Introduction
The tumor-node-metastasis (TNM) classification that
was established by the Union for International Cancer Control is
universally used to reliably predict the prognosis of patients with
malignant tumors, and is based on the anatomical distribution of
malignant tumors (1). Typically,
tumors at relatively early stages (stage I/II) are distributed in a
limited area and should have a better prognosis, compared with
those at more advanced stages (III/IV). However, certain patients
with early-stage tumors may have a poor prognosis, whereas others
with advanced tumors may have a good prognosis. The aim of the
present study was to create models for predicting the prognosis of
malignant tumors in a manner that may be auxiliary to TNM
staging.
The present study investigated the prognostic
predictors of oral squamous cell carcinoma (OSCC) based on
information about proteins expressed in OSCC cells combined with
patients' clinical information. Vascular endothelial growth factors
(VEGFs) are associated with tumor angiogenesis or
lymphovasculogenesis (2–4), and a number of previous studies of oral
pathology have attempted to use intracellular levels of VEGF
expression in OSCC cells to predict the prognosis of patients with
OSCC; however, these results were variable (5–12). In a
previous study, a novel model was proposed to predict the prognosis
of patients with OSCC using their age and the expression levels of
VEGF-A and VEGF-C (13). It has also
been revealed that podoplanin, a platelet-aggregation glycoprotein
(14,15) that is expressed by OSCC cells
(16–20), may serve as a prognostic predictor of
OSCC (21). Thus the present study
analyzed molecules expressed by OSCC tumor cells at all stages.
The present study also aimed to identify low-risk
populations among patients with advanced OSCC (stage III/IV) using
logic regression analysis, as previously described (13). Proteins that are expressed by tumor
cells and the host responses raised against these cells can
influence the prognosis of malignant tumors (22). Immunoreactions are important host
responses against tumor invasion, and inflammatory cell
infiltration is a key histological factor for assessing host
immunoreactions against tumor infiltration (23). Variable mucosal inflammation
frequently occurs in the oral cavity and is visible in a number of
histopathological tissue samples (24). Considering the variability of
inflammatory lesions in the oral mucosa, the prognosis of OSCC with
or without inflammatory reactions may be challenging to evaluate.
However, it may have specific value for separately detecting innate
and adaptive immunity if included in the logic regression analysis.
The present study analyzed the ability of neutrophils and natural
killer (NK) cells to detect innate immunity, and of lymphocytes to
detect adaptive immunity, and then applied these to a statistical
model to predict low-risk populations in patients with stage III/IV
OSCC.
Materials and methods
Patients and materials
The present study analyzed data from 41 patients
(male, n=30; female, n=11; age range, 31–85-years-old) who were
admitted to Nagasaki University Dental Hospital (Nagasaki, Japan)
between October 1986 and September 2002, with histopathologically
diagnosed stage III/IV OSCC and underwent surgical tumor resection.
Histopathological analysis diagnosed 31 patients with
well-differentiated OSCC and 5 each with moderately- and
poorly-differentiated OSCC, respectively. A total of 22 patients
were treated with preoperative irradiation (total, 30 Gy) using a
Clinac 2100C linear accelerator (Varian Medical Systems, Palo Alto,
California, USA) and pepleomycin. Of the 41 aforementioned
patients, 4 patients were treated with preoperative irradiation
only, 8 patients were treated with preoperative pepleomycin only
and 7 patients were not treated prior to surgery. All patients were
followed up at the hospital until 2005; overall survival was
analyzed, and 34 patients succumbed to disease and 7 survived
during the follow-up period. The present study was approved by the
Ethics Committee of Nagasaki University Hospital (Nagasaki, Japan;
approval no. 16020839).
Histopathological analyses
Tissue sections (3-µm thick) were cut from
paraffin-embedded blocks of biopsy or surgical tissue samples.
Sections from patients with OSCC were stained with hematoxylin and
eosin (HE) using a standard protocol. After deparaffinization,
sections were stained with Meyer's hematoxylin solution at room
temperature for 5 min. After rinsing under running water for 15
min, sections were further stained with eosin solution for 3 min at
room temperature. Lymphocyte infiltration was then graded by light
microscopy (magnification, ×40) as follows: 1, tissue samples with
band-like or follicular lymphocyte infiltration at ×40
magnification were categorized as having dense infiltration; 0, no
band-like or follicular lymphocyte infiltration. Neutrophil
infiltration was evaluated as follows: 1, presence or 0, absence of
intraepithelial micro-abscesses of OSCC in HE-stained tissue
samples. Tumors were also assessed in HE-stained sections as well,
moderately or poorly differentiated, which were categorized as 0, 1
and 2, respectively. The mode of invasion was categorized as 0, 1
or 2, which representing Jakobsson's grade 1/2, 3 and 4,
respectively (25). NK cells were
stained using an antibody against NKp46 (dilution, 1:50; cat. no.
MAB1850; R&D Systems, Inc., Minneapolis, MN, USA), as
previously described (13) and
samples were graded as -, 1 or 2 using light microscopy, indicating
sample staining was undetectable, in <5 or ≥5 cells,
respectively, in each whole specimen. In brief, deparaffinized
sections were treated with 2% bovine serum albumin diluted with PBS
at room temperature for 30 min, prior to incubation with antibody
overnight at 4°C. Immunohistochemical detection was carried out
using the EnVision+ system (Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA). Specimens were analyzed by light microscopy.
Statistical analysis
Prognostic status was defined for patients who
succumbed during the observation period as poor and for patients
who survived as good. The overall survival time was defined as the
duration from the date of the initial surgery and the date of
mortality from any cause, or the date of last contact during the
follow-up period for survivors. To identify the prognostic factors,
Cox's proportional hazards regression model was used for univariate
and multivariate analyses, as previously reported (13). Factors were selected from a
combination of prognostic factors to create a logic covariate
model, and Akaike's Information Criterion (AIC) was used for the
model selection (26). Log-rank test
with χ2 statistics was used to test survival curves. Statistical
procedures were performed using Statistical Language R-3.2.5
(27). P<0.05 was considered to
indicate a statistically significant difference.
Results
Histopathological analyses
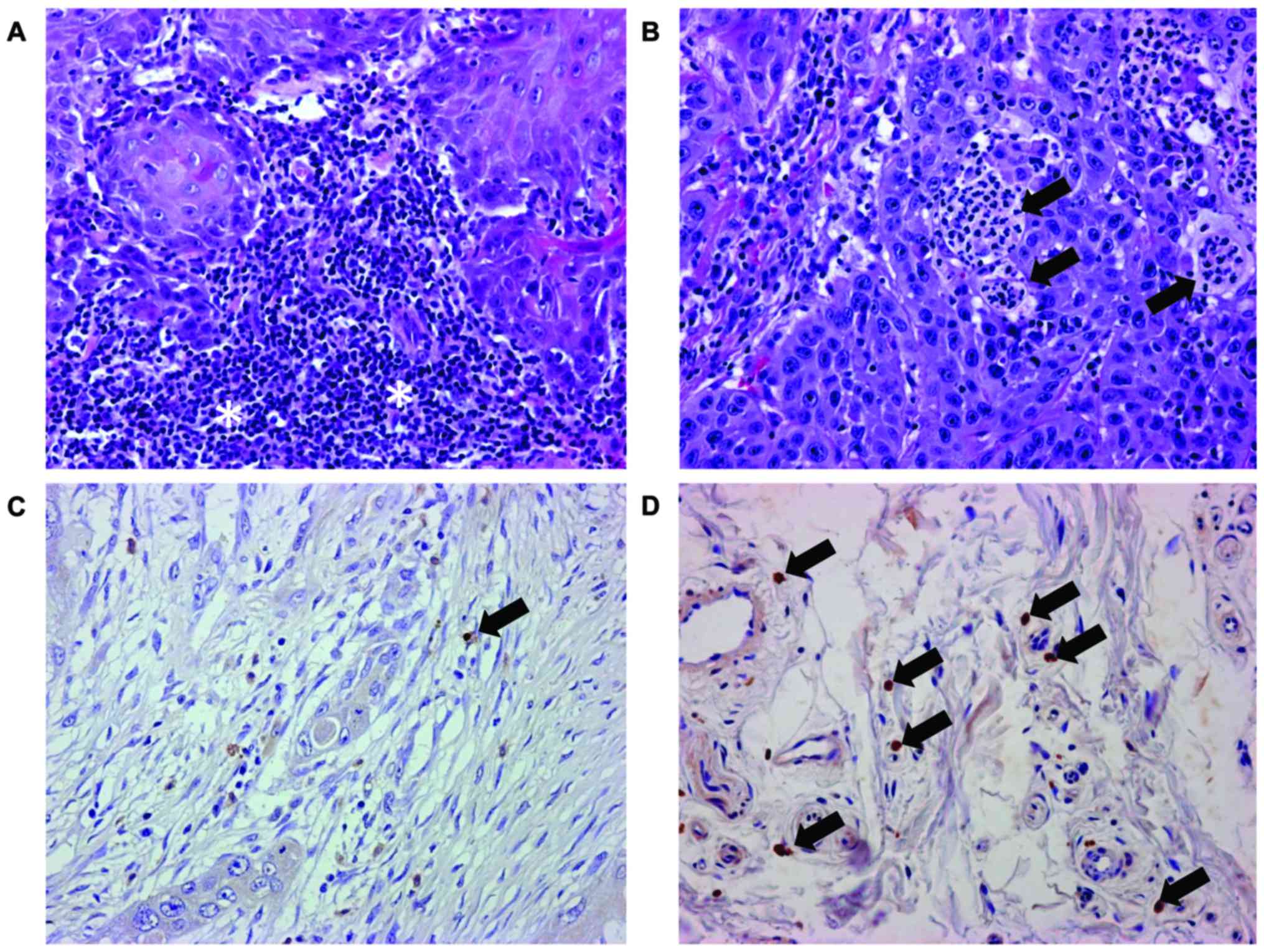
Lymphocytes and neutrophils were detected in all
analyzed tissue samples. Lymphocytes formed band-like or follicular
infiltrates in tissue samples with dense infiltration (Fig. 1A). However, few tissue samples
exhibited intraepithelial micro-abscesses (Fig. 1B). Table
I summarizes the histopathological findings. NKp46-positive
cells were detected in 14 of the 41 tissue samples (Table I). Cells positive for NKp46 were
sparse even in tissue samples categorized as 2 (Fig. 1D). NKp46-potitive cells were absent in
27 of the 41 tissue samples (Table
I). NKp46-positive cells were identified in the stromal regions
of biopsy and surgical tissue samples; the positive cells were not
always contact with tumor cells (Fig. 1C
and D).
 | Table I.Study subjects by factors and their
estimated RR. |
Table I.
Study subjects by factors and their
estimated RR.
|
|
|
| Prognosis | Cox Regression |
|---|
|
|
|
|
|
|
|---|
| Factor | Category | Sample size | Good | Poor | RR | P-value |
|---|
| Sex | 0 | 11 | 1 | 10 | 1.000 | – |
|
| 1 | 30 | 6 | 24 | 0.733 | 0.416 |
| Age | −69 | 22 | 5 | 17 | 1.000 | – |
|
| 70- | 19 | 2 | 17 | 1.577 | 0.187 |
| Stage | III | 9 | 2 | 7 | 1.000 | – |
|
| IV | 32 | 5 | 27 | 1.475 | 0.362 |
| T-stage | 1, 2 | 13 | 3 | 10 | 1.000 | – |
|
| 3 | 4 | 1 | 3 | 1.012 | 0.985 |
|
| 4 | 24 | 3 | 21 | 1.689 | 0.176 |
| N-stage | 0 | 6 | 1 | 5 | 1.000 | – |
|
| 1 | 13 | 2 | 11 | 0.777 | 0.640 |
|
| 2 | 22 | 4 | 18 | 0.761 | 0.590 |
| Dense lymphocyte
infiltration | 0 | 24 | 1 | 23 | 1.000 | – |
|
| 1 | 17 | 6 | 11 | 0.471 | 0.044 |
| Microabscess | 0 | 33 | 2 | 31 | 1.000 | – |
|
| 1 | 8 | 5 | 3 | 0.221 | 0.014 |
| Differentiation
grade | 0 | 5 | 2 | 3 | 1.000 | – |
|
| 1 | 5 | 1 | 4 | 1.770 | 0.458 |
|
| 2 | 31 | 4 | 27 | 1.571 | 0.460 |
| Mode of
invasion | 0 | 20 | 2 | 18 | 1.000 | – |
|
| 1, 2 | 21 | 5 | 16 | 0.732 | 0.368 |
| NKp46 | 0 | 27 | 6 | 21 | 1.000 | – |
|
| 1 | 10 | 1 | 9 | 1.222 | 0.619 |
|
| 2 | 4 | 0 | 4 | 1.496 | 0.473 |
Statistical findings
Univariate analysis of sex, age, clinical stage,
T-stage, N-stage, dense lymphocyte infiltration, microabscess,
differentiation grade, mode of invasion and NKp46 expression
revealed that dense lymphocyte infiltration and the presence of
intraepithelial micro-abscesses predicted the good prognosis of
patients with OSCC, whereas NKp64 alone was not significantly
associated with a good prognosis (Table
I). Logic combinations of covariates used in the univariate
analysis, including dense lymphocyte infiltration, micro-abscesses,
NKp46 and patients' age were further analyzed using the Cox's
proportional hazards model. It was revealed that logic combinations
of NKp46 and micro-abscesses had a smaller P-value (0.001) and a
smaller AIC (201.8), compared with the other univariate and
multivariate models. Logic combinations that were significantly
associated with the lowest P-value (0.001) in patients with
advanced OSCC were named important prognostic factors (IPF). An IPF
had a value of 1 when NKp46 expression <2 (when the number of
positive cells <5 at ×100 magnification in each specimen) and
micro-abscesses=1 (presence of intraepithelial micro-abscesses of
OSCC), and a value of 0 otherwise (Table
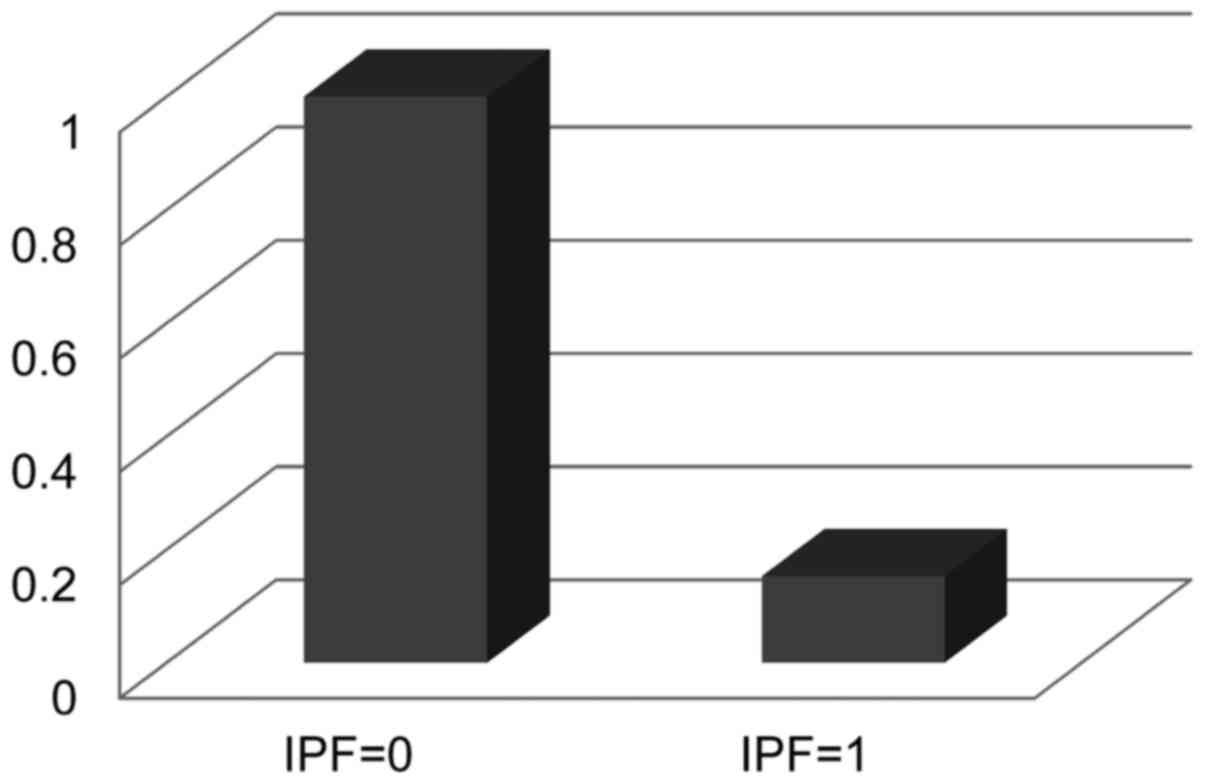
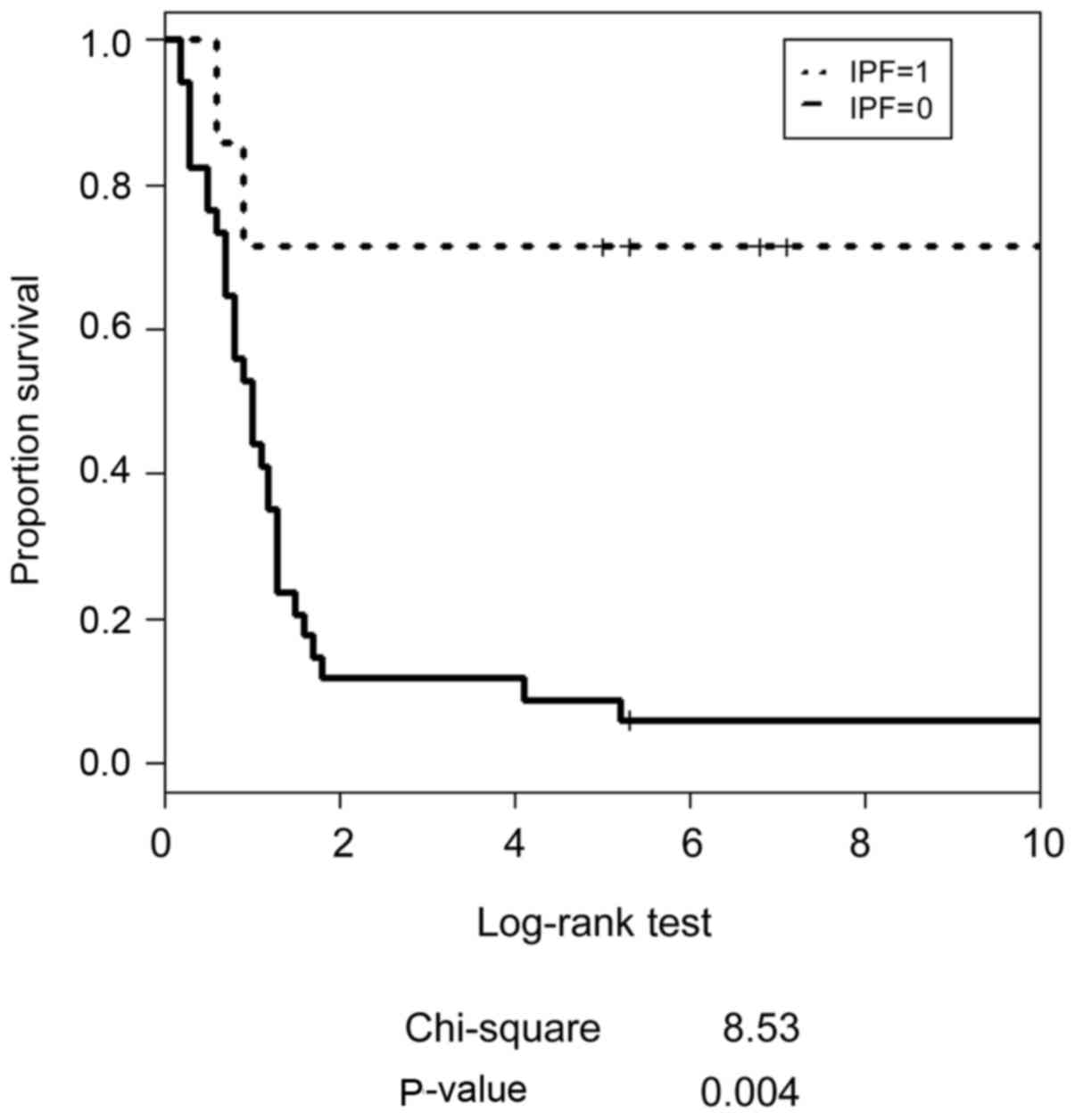
II). Fig. 2 presents the
estimated relative risk for stage III/IV OSCC evaluated by IPF, and
Fig. 3 depicts the estimated survival
curves for patients with stage III/IV OSCC and each IPF status.
Patients with an IPF of 1 had statistically significant favorable
survival as calculated using a Log-rank test (P=0.004).
 | Table II.Results of Cox proportional hazards
regression models (N=41). |
Table II.
Results of Cox proportional hazards
regression models (N=41).
|
|
|
|
| 95% confidence
interval |
|
|---|
|
|
|
|
|
|
|
|---|
| Covariate | Estimated
coefficients | SE | P-value | RR | Lower | Upper | AIC |
|---|
| Selected logic
regression model |
|
|
|
|
|
|
|
|
IPFa | −1.875 | 0.74 | 0.11 | 0.15 | 0.04 | 0.65 | 201.8 |
| Other univariate
and multivariate models with a linear combination of
covariates |
|
|
|
|
|
|
|
| Univariate model
1 |
|
|
|
|
|
|
|
| Dense
lymphocyte infiltration | −0.752 | 0.37 | 0.044 | 0.47 | 0.23 | 0.98 | 208.4 |
| Univariate model
2 |
|
|
|
|
|
|
|
|
Microabscess | −1.510 | 0.61 | 0.014 | 0.22 | 0.07 | 0.73 | 203.8 |
| Multivariate
model |
|
|
|
|
|
|
|
| Dense
lymphocyte infiltration | −0.170 | 0.41 | 0.681 | 0.84 | 0.38 | 1.90 | 205.6 |
|
Microabscess | −1.390 | 0.68 | 0.041 | 0.25 | 0.07 | 0.95 |
|
Discussion
In a cohort of patients with OSCC, the host defense
system was analyzed based on inflammatory reactions and NK cell
infiltration; subsequently, a novel logic combination model was
proposed using intraepithelial micro-abscess formation and NK cell
infiltration as covariates to identify low risk populations in
patients with advanced OSCC. In a previous study from our group, a
logic combination model was proposed for predicting the prognosis
of patients OSCC using the expression levels of VEGF-A and VEGF-C:
This combination model appeared to predict the prognosis of
patients with stage I and II OSCC (12). Therefore, the previous study (12) hypothesized that proteins expressed by
tumor cells that correlate with tumor malignancy may predict the
poor prognosis of patients with early-stage OSCC. The present study
proposed a logic combination model using host responses as
covariates to predict the good prognosis of patients with stage
III/IV OSCC. Host responses, including inflammatory reactions may
also have been more applicable in predicting the prognosis of
patients with advanced, rather than early-stage, disease when the
host responses against tumors were evident.
Lymphocytes use numerous mechanisms to eliminate
tumor cells, including direct attack by cytotoxic lymphocytes
(23). Infiltration by lymphocytes
may be associated with the prognosis of patients with malignant
tumors, including OSCC (28). In the
present study, univariate analysis revealed an association between
dense lymphocyte infiltration and an improved prognosis. The
present study immunohistochemically distinguished other lymphocyte
populations from NK cells, which attack tumor cells without
recognizing major histocompatibility complex class I molecules and
are part of the innate immune response (23). In the present study, NK cells were
sparse in the biopsy and surgical tissue samples, as previously
reported (29,30), despite reports of dense infiltration
of NK cells in OSCC (31). Therefore,
NK cells may have a limited ability to eliminate OSCC cells at
advanced stages. In contrast to dense lymphocyte infiltration,
univariate analysis did not reveal an association between NK cell
density and the prognosis of patients with OSCC. The present study
also analyzed host response based on the presence of
intraepithelial micro-abscesses. Direct evidence of neutrophil
contribution to protection against tumors has not previously been
reported. In this study, univariate analysis has demonstrated a
close associated of neutrophils with prognosis of OSCC. Thus, the
present study assumed that intraepithelial micro-abscesses do not
directly eliminate tumor cells, but reflect the immune competence
of patients with OSCC to a certain extent.
Variables revealed to be significantly associated by
univariate analysis usually serve as covariates in further
analyses. Lymphocyte infiltration and micro-abscess formation were
significantly associated with the prognosis of patients with OSCC
in univariate analysis, and the present study also further analyzed
NK cell density. The logic combination model named IPF, in which
patients with OSCC exhibited intraepithelial micro-abscesses and a
lower density of NK cells, revealed the lowest AIC scores and a
predicted 6-fold lower risk of a poor prognosis, compared with
other patients. Furthermore, considering the biological function of
NK cells in tumor immunity, patients with a higher density of NK
cells experienced a markedly higher risk of having a poor
prognosis. The biological explanation may suggest that NK cells
tend to appear in response to highly malignant tumor cells at
advanced stages.
The biological mechanisms underlying
inflammation-associated carcinogenesis have been investigated in
association with chemokines (32,33).
Crosstalk via chemokines and their receptors among tumor cells,
tumor stromal cells and endothelial cells of tumor-associated blood
vessels contribute to the progression of tumors in vivo
(34). However, the inhibition of the
chemokine C-C motif chemokine ligand 2 accelerated breast cancer
metastasis in animal models; therefore, understanding of the roles
of chemokines on carcinogenesis and tumor progression must be
improved (22). In addition,
lymphocytes may promote tumor metastasis in association with their
affinity for endothelial cells by forming tumor cell-lymphocyte
chimeras (35). These hypotheses do
not comply with the notion of tumor immunity, which is an
advantageous biological reaction to remove dangerous transformed
tumor cells, and indicates a biphasic biological role in the
inflammatory reaction to tumor cells. The biphasic role may be
associated with a relatively limited contribution of lymphocyte
infiltration to prognostic prediction for patients with OSCC.
Furthermore, oral mucosa is often accompanied by various degrees of
inflammation and the prevalence of immune-mediated diseases,
including lichen planus as well as allergic reactions, are high in
oral mucosa (24). This may partially
explain the inflammatory reaction and why the prognosis of patients
with OSCC is difficult to predict.
In conclusion, the present study developed a logic
combination model to predict low risk populations among patients
with stage III/IV OSCC using intraepithelial micro-abscesses and NK
cell density as covariates. Host responses to tumor infiltration
may be effective in evaluating the prognosis of patients with
advanced OSCC.
Acknowledgements
The present study was supported by the Japan Society
for the Promotion of Science (grant no. 25462865).
Glossary
Abbreviations
Abbreviations:
|
HE
|
hematoxylin and eosin
|
|
IPF
|
important prognostic factor
|
|
NK
|
natural killer
|
|
OSCC
|
oral squamous cell carcinoma
|
|
TNM
|
tumor-node-metastasis
|
|
UICC
|
Union for International Cancer
Control
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM Classification of Malignant Tumours. Wiley
Blackwell; Oxford: 2017
|
|
2
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lohela M, Bry M, Tammela T and Alitalo K:
VEGFs and receptors involved in angiogenesis versus
lymphangiogenesis. Curr Opin Cell Biol. 21:154–165. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsuura M, Onimaru M, Yonemitsu Y, Suzuki
H, Nakano T, Ishibashi H, Shirasuna K and Sueishi K: Autocrine loop
between vascular endothelial growth factor (VEGF)-C and VEGF
receptor-3 positively regulates tumor-associated lymphangiogenesis
in oral squamoid cancer cells. Am J Pathol. 175:1709–1721. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maeda T, Matsumura S, Hiranuma H, Jikko A,
Furukawa S, Ishida T and Fuchihata H: Expression of vascular
endothelial growth factor in human oral squamous cell carcinoma:
Its association with tumour progression and p53 gene status. J Clin
Pathol. 51:771–775. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smith BD, Smith GL, Carter D, Sasaki CT
and Haffty BG: Prognostic significance of vascular endothelial
growth factor protein levels in oral and oropharyngeal squamous
cell carcinoma. J Clin Oncol. 18:2046–2052. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shang ZJ, Li JR and Li ZB: Circulating
levels of vascular endothelial growth factor in patients with oral
squamous cell carcinoma. Int J Oral Maxillofac Surg. 31:495–498.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnstone S and Logan RM: The role of
vascular endothelial growth factor (VEGF) in oral dysplasia and
oral squamous cell carcinoma. Oral Oncol. 42:337–342. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schimming R, Reusch P, Kuschnierz J and
Schmelzeisen R: Angiogenic factors in squamous cell carcinoma of
the oral cavity: Do they have prognostic relevance? J
Craniomaxillofac Surg. 32:176–181. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shintani S, Li C, Ishikawa T, Mihara M,
Nakashiro K and Hamakawa H: Expression of vascular endothelial
growth factor A, B, C, and D in oral squamous cell carcinoma. Oral
Oncol. 40:13–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sugiura T, Inoue Y, Matsuki R, Ishii K,
Takahashi M, Abe M and Shirasuna K: VEGF-C and VEGF-D expression is
correlated with lymphatic vessel density and lymph node metastasis
in oral squamous cell carcinoma: Implications for use as a
prognostic marker. Int J Oncol. 34:673–680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki H, Fukuyama R, Hasegawa Y, Tamaki
T, Nishio M, Nakashima T and Tatematsu M: Tumor thickness, depth of
invasion, and Bcl-2 expression are correlated with FDG-uptake in
oral squamous cell carcinomas. Oral Oncol. 45:891–897. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seki S, Fujiwara M, Matsuura M, Fujita S,
Ikeda H, Asahina I and Ikeda T: Prediction of outcome of patients
with oral squamous cell carcinoma using vascular invasion and the
strongly positive expression of vascular endothelial growth
factors. Oral Oncol. 47:588–593. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kato Y, Fujita N, Kunita A, Sato S, Kaneko
M, Osawa M and Tsuruo T: Molecular identification of Aggrus/T1alpha
as a platelet aggregation-inducing factor expressed in colorectal
tumors. J Biol Chem. 278:51599–51605. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Toyoshima M, Nakajima M, Yamori T and
Tsuruo T: Purification and characterization of the
platelet-aggregating sialoglycoprotein gp44 expressed by highly
metastatic variant cells of mouse colon adenocarcinoma 26. Cancer
Res. 55:767–773. 1995.PubMed/NCBI
|
|
16
|
Yuan P, Temam S, El-Naggar A, Zhou X, Liu
DD, Lee JJ and Mao L: Overexpression of podoplanin in oral cancer
and its association with poor clinical outcome. Cancer.
107:563–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kreppel M, Drebber U, Wedemeyer I, Eich
HT, Backhaus T, Zöller JE and Scheer M: Podoplanin expression
predicts prognosis in patients with oral squamous cell carcinoma
treated with neoadjuvant radiochemotherapy. Oral Oncol. 47:873–878.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bartuli FN, Luciani F, Caddeo F, Compagni
S, Piva P, Ottria L and Arcuri C: Podoplanin in the development and
progression of oral cavity cancer: A preliminary study. Oral
Implantol (Rome). 5:33–41. 2012.PubMed/NCBI
|
|
19
|
Funayama A, Cheng J, Maruyama S, Yamazaki
M, Kobayashi T, Syafriadi M, Kundu S, Shingaki S, Saito C and Saku
T: Enhanced expression of podoplanin in oral carcinomas in situ and
squamous cell carcinomas. Pathobiology. 78:171–180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawaguchi H, El-Naggar AK,
Papadimitrakopoulou V, Ren H, Fan YH, Feng L, Lee JJ, Kim E, Hong
WK, Lippman SM and Mao L: Podoplanin: A novel marker for oral
cancer risk in patients with oral premalignancy. J Clin Oncol.
26:354–360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seki S, Fujiwara M, Matsuura M, Fujita S,
Ikeda H, Umeda M, Asahina I and Ikeda T: Prognostic value of
podoplanin expression in oral squamous cell carcinoma-a regression
model auxiliary to UICC classification. Pathol Oncol Res.
20:521–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bonapace L, Coissieux MM, Wyckoff J, Mertz
KD, Varga Z, Junt T and Bentires-Alj M: Cessation of CCL2
inhibition accelerates breast cancer metastasis by promoting
angiogenesis. Nature. 515:130–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abbas AK, Lichtman AH and Pillai S:
Cellular and Molecular Immunology 7th edition. Elsevier;
Philadelphia: pp. 389–405. 2012
|
|
24
|
Neville BWN, Damm DD, Allen CM and Chi AC:
Oral and Maxillofacial Pathology (4th). 2016.
|
|
25
|
Jakobsson PA, Eneroth CM, Killander D,
Moberger G and Mårtensson B: Histologic classification and grading
of malignancy in carcinoma of the larynx. Acta Radiol Ther Phys
Biol. 12:1–8. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akaike H: Information theory and an
extention of the maximum likelihood principle2nd International
Symposium on Information Theory. Petrov BN and Csaki F: Budapest:
1973
|
|
27
|
Ihaka R and Gentleman R: R: A language for
data analysis and graphics. J Comp Graph Stat. 5:299–314. 1996.
View Article : Google Scholar
|
|
28
|
Chatzistamou I, Rodriguez J, Jouffroy T,
Girod A, Point D, Sklavounou A, Kittas C, Sastre-Garau X and
Klijanienko J: Prognostic significance of tumor shape and stromal
chronic inflammatory infiltration in squamous cell carcinomas of
the oral tongue. J Oral Pathol Med. 39:667–671. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Halama N, Braun M, Kahlert C, Spille A,
Quack C, Rahbari N, Koch M, Weitz J, Kloor M, Zoernig I, et al:
Natural killer cells are scarce in colorectal carcinoma tissue
despite high levels of chemokines and cytokines. Clin Cancer Res.
17:678–689. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Turkseven MR and Oygür T: Evaluation of
natural killer cell defense in oral squamous cell carcinoma. Oral
Oncol. 46:e34–e37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Katou F, Ohtani H, Watanabe Y, Nakayama T,
Yoshie O and Hashimoto K: Differing phenotypes between
intraepithelial and stromal lymphocytes in early-stage tongue
cancer. Cancer Res. 67:11195–11201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Landskron G, De la Fuente M, Thuwajit P,
Thuwajit C and Hermoso MA: Chronic inflammation and cytokines in
the tumor microenvironment. J Immunol Res. 2014:1491852014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mukaida N, Sasaki S and Baba T: Chemokines
in cancer development and progression and their potential as
targeting molecules for cancer treatment. Mediators Inflamm.
2014:1703812014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qian BZ, Li J, Zhang H, Kitamura T, Zhang
J, Campion LR, Kaiser EA, Snyder LA and Pollard JW: CCL2 recruits
inflammatory monocytes to facilitate breast-tumour metastasis.
Nature. 475:222–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song G, Ren J, Stojadinovic A, Chen W,
Sahab Z, Fu SW and Man YG: Conjunction of tumor cells with
lymphocytes: Implications for tumor invasion and metastasis. Cancer
Epidemiol. 36:354–363. 2012. View Article : Google Scholar : PubMed/NCBI
|