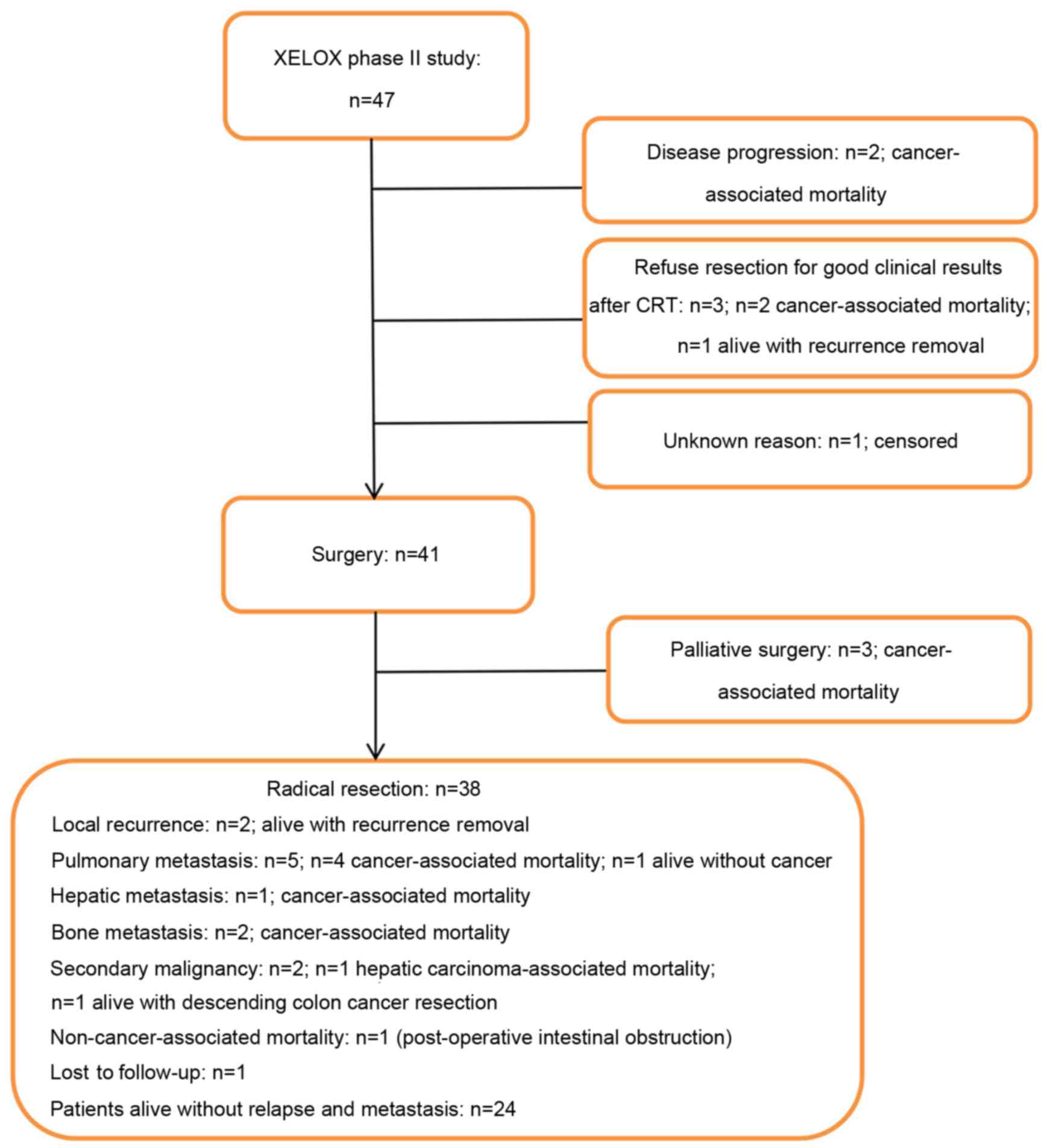
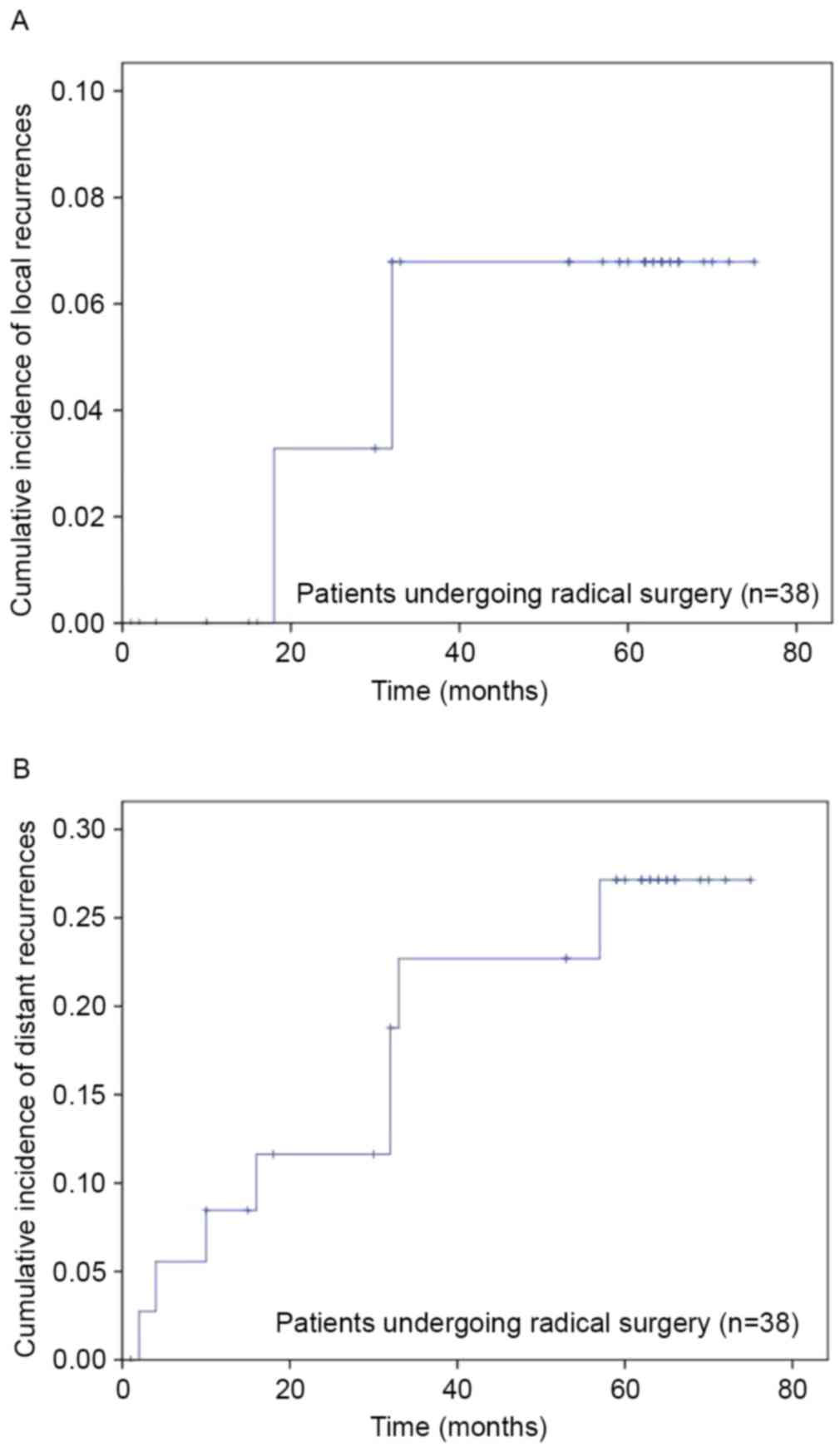
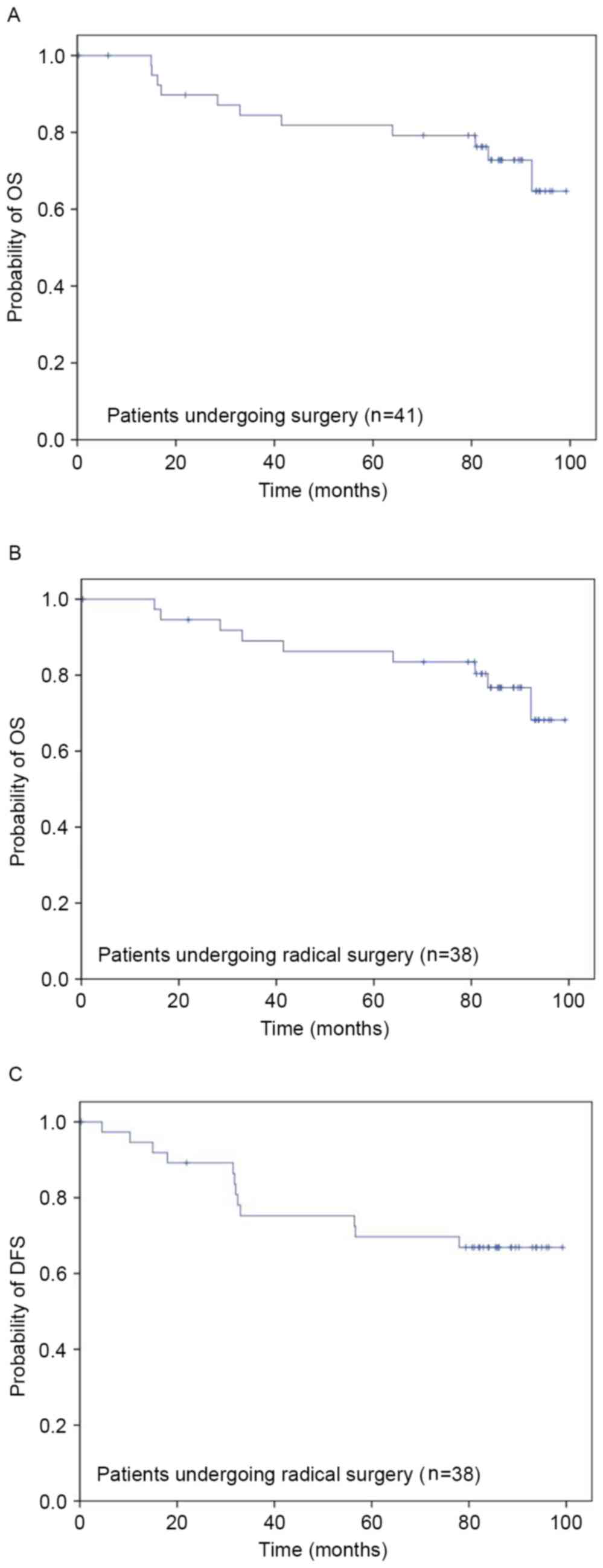
|
1
|
Bosset JF, Collette L, Calais G, Mineur L,
Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A and Ollier
JC: EORTC Radiotherapy Group Trial 22921: Chemotherapy with
preoperative radiotherapy in rectal cancer. N Engl J Med.
355:1114–1123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roh MS, Colangelo LH, O'Connell MJ,
Yothers G, Deutsch M, Allegra CJ, Kahlenberg MS, Baez-Diaz L,
Ursiny CS, Petrelli NJ and Wolmark N: Preoperative multimodality
therapy improves disease-free survival in patients with carcinoma
of the rectum: NSABP R-03. J Clin Oncol. 27:5124–5130. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sanfilippo NJ, Crane CH, Skibber J, Feig
B, Abbruzzese JL, Curley S, Vauthey JN, Ellis LM, Hoff P, Wolff RA,
et al: T4 rectal cancer treated with preoperative chemoradiation to
the posterior pelvis followed by multivisceral resection: Patterns
of failure and limitations of treatment. Int J Radiat Oncol Biol
Phys. 51:176–183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sauer R, Becker H, Hohenberger W, Rödel C,
Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF,
et al: Preoperative versus postoperative chemoradiotherapy for
rectal cancer. N Engl J Med. 351:1731–1740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin JZ, Zeng ZF, Wu XJ, Wan DS, Chen G, Li
LR, Lu ZH, Ding PR and Pan ZZ: Phase II study of pre-operative
radiotherapy with capecitabine and oxaliplatin for rectal cancer
and carcinoembryonic antigen as a predictor of pathological tumour
response. J Int Med Res. 38:645–654. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao L, Bai C, Shao Y, Guan M, Jia N, Xiao
Y, Qiu H, Zhang F, Yang T, Zhong G and Chen S: A phase II study of
neoadjuvant chemoradiotherapy with oxaliplatin and capecitabine for
rectal cancer. Cancer Lett. 310:134–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koeberle D, Burkhard R, von Moos R,
Winterhalder R, Hess V, Heitzmann F, Ruhstaller T, Terraciano L,
Neuweiler J, Bieri G, et al: Phase II study of capecitabine and
oxaliplatin given prior to and concurrently with preoperative
pelvic radiotherapy in patients with locally advanced rectal
cancer. Br J Cancer. 98:1204–1209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aschele C, Friso ML, Pucciarelli S,
Lonardi S, Sartor L, Fabris G, Urso ED, Del Bianco P, Sotti G, Lise
M and Monfardini S: A phase I–II study of weekly oxaliplatin,
5-fluorouracil continuous infusion and preoperative radiotherapy in
locally advanced rectal cancer. Ann Oncol. 16:1140–1146. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carlomagno C, Farella A, Bucci L,
D'Armiento FP, Pesce G, Pepe S, Cannella L, Pacelli R, De Stefano
A, Solla R, et al: Neo-adjuvant treatment of rectal cancer with
capecitabine and oxaliplatin in combination with radiotherapy: A
phase II study. Ann Oncol. 20:906–912. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Salazar R, Navarro M, Losa F, Alonso V,
Gallén M, Rivera F, Benavides M, Escudero P, González E, Massutí B,
et al: Phase II study of preoperative radiotherapy and concomitant
weekly intravenous oxaliplatin combined with oral capecitabine for
stages II–III rectal cancer. Clin Transl Oncol. 14:592–598. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bonnetain F, Bosset JF, Gerard JP, Calais
G, Conroy T, Mineur L, Bouché O, Maingon P, Chapet O,
Radosevic-Jelic L, et al: What is the clinical benefit of
preoperative chemoradiotherapy with 5FU/leucovorin for T3-4 rectal
cancer in a pooled analysis of EORTC 22921 and FFCD 9203 trials:
Surrogacy in question? Eur J Cancer. 48:1781–1790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Foster NR, Qi Y, Shi Q, Krook JE, Kugler
JW, Jett JR, Molina JR, Schild SE, Adjei AA and Mandrekar SJ: Tumor
response and progression-free survival as potential surrogate
endpoints for overall survival in extensive stage small-cell lung
cancer: Findings on the basis of North Central Cancer Treatment
Group trials. Cancer. 117:1262–1271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oba K, Paoletti X, Alberts S, Bang YJ,
Benedetti J, Bleiberg H, Catalano P, Lordick F, Michiels S, Morita
S, et al: Disease-free survival as a surrogate for overall survival
in adjuvant trials of gastric cancer: A meta-analysis. J Natl
Cancer Inst. 105:1600–1607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Birgisson H, Påhlman L, Gunnarsson U and
Glimelius B: Swedish Rectal Cancer Trial Group: Adverse effects of
preoperative radiation therapy for rectal cancer: Long-term
follow-up of the Swedish Rectal Cancer Trial. J Clin Oncol.
23:8697–8705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sobin LH: TNM, sixth edition: New
developments in general concepts and rules. Semin Surg Oncol.
21:19–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mohiuddin M, Mohiuddin MM, Marks J and
Marks G: Future directions in neoadjuvant therapy of rectal cancer:
Maximizing pathological complete response rates. Cancer Treat Rev.
35:547–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gérard JP, Conroy T, Bonnetain F, Bouché
O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E,
Maurel J, et al: Preoperative radiotherapy with or without
concurrent fluorouracil and leucovorin in T3-4 rectal cancers:
Results of FFCD 9203. J Clin Oncol. 24:4620–4625. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sauer R, Liersch T, Merkel S, Fietkau R,
Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann
H, et al: Preoperative versus postoperative chemoradiotherapy for
locally advanced rectal cancer: Results of the German
CAO/ARO/AIO-94 randomized phase III trial after a median follow-up
of 11 years. J Clin Oncol. 30:1926–1933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Glynne-Jones R, Falk S, Maughan TS,
Meadows HM and Sebag-Montefiore D: A phase I/II study of irinotecan
when added to 5-fluorouracil and leucovorin and pelvic radiation in
locally advanced rectal cancer: A Colorectal Clinical Oncology
Group Study. Br J Cancer. 96:551–558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao YH, An X, Sun WJ, Cai J, Cai MY, Kong
LH, Lin JZ, Liu GC, Tang JH, Wu XJ, et al: Evaluation of
capecitabine and oxaliplatin administered prior to and then
concomitant to radiotherapy in high risk locally advanced rectal
cancer. J Surg Oncol. 109:478–482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hermann RM, Rave-Fränk M and Pradier O:
Combining radiation with oxaliplatin: A review of experimental
results. Cancer Radiother. 12:61–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martin LK and Bekaii-Saab T: Optimizing
neoadjuvant therapy for rectal cancer with oxaliplatin. J Natl
Compr Canc Netw. 11:298–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chitapanarux I, Chitapanarux T,
Tharavichitkul E, Mayurasakorn S, Siriwittayakorn P, Yamada S and
Lorvidhaya V: A phase II study of oxaliplatin with 5-FU/folinic
acid and concomitant radiotherapy as a preoperative treatment in
patients with locally advanced rectal cancer. Biomed Imaging Interv
J. 7:e252011.doi: 10.2349/biij.7.4.e25. PubMed/NCBI
|
|
24
|
Chao JY, Wang HM, Chiang FF, Lin JC, Chang
CF, Lin JF and Yeh HL: Preoperative chemoradiotherapy with
oxaliplatin and tegafur-uracil in locally advanced rectal cancer:
Pathologic complete response rate and preliminary results of
overall and disease-free survival in a single institute in Taiwan.
J Chin Med Assoc. 77:128–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pucciarelli S, Urso E, DeSalvo GL, Aschele
C, Friso ML, Rugge M, Toppan P, Bruttocao A, Fabris G, Ferraro B,
et al: 5-fluorouracil and weekly oxaliplatin combined with
radiotherapy for locally advanced rectal cancer: Surgical
complications and long-term results. Arch Med Res. 37:860–865.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu L, Cao C, Zhu Y, Li D, Feng H, Luo J,
Tang Z, Liu P, Lu K, Ju H and Zhang N: Preoperative
chemoradiotherapy with 5-fluorouracil and oxaliplatin for locally
advanced rectal cancer: Long-term results of a phase II trial. Med
Oncol. 32:702015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rödel C, Graeven U, Fietkau R, Hohenberger
W, Hothorn T, Arnold D, Hofheinz RD, Ghadimi M, Wolff HA,
Lang-Welzenbach M, et al: Oxaliplatin added to fluorouracil-based
preoperative chemoradiotherapy and postoperative chemotherapy of
locally advanced rectal cancer (the German CAO/ARO/AIO-04 study):
Final results of the multicentre, open-label, randomised, phase 3
trial. Lancet Oncol. 16:979–989. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aschele C, Cionini L, Lonardi S, Pinto C,
Cordio S, Rosati G, Artale S, Tagliagambe A, Ambrosini G, Rosetti
P, et al: Primary tumor response to preoperative chemoradiation
with or without oxaliplatin in locally advanced rectal cancer:
Pathologic results of the STAR-01 randomized phase III trial. J
Clin Oncol. 29:2773–2780. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gérard JP, Azria D, Gourgou-Bourgade S,
Martel-Lafay I, Hennequin C, Etienne PL, Vendrely V, François E, de
La Roche G, Bouché O, et al: Clinical outcome of the ACCORD 12/0405
PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol.
30:4558–4565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wong SJ, Moughan J, Meropol NJ, Anne PR,
Kachnic LA, Rashid A, Watson JC, Mitchell EP, Pollock J, Lee RJ, et
al: Efficacy endpoints of radiation therapy group protocol 0247: A
randomized, phase 2 study of neoadjuvant radiation therapy plus
concurrent capecitabine and irinotecan or capecitabine and
oxaliplatin for patients with locally advanced rectal cancer. Int J
Radiat Oncol Biol Phys. 91:116–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Allegra CJ, Yothers G, O'Connell MJ, Beart
RW, Wozniak TF, Pitot HC, Shields AF, Landry JC, Ryan DP, Arora A,
et al: Neoadjuvant 5-FU or capecitabine plus radiation with or
without oxaliplatin in rectal cancer patients: A phase III
randomized clinical trial. J Natl Cancer Inst. 107:djv2482015.doi:
10.1093/jnci/djv248. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gérard JP, Azria D, Gourgou-Bourgade S,
Martel-Laffay I, Hennequin C, Etienne PL, Vendrely V, François E,
de La Roche G, Bouché O, et al: Comparison of two neoadjuvant
chemoradiotherapy regimens for locally advanced rectal cancer:
Results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin
Oncol. 28:1638–1644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grothey A: A comparison of XELOX with
FOLFOX-4 as first-line treatment for metastatic colorectal cancer.
Nat Clin Pract Oncol. 6:10–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hochster HS, Hart LL, Ramanathan RK,
Childs BH, Hainsworth JD, Cohn AL, Wong L, Fehrenbacher L, Abubakr
Y, Saif MW, et al: Safety and efficacy of oxaliplatin and
fluoropyrimidine regimens with or without bevacizumab as first-line
treatment of metastatic colorectal cancer: Results of the TREE
study. J Clin Oncol. 26:3523–3529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hong YS, Nam BH, Kim KP, Kim JE, Park SJ,
Park YS, Park JO, Kim SY, Kim TY, Kim JH, et al: Oxaliplatin,
fluorouracil, and leucovorin versus fluorouracil and leucovorin as
adjuvant chemotherapy for locally advanced rectal cancer after
preoperative chemoradiotherapy (ADORE): An open-label, multicentre,
phase 2, randomised controlled trial. Lancet Oncol. 15:1245–1253.
2014. View Article : Google Scholar : PubMed/NCBI
|