Introduction
Lung cancer, which is characterized by subclinical
activation of blood coagulation and fibrinolysis from the early
clinical stages of disease, is the most common cause of
cancer-associated mortality worldwide (1). Significant increases in the blood
concentrations of thrombin-antithrombin III complexes, fibrinogen
and D-dimer have been reported in patients with lung cancer
exhibiting extensive or limited disease. The extent of such
activation of these markers has also been associated with tumor
stage and prognosis (2,3). However, the origin of the activation of
the coagulative-fibrinolytic system in lung cancer remains
incompletely understood.
Von Willebrand factor (vWF) is a multimeric
glycoprotein produced by the vascular endothelium and
megakaryocytes that is crucial for hemostasis, and functions as a
carrier protein for factor VIII (FVIII) and as a bridge between the
subendothelial matrix and platelets (4). The distinctive properties of the
distinct molecular masses of VWF influence its ability to mediate
platelet adhesion and aggregation (5). Unusually large multimers stored in and
released from Weibel-Palade bodies in stimulated vascular
endothelial cells are the hemostatically active form of VWF
(6). A disintegrin and
metalloproteinase with a thrombospondin type 1 motif13 (ADAMTS-13),
formerly known as VWF cleavage protease, is a metalloprotease that
specifically cleaves the multimeric VWF into smaller fragments
between Tyr1605 and Met1606 within the VWF A2
domain (7). The cellular origin of
the ADAMTS-13 antigen in plasma has been described in platelets,
and in hepatic, renal and endothelial cells. Deficiency of
ADAMTS-13 activity results in an increase in circulating large VWF
multimers (8).
An association between the ABO blood group and blood
coagulation was recognized several years previously. Non-O blood
type has been associated with a significantly increased risk of
venous and arterial thromboembolic disease, including deep vein
thrombosis, pulmonary embolism, ischemic heart disease and
peripheral vascular disease (9). The
plasma levels of two coagulation glycoproteins, VWF and FVIII, have
been demonstrated as the basis of this association between the ABO
blood group and blood coagulation (10). Individuals with type O blood have
significantly decreased plasma levels of VWF and FVIII, compared
with those with non-O blood types (A, B and AB) (11). In total, ~66% of the variations in VWF
plasma levels are associated with mutations, and 30% of these are
associated with the effect of the ABO blood group (10).
Increased levels of VWF and FVIII and impaired
activity of ADAMTS-13 have been demonstrated in patients with
metastasizing and malignant tumors (12). The majority of studies have not
focused on the levels of VWF, FVIII and ADAMTS-13 in patients with
lung cancer; however, Martini et al (13) reported that VWF antigen levels are not
substantially altered in patients with non-small cell lung cancer.
However, the imbalance in the distribution of O and non-O blood
types between patients and healthy controls in that study may have
influenced the results. Therefore, the aim of the present study was
to identify possible associations between ABO blood group types and
plasma levels of VWF and ADAMTS-13, and FVIII activity in patients
with lung cancer in order to improve the characterization of
potential links between blood type and coagulative activation in
lung cancer.
Materials and methods
Patients
The present cross-sectional study included 115
patients with histologically confirmed lung carcinoma who visited
the First Affiliated Hospital of Zhejiang University School of
Medicine (Hangzhou, China) between March 2013 and May 2014, and 98
age- and gender-matched healthy controls selected from individuals
who underwent routine health examinations at the Health Center of
the First Affiliated Hospital. A pathological diagnosis of lung
cancer was derived from biopsy or cytological specimens collected
at bronchoscopy or transthoracic needle aspiration, or from
surgical specimens. Disease in all patients wasclassified according
to the revised World Health Organization classification of lung
tumors and staged according to the revised tumor-node-metastasis
staging system for lung cancer (14,15).
Patient characteristics are presented in Table I.
 | Table I.Baseline characteristics of the study
population. |
Table I.
Baseline characteristics of the study
population.
| Variable | Patients with lung
cancer (n=115) | Healthy controls
(n=98) | P-value |
|---|
| Age, years | 57.1±8.5 | 55.1±7.4 | 0.101 |
| Male/female, n | 76/39 | 58/40 | 0.299 |
| BMI,
kg/m2 | 22.2±2.7 | 23.1±2.6 | 0.024a |
| SBP, mmHg | 128.0±9.0 | 127.2±8.9 | 0.645 |
| DBP, mmHg | 80.2±6.7 | 78.5±7.5 | 0.578 |
| Smoker/non-smoker,
n | 32/83 | 20/78 | 0.216 |
| Histology |
| N/A |
|
| NSCLC, n
(%) | 89 (77.4) |
|
|
|
Squamous cell carcinoma, n
(%) | 29 (25.2) |
|
|
|
Adenocarcinoma, n (%) | 51 (44.3) |
|
|
| Others
(unclassified), n (%) | 9 (7.8) |
|
|
| SCLC, n
(%) | 26 (22.6) |
|
|
| Stage of NSCLC
disease |
| N/A |
|
| Local
(stages I + II), n (%) | 12 (10.4) |
|
|
| Locally
advanced (stage III), n (%) | 31 (27.0) |
|
|
|
Metastatic (stage IV), n
(%) | 46 (40.0) |
|
|
| SCLC |
|
|
|
|
Limited, n (%) | 17 (14.8) |
|
|
|
Extensive, n (%) | 9 (7.8) |
|
|
| Blood group |
|
|
|
| O, n
(%) | 29 (25.2) | 34 (34.7) | 0.131 |
| Non-O,
n (%) | 86 (74.8) | 64 (65.3) | 0.131 |
| A, n
(%) | 41 (35.7) | 24 (24.5) | 0.078 |
| B, n
(%) | 30 (26.1) | 33 (33.7) | 0.227 |
| AB, n
(%) | 15 (13.0) | 7 (7.1) | 0.158 |
All subjects enrolled in the present study had
normal liver, renal and cardiac function. The exclusion criteria
included a history of chronic hypertension, diabetes, renal,
hepatic, cardiovascular and autoimmune diseases, anticoagulant or
corticosteroid use, arterial or venous thrombosis, renal
transplantation, human immunodeficiency virus infection and
pregnancy. The present study was performed in accordance with the
principles embodied in the Declaration of Helsinki and was approved
by the Institutional Research Ethics Committee of the First
Affiliated Hospital of Zhejiang University. Written informed
consent was obtained from all participants.
Sample collection
Venous blood samples were collected from all
participants into 3 tubes early in the morning following overnight
fasting at the time of clinical diagnosis and prior to any
treatment. A tube containing 0.109 M trisodium citrate (9:1, v/v)
was used for measuring the plasma levels of D-dimer, fibrinogen,
VWF and ADAMTS-13, as well as FVIII activity. Plasma was obtained
by immediately centrifuging the blood in a refrigerated centrifuge
at 1,500 × g for 15 min. A second tube coated with EDTA (2.4 mg/2
ml venous blood) was used for immediate classification of the ABO
blood group, and for determination of the hemoglobin concentration
and platelet count. Blood was also collected in a third tube
without any anticoagulant and was allowed to clot for the isolation
of serum following centrifugation at 1,500 × g for 10 min at 4°C.
All plasma and serum samples were divided into 1 ml aliquots and
stored at −80°C until evaluation.
Assays
Phenotyping of the ABO blood groups was performed
using a Galileo blood group analyzer (Immucor, Inc., Norcross, GA,
USA), with anti-A and anti-B blood grouping reagents and reference
cells A1, A2, B and O (Immucor, Inc.). Serum
levels of lactate dehydrogenase (LDH) were determined using a
Hitachi 7600 automated biochemistry analyzer (Hitachi, Ltd., Tokyo,
Japan) with an International Federation of Clinical Chemistry
method (Roche Diagnostics GmbH, Mannheim, Germany) according to the
manufacturer's protocol (16). Serum
levels of carcinoembryonic antigen (CEA) were measured using a
chemiluminescent microparticle immunoassay with the ARCHITECT
i2000SR system (Abbott Laboratories, Abbott Park, IL, USA). Plasma
levels of fibrinogen (Clauss method) (17), D-dimer (immunoturbidimetric method)
(18) and FVIII activity (coagulation
method) (19) were measured using the
Sysmex CS-5100 coagulation analyzer (Sysmex Corporation, Kobe,
Japan), with the Dade® Thrombin reagent,
INNOVANCE® D-Dimer and Coagulation Factor VIII Deficient
Plasma (all from Siemens AG, Munich, Germany), respectively. Plasma
levels of VWF and ADAMTS-13 were measured using the vWF ELISA kit
and ADAMTS-13 ELISA kit (R&D Systems, Inc., Minneapolis, MN,
USA), according to the manufacturer's protocol.
Statistical analysis
All statistical analyses were performed using SPSS
for Windows software (version 16.0 J; SPSS, Inc., Chicago, IL,
USA). Shapiro-Wilk tests were used to determine whether variables
were normally distributed. Continuous variables are expressed as
the mean ± standard deviation, or as the median and the 25–75th
percentiles. Comparisons of results between two groups were made
using the Student's t-test or Mann-Whitney U test, where
appropriate. Non-parametric χ2 tests were used to
compare categorical variables. P<0.05 was considered to indicate
a statistically significant difference.
Results
Patient characteristics
Table I summarizes the
clinical characteristics of the 213 subjects enrolled in the
present study. No significant differences in age, gender, systolic
blood pressure (SBP), diastolic blood pressure (DBP) or smoking
status between patients with lung cancer and healthy controls were
identified. However, body mass index (BMI) was significantly
decreased in patients with lung cancer (P=0.024). Baseline
histopathological characteristics and lung cancer stages are also
presented in Table I.
ABO distribution
ABO blood group distribution in patients
demonstrated that 29 (25.2%) were type O, 41 (35.7%) were type A,
30 (26.1%) were type B and 15 (13.0%) were type AB. In the control
group, 34 (34.7%) subjects were type O, 24 (24.5%) were type A, 33
(33.7%) were type B and 7 (7.1%) were type AB (Table I). Subjects with type A, B and AB
blood were pooled into the non-O group. No significant difference
in the frequency of blood type between the two groups was
identified (Table I).
Hemostatic parameters
Hemostatic data are summarized in Table II. The platelet count and serum
levels of hemoglobin, LDH, CEA, D-dimer, fibrinogen and VWF, and
FVIII activity were significantly increased in all patients with
lung cancer, compared with in healthy controls (P<0.05), whereas
a significant decrease in ADAMTS-13 levels was observed in all
patients with lung cancer, compared with the healthy controls
(P=0.011). Similarly, regardless of the presence of distant
metastasis, patients with lung cancer also exhibited increased
plasma levels of hemoglobin, LDH, CEA, D-dimer, fibrinogen and VWF,
and FVIII activity when compared with healthy controls (P<0.05).
However, a significant increase in platelet count and a significant
decrease in ADAMTS-13 levels were only observed in patients with
lung cancer exhibiting distant metastasis, as compared with healthy
controls (P<0.05). Levels of hemostatic parameters were further
compared between patients with and without distant metastasis,
which demonstrated significant increases in plasma levels of LDH,
CEA, D-dimer and VWF, and in FVIII activity, and a significant
decrease in plasma levels of ADAMTS-13 (Table II).
 | Table II.Levels of hemostatic parameters in
patients with lung cancer and healthy controls. |
Table II.
Levels of hemostatic parameters in
patients with lung cancer and healthy controls.
|
| Patients with lung
cancer |
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Variable | Overall
(n=115) | Without distant
metastasis (n=60) | With distant
metastasis (n=55) | Healthy controls
(n=98) |
P-valuea |
P-valueb |
P-valuec |
P-valued |
|---|
| Hemoglobin,
g/l | 120 (106–135) | 123 (106–138) | 119 (105–135) | 147 (135–161) |
<0.001e |
<0.001e |
<0.001e | 0.558 |
| Platelet
count,x109/l | 239 (178–317) | 223 (170–298) | 249 (183–322) | 218 (192–233) | 0.033e | 0.328 | 0.011e | 0.341 |
| LDH, U/l | 186 (158–231) | 178 (149–226) | 200 (167–242) | 129 (118–141) |
<0.001e |
<0.001e |
<0.001e | 0.024e |
| CEA, ng/ml | 10.3
(3.5–38.7) | 6.0 (2.9–25.3) | 22.8
(5.3–75.7) | 1.6 (1.0–2.3) |
<0.001e |
<0.001e |
<0.001e | 0.015e |
| D-dimer, µg/l
FEU | 850
(412–2,161) | 515
(374–1,191) | 1,522
(611–2,757) | 115 (55–216) |
<0.001e |
<0.001e |
<0.001e | 0.002e |
| Fibrinogen,
g/l | 3.6 (2.9–4.7) | 3.6 (2.9–4.7) | 3.7 (2.8–4.7) | 2.4 (2.0–2.7) |
<0.001e |
<0.001e |
<0.001e | 0.990 |
| VWF, U/l | 1,562
(1,052–2,289) | 1,428
(902–1,861) | 1,892
(1,239–2,369) | 1,014
(726–1,486) |
<0.001e | 0.015e |
<0.001e | 0.026e |
| ADAMTS-13,U/l | 1,361
(1,026–1,735) | 1,402
(1,201–1,824) | 1,188
(803–1,650) | 1,464
(1,237–1,957) | 0.011e | 0.340 | 0.001e | 0.044e |
| FVIII,% | 143 (117–182) | 137 (113–174) | 154 (129–196) | 107 (91–122) |
<0.001e |
<0.001e |
<0.001e | 0.043e |
Correlation between hemostatic
parameters and ABO blood groups
All subjects in the two groups were categorized as
either type O or non-O blood groups to compare the clinical
characteristics and levels of hemostatic parameters. As presented
in Table III, no significant
differences in age, gender, BMI, SBP, DBP and smoking status
between individuals in the type O or non-O blood groups among
patients with lung cancer and the healthy controls were identified.
Similarly, no significant difference in the distribution of
patients with distant metastasis between the type O and non-O blood
groups was identified. Regarding hemostatic parameters, patients
with lung cancer from the non-O blood group demonstrated a
significant increase in VWF plasma levels (P=0.022) and FVIII
activity (P=0.014), compared with those in the type O blood group.
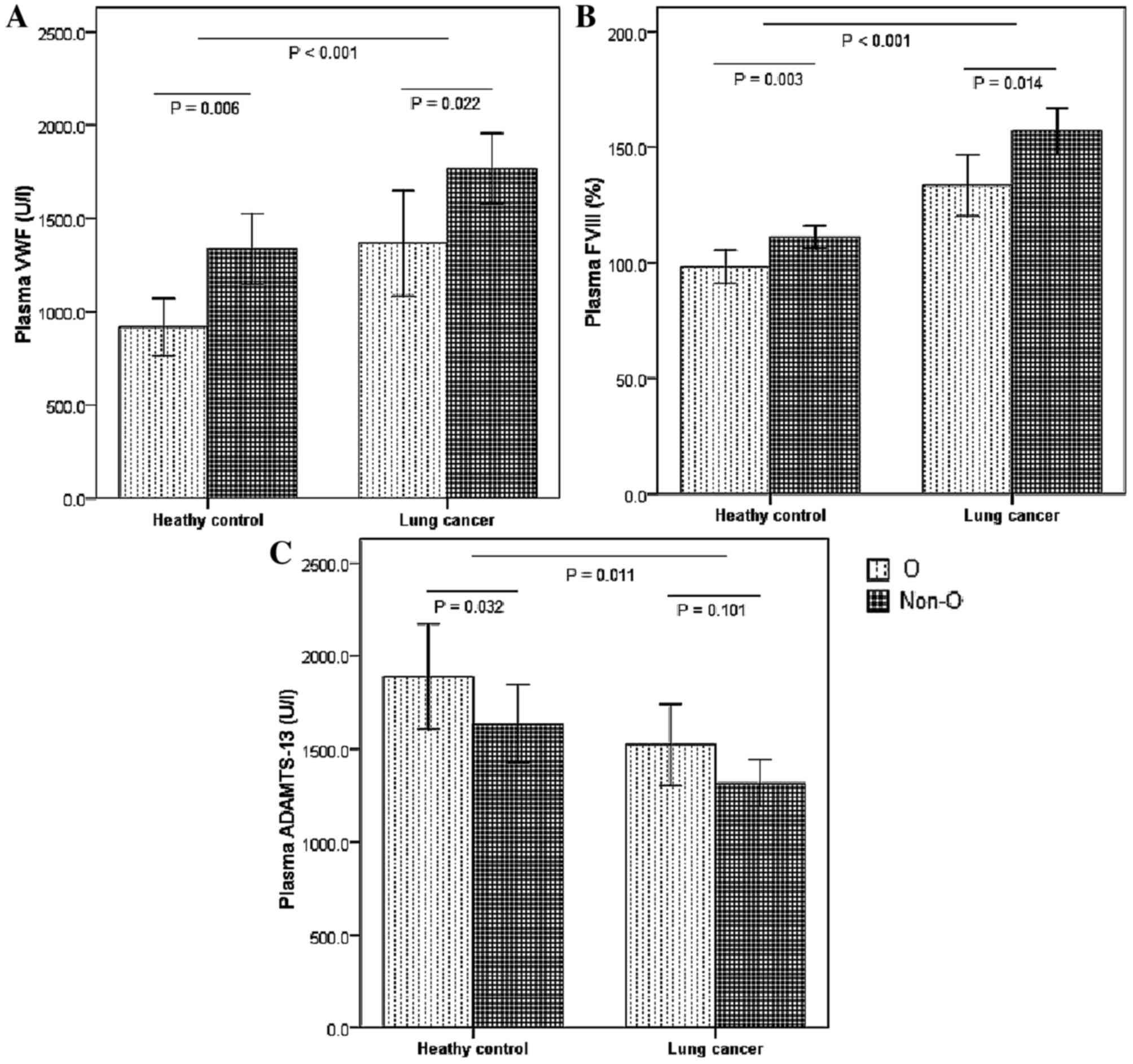
A significant increase was identified in plasma levels of
fibrinogen (P=0.004; Table III) and
VWF (P=0.006; Fig. 1A), and FVIII
activity (P=0.003; Fig. 1B) in the
control group among those with non-O type blood, compared with
those with type O. However, a significant decrease in ADAMTS-13
levels was observed only in the control group, not in the lung
cancer group, among those with non-O type blood, as compared with
those with type O blood (P<0.05; Fig.
1C). No significant differences were observed in platelet
counts or serum levels of hemoglobin, LDH, CEA and D-dimer between
the groups.
 | Table III.Comparison of hemostatic parameters
in patients with lung cancer and healthy controls, according to the
type O and non-O blood groups. |
Table III.
Comparison of hemostatic parameters
in patients with lung cancer and healthy controls, according to the
type O and non-O blood groups.
|
| Patients with lung
cancer | Healthy
controls |
|---|
|
|
|
|
|---|
| Variables | O (n=29) | Non-O (n=86) | P-value | O (n=34) | Non-O (n=64) | P-value |
|---|
| Age, years | 56.9±10.0 | 56.3±7.8 | 0.892 | 55.7±7.6 | 54.2±7.0 | 0.931 |
| Gender,
male/female | 20/9 | 56/30 | 0.705 | 18/16 | 40/24 | 0.359 |
| BMI,
kg/m2 | 22.1±2.4 | 22.3±8.0 | 0.802 | 22.4±3.0 | 23.5±2.4 | 0.751 |
| SBP, mmHg | 126.4±9.8 | 128.7±8.6 | 0.291 | 125.3±10.8 | 128.2±7.7 | 0.337 |
| DBP, mmHg | 79.1±6.5 | 80.7±6.8 | 0.347 | 76.7±9.1 | 79.5±6.4 | 0.265 |
| Smoking status,
yes/no | 9/20 | 23/63 | 0.656 | 8/26 | 12/52 | 0.576 |
| Distant metastasis,
yes/no | 13/16 | 42/44 | 0.709 | – | – | – |
| Hemoglobin,
g/l | 128 (115–138) | 118 (104–134) | 0.140 | 143 (134–158) | 149 (136–161) | 0.359 |
| Platelet count,
×109/l | 227 (175–294) | 241 (179–322) | 0.604 | 212 (186–229) | 220 (200–242) | 0.083 |
| LDH, U/l | 180 (158–238) | 189 (157–230) | 0.879 | 125 (116–142) | 131 (120–141) | 0.745 |
| CEA, ng/ml | 4.8 (2.4–30.2) | 13.6
(4.2–43.9) | 0.088 | 1.4 (1.0–2.0) | 1.7 (1.0–2.5) | 0.134 |
| D-dimer, µg/l
FEU | 759
(492–1,689) | 873
(406–2,349) | 0.828 | 78 (55–176) | 121 (63–256) | 0.298 |
| Fibrinogen,
g/l | 3.6 (2.8–4.7) | 4.1 (3.1–4.9) | 0.440 | 2.1 (2.0–2.5) | 2.6 (2.1–2.8) | 0.004a |
| VWF,U/l | 1,190
(892–1,865) | 1,676
(1,099–2,383) | 0.022a | 865
(583–1,291) | 1,117
(772–1,649) | 0.006a |
| ADAMTS-13, U/l | 1,586
(1,087–1,890) | 1,333
(974–1,576) | 0.101 | 1,572
(1,397–2,239) | 1,403
(1,116–1,771) | 0.032a |
| FVIII, % | 130 (113–161) | 145 (124–194) | 0.014a | 98 (81–113) | 112 (100–124) | 0.003a |
Discussion
Hemostatic abnormalities are present in ~50% of
patients with cancer and >90% of those with metastatic disease
(20). The results of the present
study identified significantly increased plasma levels of
hemoglobin, LDH, CEA, D-dimer, fibrinogen and VWF, and FVIII
activity, in patients with lung cancer, regardless of the presence
or absence of distant metastasis. These results confirmed an
association between the activation of blood coagulation and
fibrinolysis, and lung cancer, even though the underlying molecular
mechanisms for this association remain incompletely understood.
Additionally, significant differences in the plasma levels of LDH,
CEA, D-dimer, VWF and ADAMTS-13, and FVIII activity were observed
between patients with and without distant metastasis. These results
are in agreement with those of previous studies, which demonstrated
an increased tendency for the development of coagulative and
fibrinolytic disorders in patients with metastatic diseases
(3,21). Furthermore, these results are similar
to the observations of Oleksowicz et al (22), who identified increased VWF levels and
ADAMTS-13 deficiencies in patients with metastatic tumors.
Nevertheless, the results of the present study are in contrast with
the results obtained by Martini et al (13), who identified a similar distribution
of VWF between patients with lung cancer and control subjects. The
ABO blood group is a major determinant of plasma VWF levels
resulting in significantly decreased plasma levels in individuals
with type O blood, as compared with those with non-O blood types
(11). By contrast with the matched
age and gender, and similar distribution of blood groups between
cases and controls, as well as the strict inclusion and exclusion
criteria of the subjects in the present study, the imbalance in the
distribution of blood groups between cases and controls in the
study by Martini et al (13)
may explain the lack of differences in plasma VWF concentrations
between the two groups.
Although the biological significance of hemostatic
abnormalities in cancer remains unclear, evidence indicates that
activation of the coagulative-fibrinolytic system by neoplastic
cells may facilitate invasiveness and metastases (1). Indeed, elevated fibrinogen and D-dimer
levels have been associated with unresponsiveness to treatment and
unfavorable prognosis in patients with lung cancer (23). VWF is secreted from endothelial cells
and readily degraded into small multimeric forms, which are more
rapidly cleared from the circulation compared with the large
multimeric forms. However, only the large multimeric forms of VWF
are hemostatically active in the mediation of platelet adhesion to
cancer cells (6). As ADAMTS-13
appears to be the most important protease that degrades VWF,
ADAMTS-13 deficiency in patients with lung cancer may account for
the appearance of large VWF multimers and increased VWF levels in
circulating blood that may enhance tumor-induced platelet
aggregation, resulting in thrombus formation and metastatic
progression (24). Oleksowicz et
al (22) demonstrated that
platelet immunerelated glycoprotein Iba (GPIba) receptors may be
expressed on the membrane of cancer cells with a particular
affinity for large VWF multimers. The binding of GPIba receptors to
large VWF multimers leads to the formation of platelet-cancer cell
aggregates that allow the migration of cancer cells through vessel
walls and the formation of novel sites of metastasis (25). However, the underlying molecular
mechanism for ADAMTS-13 deficiency in cancer dissemination remains
unclear. Therefore, stringent studies are required in order to
establish a causal association between ADAMTS-13 deficiency and
malignancy and/or metastasis.
Blood group antigens are chemical components on the
erythrocyte membrane, but are also expressed on the surfaces of
cancer cells. Alterations in blood group antigens may lead to
alterations in the interactions between cells or cells and the
extracellular matrix. These alterations are hypothesized to be
important for the development of certain malignancies (26,27).
However, data concerning the role of ABO blood groups in lung
cancer are limited and inconsistent (28–30). In
the present study, no significant difference was observed between
patients with lung cancer and healthy controls in terms of the
distribution of ABO blood types, which is similar to the study by
Oguz et al (28), but
different from that of Urun et al (29), who identified an association between
non-O blood types and an increased risk of lung cancer. This
discrepancy may be due to differences in ethnicity, genetic
variants, environmental factors and lifestyle factors among
populations; therefore, inconsistent results may be obtained from
varying genetic backgrounds and ethnicities (30). Linkage disequilibrium between ABO
blood group genes and cancer-associated genes may influence cancer
risk (29). Additionally, blood type
may affect lifestyle, which was demonstrated to be associated with
the incidence and mortality of lung cancer (31).
The ABO blood group has been associated with the
development of venous thrombosis, coronary heart disease and
ischemic stroke, since they are important determinants of VWF and
FVIII plasma levels (11). It has
been suggested that the carbohydrate moiety (ABH-bearing structures
of VWF), similar to the blood group antigens A, B and H (O),
protects VWF from proteolytic degradation by ADAMTS-13 and may
affect VWF function and, indirectly, FVIII levels (32). Bowen (33) demonstrated that proteolysis of VWF by
ADAMTS-13 appears to be more rapid in patients with type O blood,
compared with that in individuals with non-O blood types. The
carbohydrate moiety also serves an important role in VWF
polymerization and function, and affects the liver-mediated
clearance of VWF (34). In addition,
the ABO blood group locus on chromosome 9q34 is the most important
genetic determinant of plasma levels of the VWF-FVIII complex
(6). As VWF serves an important role
as a carrier protein for FVIII, preventing its clearance from
plasma, the majority of the effects of blood type on FVIII plasma
levels are mediated by VWF (10).
Additionally, several other factors, including fibrinogen,
triacylglycerols, and genetic or acquired factors involved in the
biosynthesis or clearance of VWF and FVIII, are also associated
with increased plasma levels of FVIII (35). In the present study, FVIII activity
and plasma levels of VWF were significantly increased in healthy
controls with non-O blood types, compared with those with type O
blood, which is in agreement with previously published data
(10,11). Similar results for FVIII activity and
VWF levels were also observed in patients with lung cancer. As
patients with distant metastasis have increased levels of VWF and
FVIII, one would expect to observe an increased frequency of
distant metastasis in patients with lung cancer with non-O type
blood. However, no significant differences were observed in the
frequencies of distant metastasis between the type O and non-O
blood groups. Notably, decreased ADAMTS-13 levels were observed
among those with non-O blood groups only in healthy controls,
similar to a previous study that observed ADAMTS-13 levels were
increased by ~10% in individuals with type O blood, as compared
with those with non-O type blood (36). Furthermore, the results of the present
study indicated that plasma fibrinogen levels were significantly
increased in healthy controls with non-O type blood, compared with
in those with type O blood. However, no association of type O and
non-O blood groups with fibrinogen and ADAMTS-13 levels was
observed in patients with lung cancer. The small sample size of the
present study may have led to insufficient statistical power.
Another possible explanation for this result is that the state of
hypercoagulability or acute inflammatory responses in patients with
lung cancer may have surpassed the physiological influence
determined by the ABO blood groups and subsequently masked the
effect of blood type in this condition. These data confirm the
presence of complicated alterations in the coagulative-fibrinolytic
system in lung cancer, with a number of factors acting in
concert.
The present study has a number of limitations that
require addressing. First, the number of participants was small.
However, to the best of our knowledge, the present study is the
first to identify an association between ABO blood group and plasma
levels of VWF and ADAMTS-13, and FVIII activity in patients with
lung cancer. Secondly, ADAMTS-13 appears to be the most important
protease that degrades VWF, whereas several proteases from
granulocytes and platelets, which have also been demonstrated to
degrade large VWF multimers in vitro, were not detected.
Thirdly, measurement of the VWF antigen was insufficient to acquire
specific information regarding circulating VWF molecular species,
including the VWF propeptide and VWF multimers. Therefore, further
studies are required to assess VWF propeptides, multimers and
activity levels to reveal the imbalance between VWF secretion and
ADAMTS-13 levels in patients with lung cancer.
The results of the present study demonstrated
increased VWF levels and FVIII activity in patients with lung
cancer with or without distant metastasis, whereas decreased
ADAMTS-13 levels were observed in patients with disseminated
tumors. Non-O blood groups constitute a risk factor for elevated
plasma levels of FVIII and VWF activity in patients with lung
cancer. It is reasonable to carefully monitor patients with lung
cancer with non-O blood types to minimize the risk of thrombotic
events. Furthermore, future studies are required to investigate
potential associations between plasma VWF and ADAMTS-13 levels, and
FVIII activity and outcomes of the patients with lung cancer with
different ABO blood types.
Acknowledgements
The present study was financially supported by the
Science and Technology Foundation of the Public Welfare Profession
of Zhejiang Province (grant no. 2015C33198), the Department of
Education Foundation of Zhejiang Province (grant no. Y201430628)
and the Medical Science and Technology Project of Zhejiang Province
(grant no. 2016KYA074).
References
|
1
|
Gabazza EC, Taguchi O, Yamakami T,
Machishi M, Ibata H and Suzuki S: Evaluating prethrombotic state in
lung cancer using molecular markers. Chest. 103:196–200. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Masago K, Fujita S, Mio T, Togashi Y, Kim
YH, Hatachi Y, Fukuhara A, Irisa K, Sakamori Y and Mishima M:
Clinical significance of the ratio between the alpha 2 plasmin
inhibitor-plasmin complex and the thrombin-antithrombin complex in
advanced non-small cell lung cancer. Med Oncol. 28:351–356. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tas F, Kilic L, Serilmez M, Keskin S, Sen
F and Duranyildiz D: Clinical and prognostic significance of
coagulation assays in lung cancer. Respir Med. 107:451–457. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Terraube V, O'Donnell JS and Jenkins PV:
Factor VIII and von Willebrand factor interaction: Biological,
clinical and therapeutic importance. Haemophilia. 16:3–13. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sporn LA, Marder VJ and Wagner DD:
Inducible secretion of large, biologically potent von Willebrand
factor multimers. Cell. 46:185–190. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sadler JE: Biochemistry and genetics of
von Willebrand factor. Annu Rev Biochem. 67:395–424. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujikawa K, Suzuki H, McMullen B and Chung
D: Purification of human von Willebrand factor-cleaving protease
and its identification as a new member of the metalloproteinase
family. Blood. 98:1662–1666. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moake JL: Thrombotic microangiopathies. N
Engl J Med. 347:589–600. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Franchini M, Favaloro EJ, Targher G and
Lippi G: ABO blood group, hypercoagulability, and cardiovascular
and cancer risk. Crit Rev Clin Lab Sci. 49:137–149. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jenkins PV and O'Donnell JS: ABO blood
group determines plasma von Willebrand factor levels: A biologic
function after all? Transfusion. 46:1836–1844. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sousa NC, Anicchino-Bizzacchi JM,
Locatelli MF, Castro V and Barjas-Castro ML: The relationship
between ABO groups and subgroups, factor VIII and von Willebrand
factor. Haematologica. 92:236–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Terraube V, Marx I and Denis CV: Role of
von Willebrand factor in tumor metastasis. Thromb Res. 120:(Suppl
2). S64–S70. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martini F, Ferroni P, Guadagni F, Basili
S, Spila A, D'Alessandro R, Mineo D, Laudisi A, Portarena I,
Mariotti S, et al: Plasma von Willebrand factor antigen levels in
non-small cell lung cancer patients. Anticancer Res. 25:403–407.
2005.PubMed/NCBI
|
|
14
|
Brambilla E, Travis WD, Colby TV, Corrin B
and Shimosato Y: The new World Health Organization classification
of lung tumours. Eur Respir J. 18:1059–1068. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Detterbeck FC, Boffa DJ and Tanoue LT: The
new lung cancer staging system. Chest. 136:260–271. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bais R and Philcox M: Approved
recommendation on IFCC methods for the measurement of catalytic
concentration of enzymes. Part 8. IFCC method for lactate
dehydrogenase (l-lactate: NAD+oxidoreductase, EC 1.1.1.27).
International Federation of Clinical Chemistry (IFCC). Eur J Clin
Chem Clin Biochem. 32:639–655. 1994.PubMed/NCBI
|
|
17
|
Okuno T and Selenko V: Plasma fibrinogen
determination by automated thrombin time. Am J Med Technol.
38:196–201. 1972.PubMed/NCBI
|
|
18
|
Elf JL, Strandberg K and Svensson PJ:
Performance of two relatively new quantitative D-dimer assays
(Innovance D-dimer and AxSYM D-dimer) for the exclusion of deep
vein thrombosis. Thromb Res. 124:701–705. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barrowcliffe TW, Raut S, Sands D and
Hubbard AR: Coagulation and chromogenic assays of factor VIII
activity: General aspects, standardization, and recommendations.
Semin Thromb Hemost. 28:247–256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goad KE and Gralnick HR: Coagulation
disorders in cancer. Hematol Oncol Clin North Am. 10:457–484. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guadagni F, Ferroni P, Basili S, Facciolo
F, Carlini S, Crecco M, Martini F, Spila A, D'Alessandro R, Aloe S,
et al: Correlation between tumor necrosis factor-alpha and D-dimer
levels in non-small cell lung cancer patients. Lung Cancer.
44:303–310. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oleksowicz L, Bhagwati N and
DeLeon-Fernandez M: Deficient activity of von Willebrand's
factor-cleaving protease in patients with disseminated
malignancies. Cancer Res. 59:2244–2250. 1999.PubMed/NCBI
|
|
23
|
Unsal E, Atalay F, Atikcan S and Yilmaz A:
Prognostic significance of hemostatic parameters in patients with
lung cancer. Respir Med. 98:93–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sugimoto Y, Watanabe M, Oh-Hara T, Sato S,
Isoe T and Tsuruo T: Suppression of experimental lung colonization
of a metastatic variant of murine colon adenocarcinoma 26 by a
monoclonal antibody 8F11 inhibiting tumor cell-induced platelet
aggregation. Cancer Res. 51:921–925. 1991.PubMed/NCBI
|
|
25
|
Oleksowicz L, Bhagwati N, Fernandez MD,
Seno R and Etkind P: Prognostic significance of platelet
immunorelated GPIb expression in breast cancer. Cancer J Sci Am.
4:247–253. 1998.PubMed/NCBI
|
|
26
|
León-Atance P, Moreno-Mata N,
González-Aragoneses F, Cañizares-Carretero MÁ, Poblet-Martínez E,
Genovés-Crespo M, García-Jiménez MD, Honguero-Martínez AF, Rombolá
CA, Simón-Adiego CM, et al: Prognostic influence of loss of blood
group A antigen expression in pathologic stage I non-small-cell
lung cancer. Arch Bronconeumol. 48:49–54. 2012.(In English,
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Joh HK, Cho E and Choueiri TK: ABO blood
group and risk of renal cell cancer. Cancer Epidemiol. 36:528–532.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oguz A, Unal D, Tasdemir A, Karahan S,
Aykas F, Mutlu H, Cihan YB and Kanbay M: Lack of any association
between blood groups and lung cancer, independent of histology.
Asian Pac J Cancer Prev. 14:453–456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Urun Y, Utkan G, Cangir AK, Oksuzoglu OB,
Ozdemir N, Oztuna DG, Kocaman G, Coşkun HŞ, Kaplan MA, Yuksel C, et
al: Association of ABO blood group and risk of lung cancer in a
multicenter study in Turkey. Asian Pac J Cancer Prev. 14:2801–2803.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amundadottir L, Kraft P,
Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA,
Bueno-de-Mesquita HB, Gross M, Helzlsouer K, Jacobs EJ, et al:
Genome-wide association study identifies variants in the ABO locus
associated with susceptibility to pancreatic cancer. Nat Genet.
41:986–990. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suadicani P, Hein HO and Gyntelberg F: ABO
phenotypes and inflammation-related predictors of lung cancer
mortality: The Copenhagen Male Study-a 16-year follow-up. Eur
Respir J. 30:13–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Klarmann D, Eggert C, Geisen C, Becker S,
Seifried E, Klingebiel T and Kreuz W: Association of ABO(H) and I
blood group system development with von Willebrand factor and
factor VIII plasma levels in children and adolescents. Transfusion.
50:1571–1580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bowen DJ: An influence of ABO blood group
on the rate of proteolysis of von Willebrand factor by ADAMTS13. J
Thromb Haemost. 1:33–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wagner DD, Mayadas T and Marder VJ:
Initial glycosylation and acidic pH in the Golgi apparatus are
required for multimerization of von Willebrand factor. J Cell Biol.
102:1320–1324. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi Q, Kim JE, Kim SY, Han KS and Kim HK:
Influence of ABO type on global coagulation assay results: Effect
of coagulation factor VIII. Clin Chem Lab Med. 53:1425–1432. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mannucci PM, Capoferri C and Canciani MT:
Plasma levels of von Willebrand factor regulate ADAMTS-13, its
major cleaving protease. Br J Haematol. 126:213–218. 2004.
View Article : Google Scholar : PubMed/NCBI
|