG promoted apoptosis in
MCF-7 cells (Table III).
 | Table III.The effect of 30 µmol/l EGCG on the
apoptotic rate of MCF-7 after 48 h was detected using a flow
cytometer. |
Table III.
The effect of 30 µmol/l EGCG on the
apoptotic rate of MCF-7 after 48 h was detected using a flow
cytometer.
| Group | Apoptosis rate (%)
(mean ± SD) | t | P-value |
|---|
| NC | 1.38±0.19 |
|
|
| EGCG | 5.65±0.64 | 8.9 | 0.0124 |
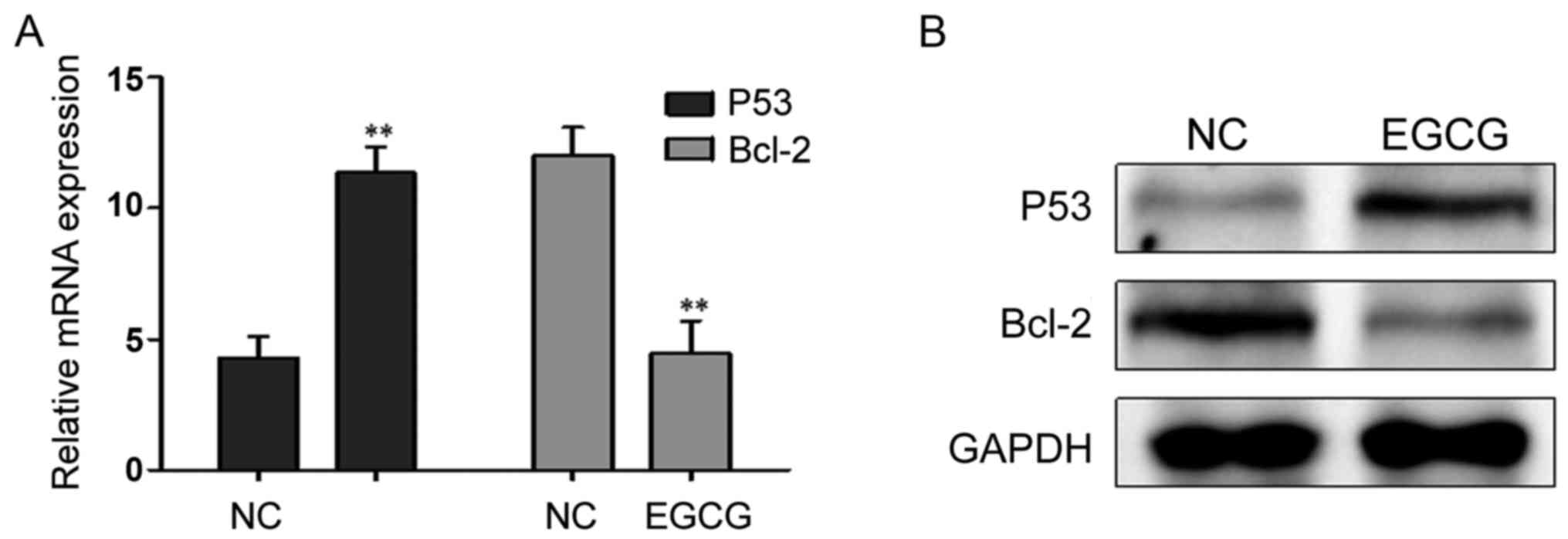
Effects of EGCG treatment on the expression of
P53 and Bcl-2
After treatment with 30 µmol/l EGCG, P53 mRNA and
protein expression levels were significantly higher, while the
Bcl-2 mRNA and protein expression levels decreased significantly
(Fig. 3; Table IV).
 | Table IV.The effect of 30 µmol/l EGCG on the Cq
and F value of P53 and Bcl-2 in MCF-7 cells. |
Table IV.
The effect of 30 µmol/l EGCG on the Cq
and F value of P53 and Bcl-2 in MCF-7 cells.
|
| 2−ΔΔCq
(mean ± SD) |
|
|
|---|
|
|
|
|
|
|---|
| Gene | NC | EGCG | t | P-value |
|---|
| P53 | 4.289±0.831 | 11.352±0.992 | 9.451 | <0.001 |
| Bcl-2 | 12.013±1.082 | 4.465±1.228 | 7.985 | <0.001 |
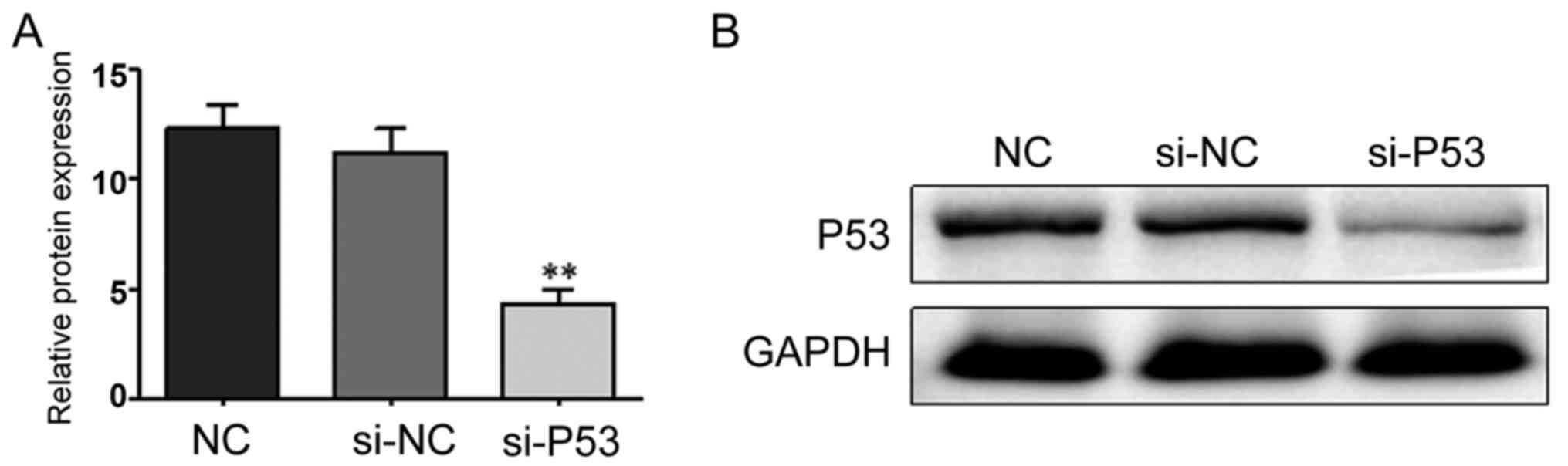
si-P53 interference effect
The RNA interference technique was used to silence
P53 expression. The interference effect was studied using RT-qPCR
and western blot analysis. As shown in Fig. 4, the interference effect was
significant and could be further tested.
Changes in expression levels of P53 and Bcl-2
proteins in MCF-7 cells transfected with si-P53
P53 and Bcl-2 expression levels in
si-P53-transfected MCF-7 cells treated with EGCG were detected
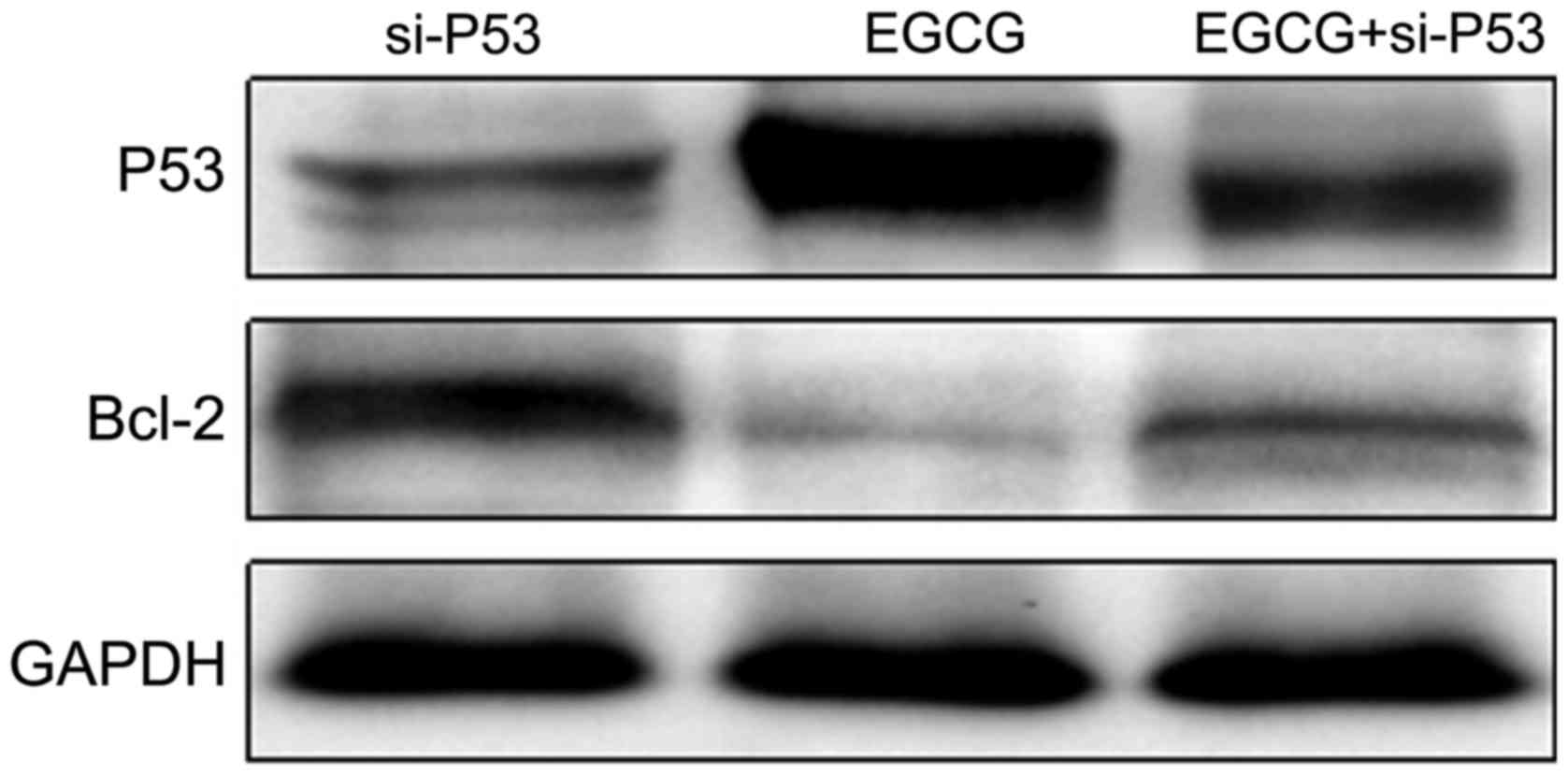
using western blot analysis. As shown in Fig. 5, the expression of P53 in the EGCG +
si-P53 group was higher than that in the si-P53 group but lower
than that in the EGCG group. The expression of Bcl-2 in the EGCG +
si-P53 group was lower than that in the si-P53 group but higher
than that in the EGCG group. The results suggested that EGCG
promoted the expression of P53 (Table
V).
 | Table V.The Cq value of P53 was detected by
RT-qPCR after transfection with si-P53. |
Table V.
The Cq value of P53 was detected by
RT-qPCR after transfection with si-P53.
| Group | 2−ΔΔCq
(mean ± SD) | F | P-value |
|---|
| NC | 12.332±1.063 |
|
|
| si-NC | 11.219±1.097 |
|
|
| si-P53 |
4.329±0.658 | 61.450 | <0.001 |
Discussion
P53 is an important tumor suppressor gene. It is
involved in tumor occurrence and development through DNA repair,
cell cycle regulation, angiogenesis inhibition and cell apoptosis
(10). The P53 gene has been
found to be mutated in more than 50% of malignant tumors (11). Mutations and deletions of the
P53 gene have been reported to be closely related to breast
cancer (12–15). Apoptosis is a spontaneous cell death
process regulated by a series of genes. The proto-oncogene Bcl-2 is
a member of the Bcl-2 family. The oncoproteins located in the
nuclear membrane, part of the endoplasmic reticulum and
mitochondrial outer membrane, can negatively regulate cell
apoptosis and prolong cell life (16). Previous findings have shown that P53
can inhibit the expression of Bcl-2 protein (17,18).
EGCG is the most active substance in green tea,
which has great developmental value, and its anticancer effect has
been widely studied. Previous results have shown that EGCG can
promote apoptosis in a variety of malignant tumor cells, including
pancreatic and gastric cancer cells (18,19). In
the present study, we showed that the apoptotic rate in MCF-7 cells
treated with EGCG was significantly higher than that of the control
group. In order to explore the mechanism of apoptosis, P53 and
Bcl-2 expression levels were determined. The RT-qPCR and western
blot results showed that EGCG induced the expression of P53 but
inhibited the expression of Bcl-2. The EGCG + si-P53 group had a
higher P53 expression level compared with the si-P53 group. The P53
expression level was lower than that of the EGCG group, while the
Bcl-2 expression was lower than that in the si-P53 group but higher
than that in the EGCG group. These results suggest that EGCG
inhibited the expression of Bcl-2 and promoted the expression of
P53.
In conclusion, the findings show that EGCG inhibited
the proliferation of MCF-7 cells and promoted apoptosis in these
cells. The underlying mechanism involved may be related to the
P53/Bcl-2 signaling pathway, which can provide a theoretical basis
for EGCG to be used in the new target drug development for breast
cancer.
References
|
1
|
Yang CS and Wang ZY: Tea and cancer. J
Natl Cancer Inst. 85:1038–1049. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan XH, Zhou DY and Zhang YL: Advances in
research on anticancer mechanism of tea. Foreign Medical Oncology.
25:41998.
|
|
3
|
Yang GY, Liao J, Kim K, Yurkow EJ and Yang
CS: Inhibition of growth and induction of apoptosis in human cancer
cell lines by tea polyphenols. Carcinogenesis. 19:611–616. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahmad N, Feyes DK, Nieminen AL, Agarwal R
and Mukhtar H: Green tea constituent epigallocatechin-3-gallate and
induction of apoptosis and cell cycle arrest in human carcinoma
cells. J Natl Cancer Inst. 89:1881–1886. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang G, Wang Y, Zhang Y, Wan X, Li J, Liu
K, Wang F, Liu K, Liu Q, Yang C, et al: Anti-cancer activities of
tea epigallocatechin-3-gallate in breast cancer patients under
radiotherapy. Curr Mol Med. 12:163–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang YC, Lin-Shiau SY, Chen CF and Lin
JK: Inhibition of cyclin-dependent kinases 2 and 4 activities as
well as induction of Cdk inhibitors p21 and p27 during growth
arrest of human breast carcinoma cells by
(−)-epigallocatechin-3-gallate. J Cell Biochem. 75:1–12. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sartippour MR, Shao ZM, Heber D, Beatty P,
Zhang L, Liu C, Ellis L, Liu W, Go VL and Brooks MN: Green tea
inhibits vascular endothelial growth factor (VEGF) induction in
human breast cancer cells. J Nutr. 132:2307–2311. 2002.PubMed/NCBI
|
|
8
|
Masuda M, Suzui M, Lim JT, Deguchi A, Soh
JW and Weinstein IB: Epigallocatechin-3-gallate decreases VEGF
production in head and neck and breast carcinoma cells by
inhibiting EGFR-related pathways of signal transduction. J Exp Ther
Oncol. 2:350–359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Farabegoli F, Papi A and Orlandi M:
(−)-Epigallocatechin-3-gallate down-regulates EGFR, MMP-2, MMP-9
and EMMPRIN and inhibits the invasion of MCF-7 tamoxifen-resistant
cells. Biosci Rep. 31:99–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levine AJ, Momand J and Finlay CA: The p53
tumour suppressor gene. Nature. 351:453–456. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pakos EE, Kyzas PA and Ioannidis JP:
Prognostic significance for TP53 tumor suppressor gene expression
and mutations in human motations in human osteosarcoma: A
meta-analysis. Clin Cancer Res. 10:6208–6214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barnes DM, Dublin EA, Fisher CJ, Levison
DA and Millis RR: Immunohistochemical detection of p53 protein in
mammary carcinoma: An important new independent indicator of
prognosis? Hum Pathol. 24:469–476. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dastjerdi MN, Rarani MZ, Valiani A and
Mahmoudieh M: The effect of adenosine A1 receptor agonist and
antagonist on p53 and caspase 3, 8, and 9 expression and apoptosis
rate in MCF-7 breast cancer cell line. Res Pharm Sci. 11:303–310.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Synnott NC, Murray A, McGowan PM, Kiely M,
Kiely PA, O'Donovan N, O'Connor DP, Gallagher WM, Crown J and Duffy
MJ: Mutant p53: A novel target for the treatment of patients with
triple-negative breast cancer? Int J Cancer. 140:234–246. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Darb-Esfahani S, Denkert C, Stenzinger A,
Salat C, Sinn B, Schem C, Endris V, Klare P, Schmitt W, Blohmer JU,
et al: Role of TP53 mutations in triple negative and HER2-positive
breast cancer treated with neoadjuvant anthracycline/taxane-based
chemotherapy. Oncotarget. 7:67686–67698. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schinzel A, Kaufmann T and Borner C: Bcl-2
family members: Integrators of survival and death signals in
physiology and pathology. Biochim Biophys Acta. 1644:95–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mihara M, Erster S, Zaika A, Petrenko O,
Chittenden T, Pancoska P and Moll UM: p53 has a direct apoptogenic
role at the mitochondria. Mol Cell. 11:577–590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu BH, Zhan WH, Li ZR, Wang Z, He YL,
Peng JS, Cai SR, Ma JP and Zhang CH: (−)-Epigallocatechin-3-gallate
inhibits growth of gastric cancer by reducing VEGF production and
angiogenesis. World J Gastroenterol. 13:1162–1169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qanungo S, Das M, Haldar S and Basu A:
Epigallocatechin-3-gallate induces mitochondrial membrane
depolarization and caspase-dependent apoptosis in pancreatic cancer
cells. Carcinogenesis. 26:958–967. 2005. View Article : Google Scholar : PubMed/NCBI
|