Introduction
Metastasis is the most lethal aspect of cancer, due
to the challenges in treating the metastasis and spread of cancer
to key organs, including the lung, liver and brain. Consequently,
metastasis remains a major challenge for the clinical management
and prognosis of patients with cancer. Therefore, novel systemic
therapies for tumor metastasis are required. RNA interference can
be used as a novel therapeutic modality for metastasis through
specific silencing of therapeutically relevant genes in vivo
(1–3).
Synthetic small interfering RNAs (siRNAs), which are small,
double-stranded RNAs, are substrates for the RNA-induced silencing
complex. However, there are challenges associated with the in
vivo delivery of siRNA, such as enzymatic instability and low
cellular uptake (1).
Protein kinase N3 (PKN3) has been identified as a
downstream effector of the phosphoinositide-3-kinase signaling
pathway (4). Loss of PKN3 function in
vascular and lymphatic endothelial cells results in the inhibition
of tumor progression and lymph node metastasis (5), although the molecular mechanism by which
PKN3 contributes to malignant growth and tumorigenesis is not well
understood. Gene silencing of PKN3 expression by liposomal PKN3
siRNA (Atu027) inhibited tumor growth and metastasis formation by
modulating tumor-associated angiogenesis in various cancer mouse
models (5,6). Atu027 is currently being tested in a
Phase I clinical trial in cancer therapy (7). Therefore, PKN3 is considered a promising
therapeutic target for metastasis.
For in vitro and in vivo gene
delivery, cationic liposomes composed
1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate salt
(DOTAP)/cholesterol (Chol) or
DOTAP/1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) have
often been used (8–10). Systemic injection of a cationic
liposome/siRNA complex (lipoplex) can efficiently deliver siRNA to
the lungs (11). Electrostatic
interactions between positively charged lipoplexes and negatively
charged erythrocytes cause agglutination (12), and the agglutinates contribute to high
entrapment of lipoplexes in the highly extended lung capillaries
(13). Previously, it was reported
that intravenous injection of DOTAP/DOPE lipoplexes could deliver
siRNA into lung metastasis and suppress the expression of a target
gene (11). Furthermore, it was
demonstrated that intravenous sequential injection of chondroitin
sulfate plus DOTAP/Chol lipoplex into mice with liver metastasis
could deliver siRNA efficiently to the metastasis without
accumulation in the lungs, and suppress expression of a target gene
in tumor cells (14,15). Therefore, in the present study, the
therapeutic efficacy of treatment with PKN3 siRNA was evaluated
using the aforementioned transfection methods against liver and
lung metastases. Furthermore, it was examined whether a combination
of PKN3 siRNA and doxorubicin (DXR) could increase therapeutic
efficacy against metastases.
Materials and methods
Materials
DOTAP was obtained from Avanti Polar Lipids Inc.
(Alabaster, AL, USA). Chol and chondroitin sulfate C sodium salt
were purchased from Wako Pure Chemical Industries, Ltd. (Osaka,
Japan). DOPE was obtained from NOF Corporation (Tokyo, Japan). DXR
was purchased from Wako Pure Chemical Industries Inc.
siRNA
Mouse/human specific PKN3 siRNA and pGL2-luciferase
siRNA (Cont siRNA), as a negative control for PKN3 siRNA
(blunt-ended 23-mer, alternating 2′-O-methyl modified), was
synthesized by Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The
siRNA sequences of the PKN3 siRNA were as follows: Sense, 5′-AgA
cUu GaG gAc UuC cUg GaC aA-3′ and antisense, 5′-uUg UcC aGg AaG uCc
UcA aGu Cu-3′ (5). The siRNA
sequences of the Cont siRNA were as follows: Sense, 5′-AuC aCg UaC
gCg GaA uAc UuC gA-3′ and antisense, 5′-uCg AaG uAu UcC gCg UaC gUg
Au-3′ (5). The lower-case letters
represent 2′-O-methyl-modified nucleotides.
Cell culture
Human breast cancer MDA-MB-231/Luc cells stably
expressing firefly luciferase were obtained from Cell BioLabs, Inc.
(San Diego, CA, USA). Tamoxifen-resistant human breast cancer
MCF-7-Luc (TamR-Luc#1) cells stably expressing firefly
pGL3-luciferase were donated by Dr. Kazuhiro Ikeda (Division of
Gene Regulation and Signal Transduction, Research Center for
Genomic Medicine, Saitama Medical University, Saitama, Japan).
Human hepatocellular carcinoma HepG2 and human cervical carcinoma
HeLa cells were obtained from the European Collection of Cell
Cultures (Salisbury, UK). Human pancreatic carcinoma PANC-1 cells
were provided by Dr. Fumitaka Takeshita (Division of Molecular and
Cellular Medicine, National Cancer Center Research Institute,
Tokyo, Japan). Murine Lewis lung carcinoma LLC and murine
colon carcinoma Colon 26 cells were obtained from the Cell Resource
Center for Biomedical Research, Tohoku University (Sendai,
Japan).
LLC and Colon 26 cells were cultured in RPMI-1640
medium (Wako Pure Chemical Industries Inc.) with 10%
heat-inactivated fetal bovine serum (FBS, Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and 100 µg/ml kanamycin (Wako
Pure Chemical Industries Inc.) in a humidified atmosphere
containing 5% CO2 at 37°C. MCF-7-Luc cells were cultured
in Dulbecco's modified Eagle's medium (DMEM; Wako Pure Chemical
Industries Inc.) supplemented with 10% FBS, 100 µg/ml kanamycin and
0.5 mg/ml G418 at 37°C in a 5% CO2 humidified
atmosphere. MDA-MB-231/Luc and HepG2 cells were cultured in DMEM
supplemented with 10% FBS and 100 µg/ml kanamycin at 37°C in a 5%
CO2 humidified atmosphere. HeLa cells were cultured in
Eagle's Minimum Essential Medium (Wako Pure Chemical Industries
Inc.) supplemented with 10% FBS and 100 µg/ml kanamycin at 37°C in
a 5% CO2 humidified atmosphere.
PKN3 expression level in vitro
To investigate the PKN3 mRNA levels in tumor cells,
total RNA was isolated from MDA-MB-231, MCF-7, PANC-1, HeLa, HepG2,
LLC and Colon 26 cells using NucleoSpin RNA (Macherey-Nagel, Düren,
Germany). For reverse transcription-polymerase chain reaction
(RT-PCR), the 20-µl reaction volume contained 1 µl synthesized
cDNA, 10 pmol of each specific primer pair (Fasmac Co., Ltd.,
Kanagawa, Japan) and 0.25 U Ex Taq DNA polymerase (Takara Bio,
Inc., Otsu, Japan) with PCR buffer containing 1.5 mM
MgCl2 and 0.25 mM of each dNTP (Takara Bio, Inc., Otsu,
Japan). The profile of PCR amplification consisted of denaturation
at 95°C for 0.5 min, primer annealing at 55°C for 0.5 min, and
elongation at 72°C for 1 min for 30 cycles. Human PKN3 cDNA (115
bp) was amplified using the following primers: Forward,
5′-CACTTTGGGAAGGTCCTCCTG-3′ and reverse,
5′-CGCAGTACAGGCTCTCTATCT-3′. Mouse PKN3 cDNA (88 bp) was amplified
using the following primers: Forward, 5′-TCACCTTCTGCGAGCCTGTC-3′
and reverse, 5′-ATGAAATCCCGGCCTCTCCGC-3′. Human GAPDH cDNA (820 bp)
was amplified using the following primers: Forward,
5′-ATGACCCCTTCATTGACCTC-3′ and reverse, 5′-AAGTGGTCGTTGAGGGCAAT-3′.
Mouse β-actin cDNA (514 bp) was amplified using the following
primers: Forward, 5′-TGTGATGGTGGGAATGGGTCAG-3′ and reverse,
5′-TTTGATGTCACGCACGATTTCC-3′. The PCR products were analyzed by 18%
acrylamide gel electrophoresis in a Tris-borate-EDTA buffer (Wako
Pure Chemical Industries Inc.), and were visualized by ethidium
bromide staining.
For knockdown of PKN3 mRNA by transfection of cells
with PKN3 siRNA, MDA-MB-231, PANC-1, HeLa, LLC and Colon 26 cells
were plated into 6-well culture dishes at a density of
3×105 cells per well. The cells were transfected with 50
nM Cont siRNA or PKN3 siRNA using Lipofectamine RNAiMax reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was
isolated and quantitative RT-PCR was performed using a iCycler MyiQ
detection system (Bio-Rad Laboratories, Hercules, CA, USA) and a
SYBR Green I assay (iQ™ SYBER Green Supermix; Bio-Rad Laboratories)
48 h subsequent to transfection. Human and mouse PKN3 cDNAs were
amplified using the aforementioned primers. Human β-actin cDNA (186
bp) was amplified using the following primers: Forward,
5′-TGGCACCCAGCACAATGAA-3′ and reverse,
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′. Mouse β-actin cDNA (171 bp) was
amplified using the following primers: Forward,
5′-CATCCGTAAAGACCTCTATGCCAAC-3′ and reverse,
5′-ATGGAGCCACCGATCCACA-3′. Samples were run in triplicate and the
expression levels of human PKN3 and mouse PKN3 mRNA were normalized
to the quantity of human β-actin and mouse β-actin mRNA,
respectively, in the same sample, and analyzed using the
comparative Cq method (16).
Cell growth inhibition
MCF-7, MDA-MB-231, LLC and Colon 26 cells were
seeded in 96-well plates at a density of 2×104
cells/well 24 h prior to transfection. Cells at 50% confluency were
transfected with 50 nM Cont siRNA or PKN3 siRNA using Lipofectamine
RNAiMax reagent and then incubated for 48 h. In the PKN3 siRNA or
Cont siRNA with DXR combined treatment conditions, the cells were
incubated for 2 h subsequent to transfection with 50 nM PKN3 siRNA
or Cont siRNA using Lipofectamine RNAiMax reagent, and then treated
with various concentrations (0.016–0.5 µM) of DXR for an additional
48 h. The cell number was determined using Cell Counting Kit-8
(Dojindo Laboratories, Kumamoto, Japan). Cell viability was
expressed relative to the absorbance of untreated cells at 450
nm.
Preparation of liposome and
lipoplexes
Cationic liposomes were prepared from DOTAP/Chol or
DOTAP/DOPE at a molar ratio of 1:1 using a thin-film
hydration method, as reported previously (17). To prepare the cationic liposome/siRNA
complex (cationic lipoplex), cationic liposome suspension was mixed
with siRNA by vortexing for 10 sec at a charge ratio (+:-) of 4:1,
and left for 15 min at room temperature. The theoretical charge
ratio (+:-) of cationic liposome to siRNA was calculated as the
molar ratio of DOTAP nitrogen to siRNA phosphate.
The particle size distributions of liposomes and
lipoplexes were measured by the cumulant method using a
light-scattering photometer (ELS-Z2, Otsuka Electronics Co., Ltd.,
Osaka, Japan) at 25°C subsequent to diluting the dispersion to an
appropriate volume (~1.5 ml) with water. The zeta-potentials were
measured by electrophoresis light-scattering methods using ELS-Z2
at 25°C subsequent to diluting the dispersion to an appropriate
volume (~1.5 ml) with water. Cationic liposomes were ~100 nm in
size and had a zeta-potential of ~53 mV. The lipoplex size was ~340
nm and the zeta-potential was ~41 mV.
Liver and lung metastasis model
All animal experiments were performed with approval
from the Institutional Animal Care and Use Committee of Hoshi
University (Tokyo, Japan). To generate the mouse model of liver
metastasis, 1.0×106 MCF-7 or MDA-MB-231 cells suspended
in 50 µl PBS (pH 7.4) containing 50% reconstituted basement
membrane (Matrigel; BD Biosciences, Franklin Lakes, NJ, USA) were
inoculated into the spleen of female BALB/c nu/nu mice (18–20 g; 8
weeks of age; CLEA Japan, Inc., Tokyo, Japan). To generate mice
with lung metastases, 1.0×106 MCF-7 and LLC cells were
injected intravenously into the tail vein of female BALB/c nu/nu
mice and female C57BL/6 N mice (18–20 g; 8 weeks of age; Sankyo
Laboratory Service Corporation, Tokyo, Japan), respectively.
In vivo therapy for liver MDA-MB-231
metastasis
For efficient delivery of siRNA into liver
metastasis, DOTAP/Chol lipoplex with 50 µg siRNA was administered
intravenously into mice bearing liver metastases 1 min subsequent
to the intravenous injection of 1 mg chondroitin sulfate
(sequential injection method) (14).
On day 32 subsequent to inoculation of MDA-MB-231/Luc cells into
the spleen of mice, DXR was administered intravenously at 3 mg/kg
via the lateral tail vein, and then Cont siRNA or PKN3 siRNA was
administered to mice using a sequential injection method at 1 h
subsequent to injection of DXR. On days 34, 36 and 38 subsequent to
inoculation, Cont siRNA or PKN3 siRNA was administered to mice
using a sequential injection method. On day 40 subsequent to
inoculation, mice were sacrificed by cervical dislocation, and the
excised livers were then weighed.
To examine the expression level of PKN3 and vascular
endothelial growth factor (VEGF) mRNA in MDA-MB-231 tumors that
metastasized to the liver, total RNA was isolated from the excised
livers using TRI Reagent (Molecular Research Center, Inc.,
Cincinnati, OH, USA). RNA yield and purity were checked by
spectrometric measurements at 260 and 280 nm. cDNA was synthesized
from total RNA using a PrimeScript RT Reagent kit with gDNA Eraser
(Takara Bio Inc., Otsu, Japan). Quantitative PCR was performed
using Takara Thermal Cycler Dice (Takara Bio Inc.) and TaqMan Gene
expression assays (Pkn3, Mm00464275_m1; Vegfa, Mm00437306_m1;
Gapdh, Mm99999915_g1; Applied Biosystems®; Thermo Fisher
Scientific, Inc.). Samples were run in triplicate, and the
expression levels of PKN3 and VEGF mRNA were normalized to the
amount of GAPDH mRNA in the same sample, and analyzed using the
comparative Cq method (16).
Furthermore, to examine the antiangiogenic effect of
PKN3 siRNA on MDA-MB-231 tumors that metastasized to the liver,
excised livers were frozen on dry ice and sliced to 16 µm
thickness. The sections were incubated with rat anti-mouse CD31
(PECAM-1) monoclonal antibody (cat. no. 550274; dilution 1:50;
clone, MEC 13.3; BD Pharmingen, San Diego, CA, USA), for the
detection of mouse endothelial cells, and subsequently incubated
with goat anti-rat IgG conjugated to Alexa Fluor 488 (cat. no.
A11006; dilution, 1:1,000; Invitrogen; Thermo Fisher Scientific,
Inc.), as a secondary antibody. Immunofluorescence was examined
microscopically using an ECLIPSE TS100-F microscope at a
magnification of ×100 (Nikon, Tokyo, Japan).
In vivo therapy for liver MCF-7
metastases
On day 8 subsequent to inoculation of MCF-7-Luc
cells into the spleen of mice, 3 mg/kg DXR was administered
intravenously via the lateral tail vein and then 50 µg Cont siRNA
or PKN3 siRNA was administered to mice using sequential injections
at 1 h subsequent to the injection of DXR. On days 10, 12, and 14
following inoculation, 50 µg Cont siRNA or PKN3 siRNA was
administered into the mice using sequential injections. At day 16
subsequent to inoculation, mice were sacrificed by cervical
dislocation, and the excised livers were weighed. The luciferase
activities in the livers were measured as previously described
(14). Luciferase activity (%) was
calculated relative to the luciferase activity (cps/organ) of
untreated liver.
Distribution of DXR in MCF-7 tumors
that metastasized to the liver
On day 15 subsequent to the inoculation of MCF-7-Luc
cells into the spleen of mice, 3 mg/kg DXR was administered
intravenously via the lateral tail vein. At 1 h after injection of
DXR, 50 µg Cont siRNA or PKN3 siRNA was administered to the mice
using sequential injections with chondroitin sulfate and DOTAP/Chol
lipoplexes. Twenty-four h after injection of cationic lipoplex, the
mice were sacrificed by cervical dislocation. The tumors that
metastasized to the liver were excised, and then homogenized in 0.1
M NH4Cl/NH3 buffer (pH 9.0). DXR was
extracted with chloroform/methanol (2:1 v/v) and analyzed by
high-performance liquid chromatography, as previously described
(18).
In vivo therapy for lung MCF-7 and LLC
metastatic tumors
For efficient delivery of siRNA to lung metastases,
DOTAP/DOPE lipoplexes were used (11). On day 5 after inoculation of MCF-7-Luc
or LLC cells by intravenous injection into mice, DXR was
administered intravenously at 3 mg/kg via the lateral tail vein,
and then DOTAP/DOPE lipoplexes with 50 µg of Cont siRNA or PKN3
siRNA were administered intravenously to mice at 1 h after DXR
administration. On days 7, 9, and 11 after inoculation, Cont siRNA
or PKN3 siRNA was administered intravenously by injection of
DOTAP/DOPE lipoplexes. At day 13 after inoculation, the mice were
sacrificed by cervical dislocation, and then the excised lungs were
weighed.
Statistical analysis
The statistical significance of differences between
mean values was determined by Student's t-test using GraphPad Prism
(version 4.0; GraphPad Software Inc., La Jolla, CA, USA). P≤0.05
was considered to indicate a statistically significant
difference.
Results
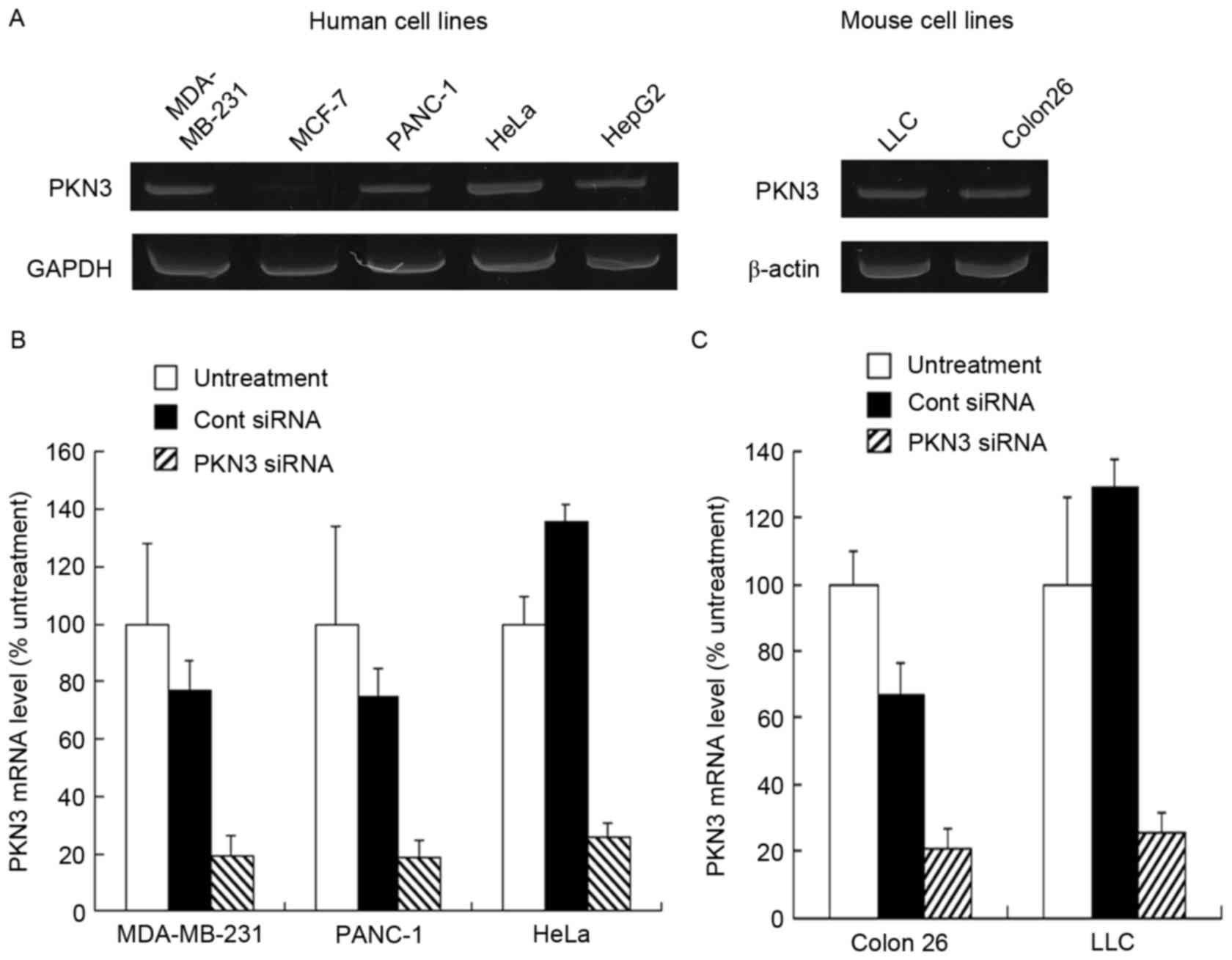
Expression level of PKN3 mRNA
To investigate PKN3 mRNA expression in culture
cells, the human tumor MDA-MB-231, MCF-7, PANC-1, HeLa and HepG2
cell lines, and mouse tumor Colon 26 and LLC cell lines were used.
Human PKN3 mRNA was expressed in MDA-MB-231, PANC-1, HeLa and HepG2
cells, but not in MCF-7 cells (Fig.
1A). By contrast, mouse PKN3 mRNA was expressed in LLC and
Colon 26 cells (Fig. 1A). Previously,
it has been reported that PKN3 is expressed in breast tumor cell
lines, including MDA-MB-231 and MCF-7 cells (19); however, certain studies have reported
that PKN3 is expressed in MDA-MB-231 cells, but not in MCF-7 cells
(20,21), which was consistent with the present
results. Therefore, in subsequent experiments, the MDA-MB-231,
PANC-1, HeLa, Colon 26 and LLC cells were used as PKN3-positive
cell lines and MCF-7 cells as the PKN3-negative cell line.
Subsequently, whether transfecting PKN3 siRNA into
the cells decreased the expression level of PKN3 mRNA was
investigated. Lipofectamine RNAiMax was used as an in vitro
transfection reagent for siRNA. When transfected into MDA-MB-231,
PANC-1, HeLa, Colon 26 and LLC cells, PKN3 siRNA significantly
inhibited the expression of human and mouse PKN3 mRNA compared with
Cont siRNA (P<0.01) (Fig. 1B and
C). By contrast, Cont siRNA did not markedly affect the
expression of PKN3 mRNA in the cells.
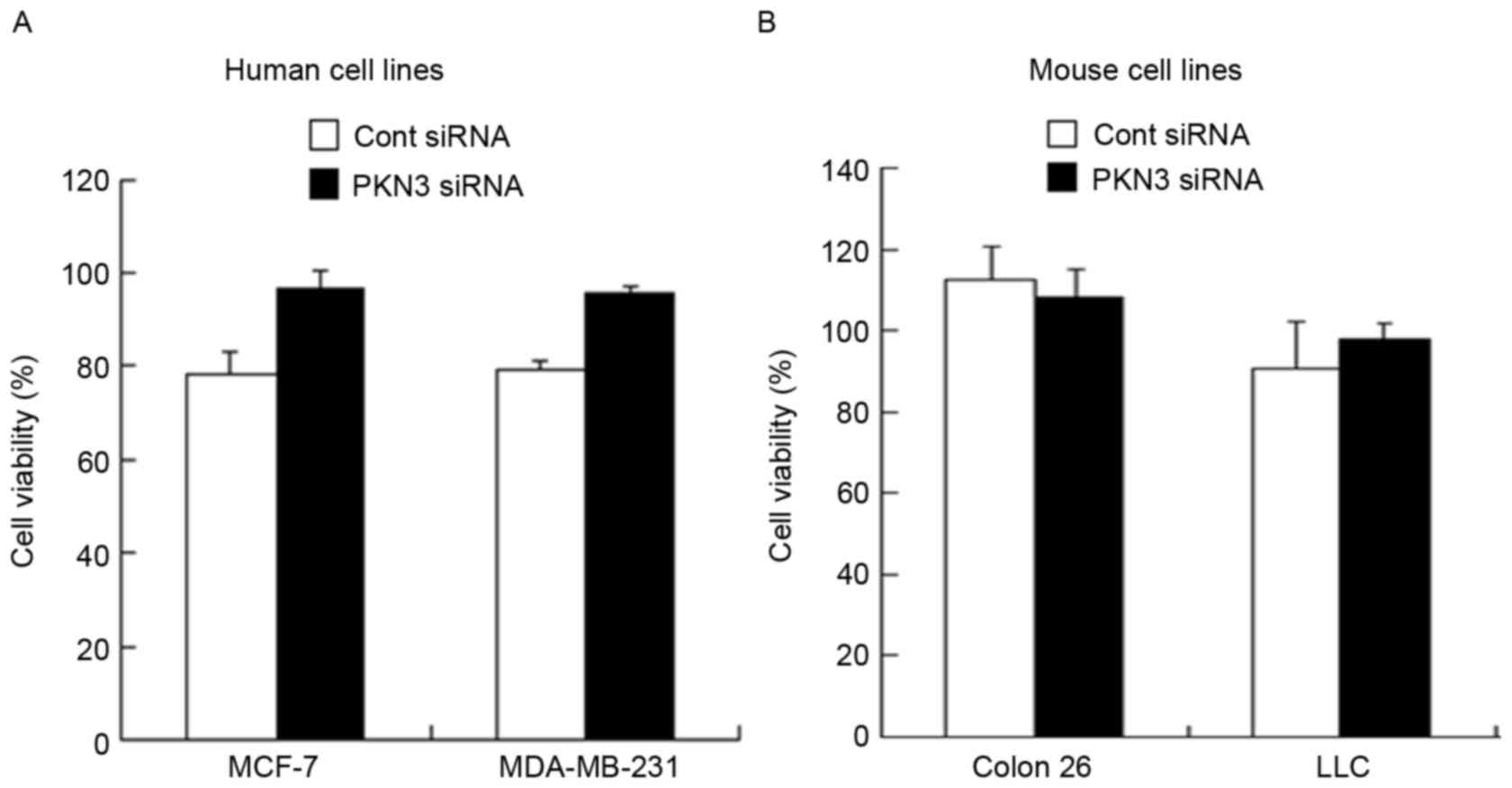
Antiproliferative activity
To examine whether transfection with PKN3 siRNA
could induce suppression of cell growth, cell viability was
measured 48 h subsequent to transfection of MDA-MB-231, MCF-7,
Colon 26 and LLC cells with PKN3 siRNA. However, transfection with
PKN3 siRNA did not inhibit tumor growth in all the cell lines
(Fig. 2A and B), indicating that
suppression of PKN3 expression did not affect tumor growth in
vitro.
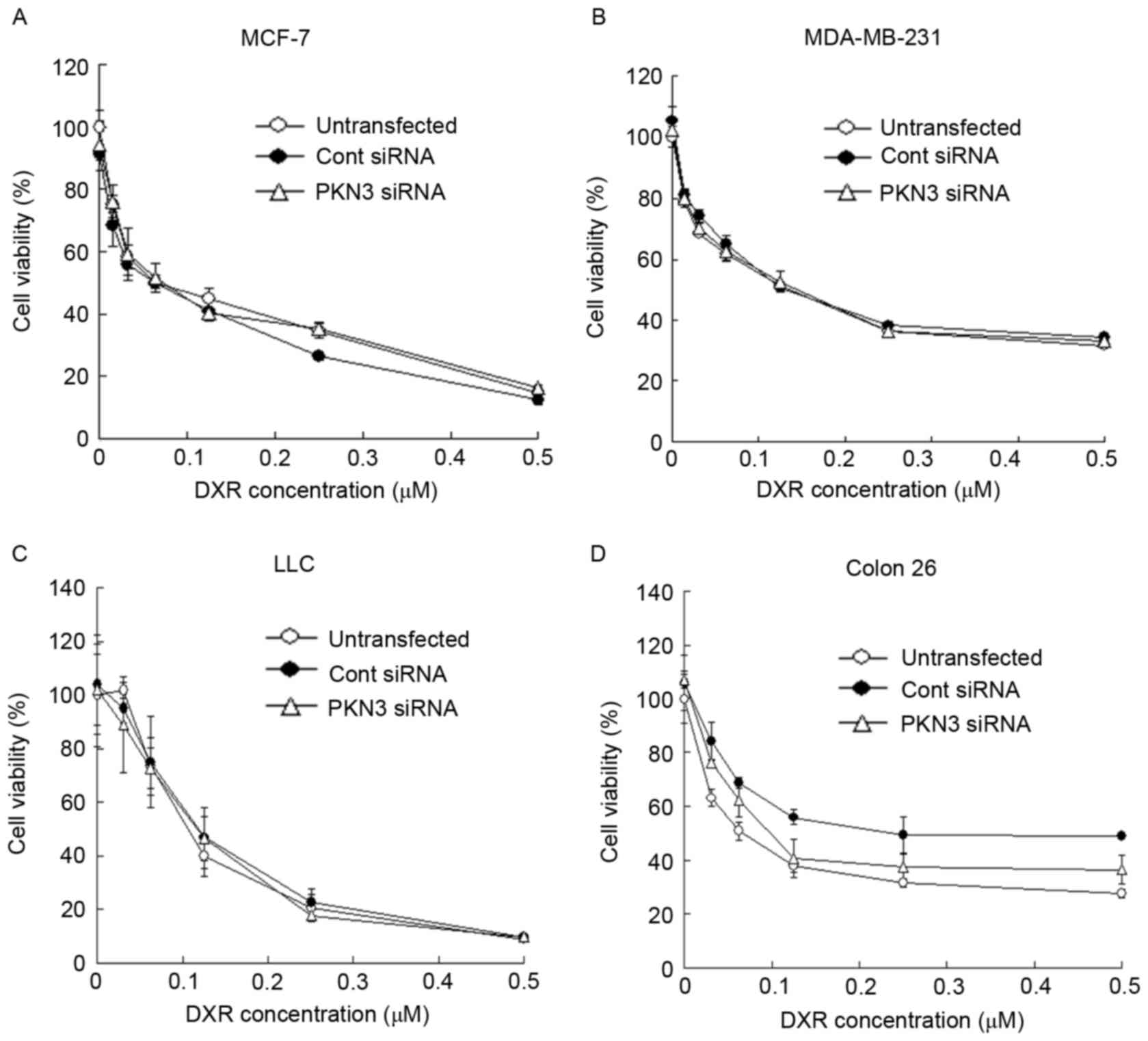
Subsequently, whether transfection with PKN3 siRNA
could increase the growth inhibitory effect of DXR treatment in
tumor cells was evaluated. MCF-7, MDA-MB-231, Colon 26 and LLC
cells were transfected with PKN3 siRNA, and 2 h later the cells
were treated with various concentrations of DXR for 48 h. However,
the transfection of PKN3 siRNA did not affect the cytotoxicity of
DXR in any cell lines (Fig. 3A-D).
These data indicated that a reduction of PKN3 expression did not
increase the sensitivity to DXR in tumor cells.
Therapeutic efficacy against liver
MDA-MB-231-metastasized tumors
Subsequently, the in vivo efficacy of PKN3
siRNA and DXR combination therapy in inhibiting the growth of
MDA-MB-231 tumors that metastasized to the liver was investigated.
Injection of PKN3 siRNA was performed a total of four times, with 2
days between each injection. DXR was administered at a dose of 3
mg/kg at 1 h prior to the first injection of siRNA lipoplex. The
anti-metastatic effect was evaluated by measurement of liver weight
(mg) (Fig. 4A) or liver weight
relative to body weight (%) (Fig.
4B). A normal mouse liver (8 weeks of age) is ~1,300 mg in
weight and accounts for 6.5% of body weight (data not shown). The
four injections of PKN3 siRNA significantly suppressed the increase
in weight of livers with MDA-MB-231 metastasis (1,320±62 mg)
compared with no treatment (1,712±188 mg; P<0.01) or Cont siRNA
(1,643±173 mg; P<0.05), but PKN3 siRNA plus DXR did not reduce
liver weight (1,370±59 mg) compared with PKN3 siRNA alone (Fig. 4A and B).
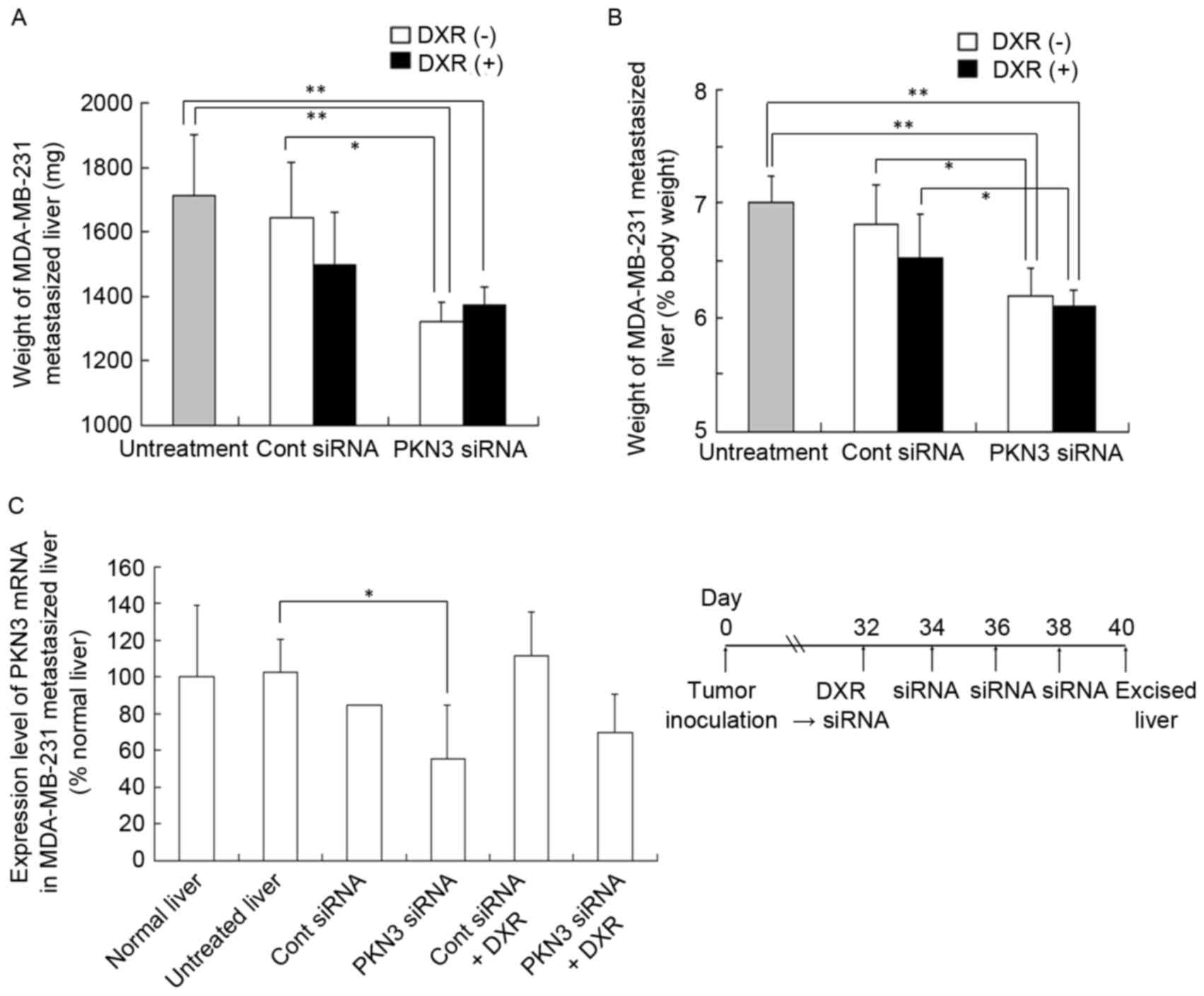 | Figure 4.In vivo combination therapy of
PKN3 siRNA and DXR for mice with liver MDA-MB-231 metastasis. In (A
and B), DXR, followed by PKN3 siRNA was administered to mice on day
32 after inoculation, and PKN3 siRNA was administered on days 34,
36, and 38. The mice were sacrificed at day 40, and then the
excised livers were weighed. Each result represents the mean ±
standard deviation (n=4-5). *P<0.05; **P<0.01. In (C), mouse
PKN3 mRNA level in tumor-metastasized liver shown in (A and B).
Each result represents the mean (n=2 for Cont siRNA) or the mean ±
standard deviation (n=4). *P<0.05. PKN3, protein kinase N3;
Cont, control; siRNA, small interfering RNA; DXR, doxorubicin. |
To examine whether this antitumor effect was
dependent on the suppression of PKN3 expression, human and mouse
PKN3 mRNA levels in livers with MDA-MB-231 metastasis were measured
by quantitative RT-PCR analysis; however, human PKN3 and GAPDH mRNA
levels in certain tumors were below the detection limit by
quantitative PCR analysis (data not shown). For mouse PKN3 mRNA
levels, repeated injections of PKN3 siRNA significantly decreased
PKN3 mRNA levels compared with those in untreated livers
(P<0.05; Fig. 4C), indicating that
PKN3 siRNA could suppress expression of PKN3 mRNA in
vivo.
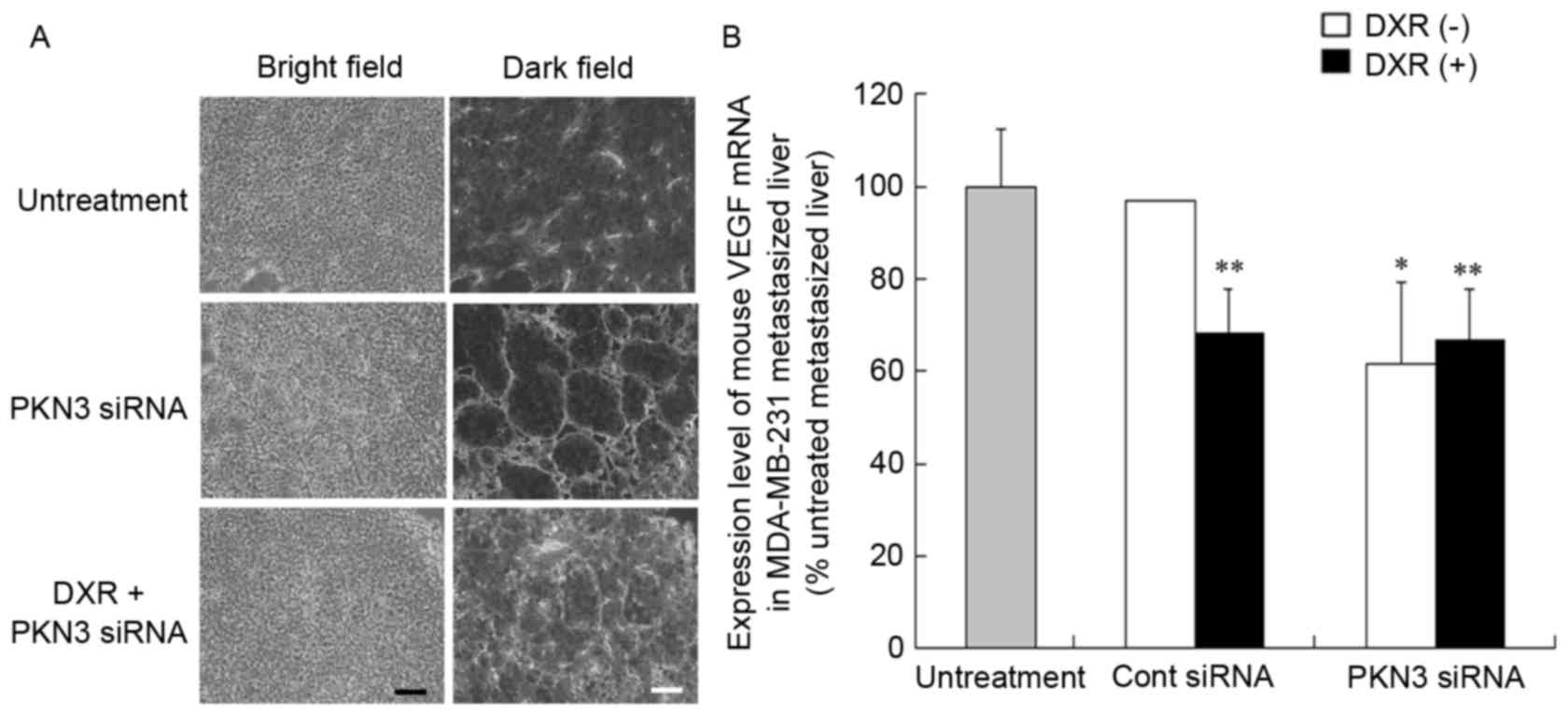
Vascular structure of tumors after
injection of PKN3 siRNA
To investigate the mechanism of the antitumor effect
of PKN3 siRNA on liver MDA-MB-231 metastases, the vascular
structure following repeated injections of PKN3 siRNA was examined.
Treatment of metastasis with PKN3 siRNA and PKN3 siRNA combined
with DXR induced apparent changes in vascular structure (Fig. 5A). Treatment with PKN3 siRNA reduced
narrow vessels in tumors and increased open vessels. This
histological change in tumor vasculature subsequent to treatment
with PKN3 siRNA appeared to be similar to ‘normalization’ of tumor
vasculature (22).
Vascular endothelial growth factor (VEGF) protein is
a prominent cytokine that promotes endothelial cell proliferation
during angiogenesis (23). Therefore,
the present study investigated whether treatment with PKN3 siRNA
affected the expression level of VEGF mRNA in livers with
MDA-MB-231 metastases by quantitative RT-PCR analysis. Treatment
with PKN3 siRNA significantly decreased mouse VEGF mRNA levels in
the liver compared with that in untreated livers (P<0.05;
Fig. 5B), indicating that the change
in vascular structure may be caused by a decrease in VEGF
expression.
Therapeutic efficacy against liver
MCF-7-metastasized tumors
To examine whether PKN3 siRNA could exhibit
antitumor effects, regardless of the expression of PKN3 mRNA in
tumor cells, the efficacy of PKN3 siRNA combined with DXR against
liver MCF-7-Luc metastasis was investigated. Since this liver
metastasis model rapidly led to moribund recipient mice,
combination therapy was commenced at 8 days subsequent to tumor
cell challenge. Repeated injections of PKN3 siRNA or a single
injection of DXR could not suppress the increase in weight of
livers with MCF-7-Luc metastasis (2,230±303 and 1,983±356 mg,
respectively), compared with no treatment (2,160±174 mg) or Cont
siRNA (2,163±289 mg); however, combination treatment significantly
inhibited the increase in liver weight (1,593±131 mg; P<0.01)
(Fig. 6A and B).
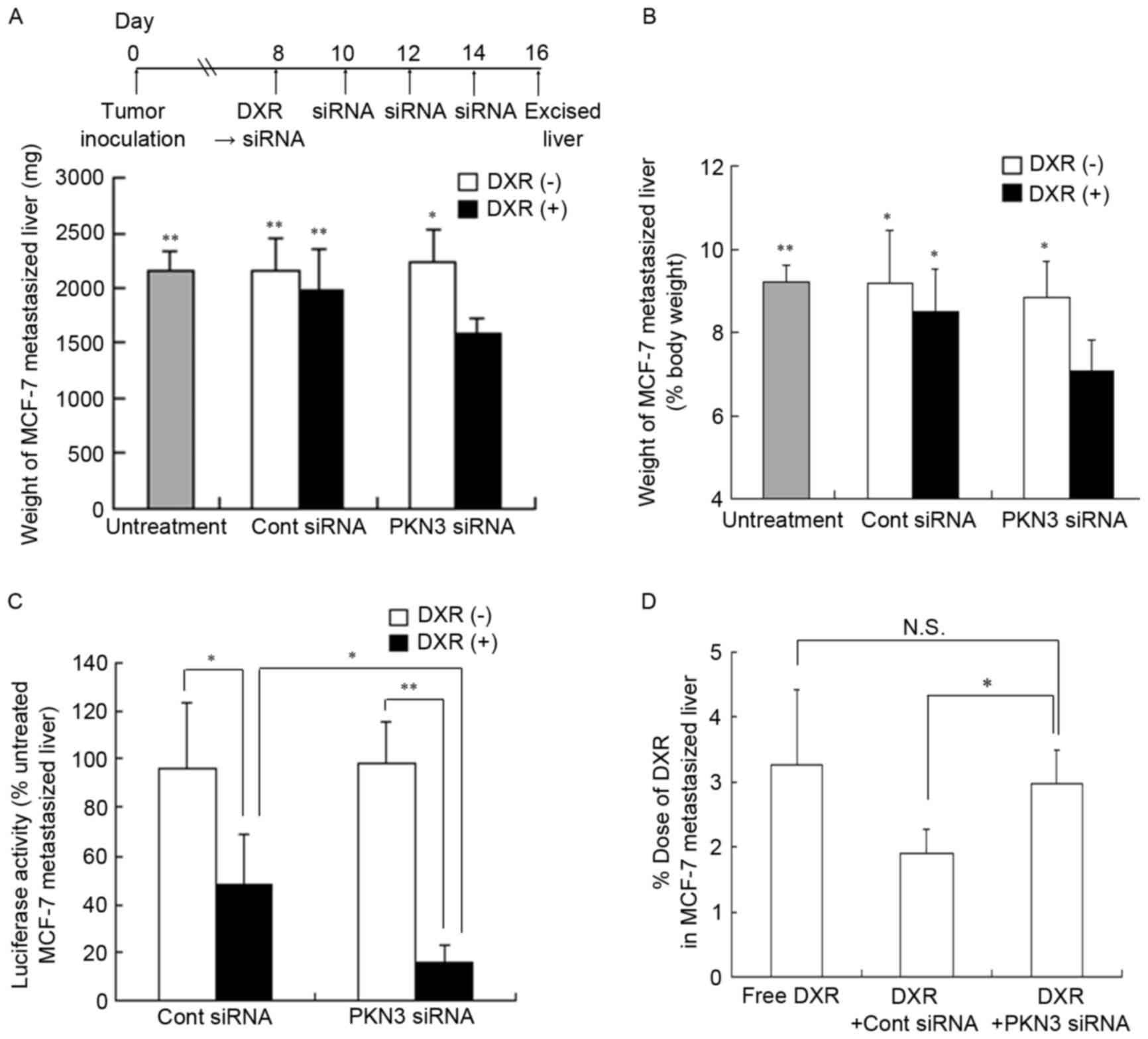 | Figure 6.In vivo combination therapy
with PKN3 siRNA and DXR in mice with liver MCF-7 metastasis. In
(A-C), DXR, followed by PKN3 siRNA was administered to mice on day
8 after inoculation, and PKN3 siRNA was administered on days 10,
12, and 14. The mice were sacrificed at day 16 after inoculation,
and then the excised livers were weighed. In (C), the luciferase
activities in livers shown in (A and B) were measured. Luciferase
activity (%) was calculated relative to the luciferase activity
(cps/organ) of untreated liver. In (A-C), each result represents
the mean ± standard deviation (n=4-6). In (A and B), *P<0.05,
**P<0.01, compared with PKN3 siRNA and DXR. In (D), on day 15
after the inoculation, DXR, followed by PKN3 siRNA was administered
to mice. DXR concentrations in livers were measured at 24 h after
injection of siRNA by high-performance liquid chromatography
(HPLC). Each column shows the mean ± standard deviation (n=3). In
(C and D), *P<0.05, **P<0.01. N.S., not significant; PKN3,
protein kinase N3; Cont, control; siRNA, small interfering RNA;
DXR, doxorubicin. |
Luciferase activity in livers with MCF-7-Luc
metastasis indicated the number of living tumor cells. Therefore,
the present study measured luciferase activity in the livers
subsequent to combination therapy. Treatment with PKN3 siRNA did
not decrease luciferase activity in the metastatic tumors, but a
combination of Cont siRNA plus DXR significantly reduced luciferase
activity compared with Cont siRNA (P<0.05) (Fig. 6C). However, the liver weight was not
significantly different between the groups (Fig. 6A), indicating that a single injection
of DXR may be effective for the treatment of liver MCF-7
metastasis. Furthermore, the luciferase activity following
combination therapy with PKN3 siRNA and DXR was significantly
decreased compared with the activity subsequent to injections of
PKN3 siRNA (P<0.01) and subsequent to combination therapy with
Cont siRNA and DXR (P<0.05) (Fig.
6C), indicating that PKN3 siRNA could increase the therapeutic
efficacy of DXR for liver MCF-7-Luc metastasis. These findings
suggested that PKN3 siRNA may increase antitumor effects by
suppression of PKN3 in mouse stroma cells, such as endothelial
cells, but not tumor cells, as MCF-7 cells do not express PKN3
(Fig. 1A).
Additionally, to investigate the mechanism of how
PKN3 siRNA enhanced antitumor activity by DXR in vivo, the
concentration of DXR in liver MCF-7 metastasis was measured at 24 h
subsequent to a single injection of PKN3 siRNA that was
administered following DXR administration. However, PKN3 siRNA did
not significantly increase the accumulation of DXR in the liver
compared with the administration of free DXR solution (Fig. 6D), indicating that treatment with PKN3
siRNA did not affect the accumulation of DXR in liver MCF-7
metastasis.
Therapeutic efficacy against lung
MCF-7 and LLC metastasis
To investigate whether treatment with PKN3 siRNA
could inhibit lung metastasis, combination therapy with PKN3 siRNA
and DXR for lung MCF-7 and LLC metastases was examined. Since the
two lung metastasis models rapidly led to moribund recipient mice,
combination therapy was started on day 5 after tumor cell
challenge. Normal mouse lungs are ~150 mg in weight and represent
0.75% of body weight (data not shown). In lung MCF-7 metastasis,
repeated injections of PKN3 siRNA significantly suppressed the
increase in weight of lungs with metastasis (305±45 mg), compared
with no treatment (446±16 mg; P<0.01) or Cont siRNA (384±30 mg;
P<0.05) (Fig. 7A and B). However,
the combination of PKN3 siRNA and DXR did not induce a decrease in
lung weight (312±93 mg) compared with PKN3 siRNA alone. In lung LLC
metastasis, repeated injections of PKN3 siRNA or a single injection
of DXR significantly suppressed the increase in the weight of lungs
with LLC metastasis (323±79 mg and 396±77 mg, respectively),
compared with Cont siRNA (630±26 mg; P<0.01) (Fig. 7C and D). However, the combination with
PKN3 siRNA and DXR could not reduce lung weight (378±88 mg)
compared with PKN3 siRNA. These findings suggested that PKN3 siRNA
could suppress tumor growth in lung metastases, but could not
increase the antitumor effect of DXR.
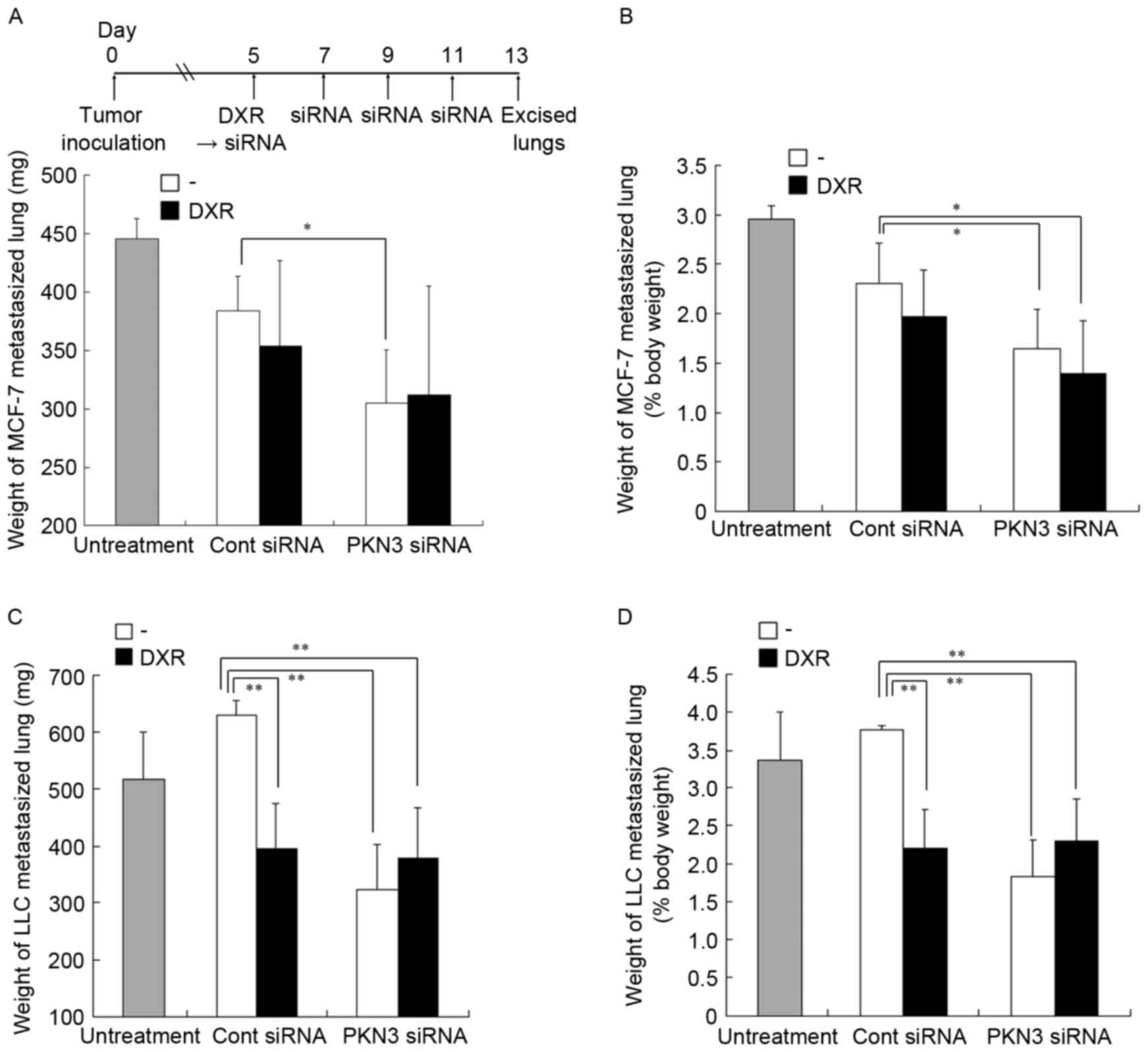 | Figure 7.In vivo combination therapy of
PKN3 siRNA and DXR for lung MCF-7-(A and B) and LLC-metastasized
mice (C and D). (A-D) DXR, followed by PKN3 siRNA was administered
to mice on day 5 subsequent to inoculation, and PKN3 siRNA was
administered on days 7, 9 and 11. The mice were sacrificed at day
13 after inoculation, and then the excised livers were weighed. In
(A and B), each result represents the mean + standard deviation
(n=4-5). In (C and D), each result represents the mean ± standard
deviation (n=3-5). *P<0.05, **P<0.01. PKN3, protein kinase
N3; siRNA, small interfering RNA; DXR, doxorubicin. |
Discussion
The present study investigated whether PKN3 siRNA
and DXR combination therapy could increase the inhibition of tumor
growth induced by DXR or PKN3 siRNA alone in vitro and in
vivo. It has been reported that the expression of PKN3 mRNA was
relatively restricted to specific tissues, including the skeletal
muscle, heart and liver in normal tissues (20), primary vascular endothelial HUVEC
cells and endothelial lymphatic HMVEC-LLy cells (5). Aleku et al (5) reported that transfection of PKN3 siRNA
into cultured primary endothelial cells revealed an essential role
of PKN3 in endothelial tube formation and migration. Furthermore,
liposomal PKN3 siRNA (Atu027) showed an inhibitory effect on
invasive tumor growth and regional lymph node metastasis in mice
bearing orthotropic pancreatic and prostate cancer (6). In the present study, treatment of liver
or lung metastases with PKN3 siRNA could suppress tumor growth
in vivo (Figs. 4 and 7), although it did not exhibit inhibition of
cell growth in vitro (Fig. 2).
Furthermore, PKN3 siRNA reduced tumor growth of lung PKN3-negative
metastasis, as well as PKN3-positive metastasis (Fig. 7). These findings suggested that the
antitumor effect of PKN3 siRNA on the metastases may be due to
inhibition of endothelial function via knockdown of PKN3 expression
in endothelial cells.
Adhesive interactions between tumor cells and
endothelial cells are initial key events in the extravasation of
tumor cells from the blood stream into underlying tissues. Firm
adhesion of tumor cells to endothelial cells is mediated by cell
adhesion molecules, such as intercellular adhesion molecule-1
(ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), leading to
tumor invasion (24,25). ICAM-1 is a central adhesion molecule
that is important for binding and signaling between vascular
endothelial cells and tumor cells during tumor metastasis (26). Recently, it has been reported that
PKN3-knockdown mice are viable and develop normally, but the mice
exhibited impaired lung metastasis of tumor cells subsequent to
intravenous injection (27). It was
also found that PKN3 knockdown induced a glycosylation defect in
cell-surface glycoproteins, including ICAM-1, integrin β1, and
integrin α5 in HUVEC cells. Furthermore, Atu027-treated HUVEC cells
showed elevated expression of VE-cadherin, a major protein involved
in adherence junction integrity, indicating that knockdown of PKN3
siRNA induced an elevation of VE-cadherin in vascular endothelial
cells and prevented tumor metastasis through enhancement of the
endothelial barrier (6). These
findings suggested that knockdown of PKN3 in stroma cells, such as
endothelial cells, may inhibit tumor metastasis via an increase in
the endothelial barrier or a decrease in binding between vascular
endothelial cells and tumor cells. Following the present study, to
elucidate the mechanism of the antitumor effect of PKN3 siRNA,
additional studies should be performed to investigate the
expression level of VE-cadherin and changes in glycosylation in
ICAM-1 in the metastasis subsequent to treatment with PKN3
siRNA.
Repeated injections of PKN3 siRNA were effective
against liver MDA-MB-231 metastasis and lung MCF-7 and LLC
metastases, but not liver MCF-7 metastasis (Figs. 4, 6 and
7). The therapeutic efficacy against
lung LLC metastasis in the present study (2.5 mg PKN3 siRNA/kg
mouse, a total of four times) was extremely similar to a previous
finding (6) that LLC metastasis in
the lungs was significantly inhibited in mice repeatedly treated
with Atu027 at 2.8 mg PKN3 siRNA/kg mouse (a total of nine times),
indicating that DOTAP/DOPE liposomes could deliver PKN3 siRNA into
lung metastasis, as well as Atu027. It was previously reported that
repeated injections of PKN3 siRNA (a total of five times) could
inhibit liver MCF-7 metastasis (14).
However, in the present study, no inhibitory effect was observed
after repeated injections of PKN3 siRNA (a total of four times) in
liver MCF-7 metastasis (Fig. 6A and
B). It was hypothesized that a difference in administration
schedule between the previous study and the present study may have
affected the therapeutic efficacy. However, it was not clear why
repeated injections of PKN3 siRNA suppressed lung MCF-7 metastasis,
but not liver MCF-7 metastasis using the same administration
schedule. In the present study, PKN3 siRNA was transfected into
liver metastasis using sequential injections of chondroitin sulfate
and DOTAP/Chol lipoplex, and into lung metastasis by injections of
DOTAP/DOPE lipoplex. The difference in transduction efficiencies
between the injection methods into MCF-7 metastases may affect the
therapeutic efficacy of PKN3 siRNA.
It has been reported that knockdown of PKN3 in
vascular endothelial cells induced an enhancement of the
endothelial barrier via an elevation of VE-cadherin (6), indicating that injection of PKN3 siRNA
may be able to decrease the efflux of DXR accumulated in tumor
metastasis into the blood flow; therefore, for in vivo
combination therapy, DXR was injected prior to injections of PKN3
siRNA. However, in vivo treatment with PKN3 siRNA could not
increase therapeutic efficacy by DXR for liver MDA-MB-231
metastasis (Fig. 4A and B) and lung
MCF-7 and LLC metastases (Fig. 7),
with the exception of liver MCF-7 metastasis (Fig. 6A and B), indicating that combination
therapy with PKN3 siRNA and DXR could not induce synergistic or
additive antitumor effects for their metastases. Additional studies
should be performed to elucidate why treatment with PKN3 siRNA
using sequential injections of chondroitin sulfate and DOTAP/Chol
lipoplexes increased the therapeutic efficacy of DXR only for liver
MCF-7 metastasis.
In conclusion, the present study demonstrated that
PKN3 siRNA reduced tumor growth of liver and lung metastases,
regardless of PKN3 expression in tumor cells, but the combination
with DXR did not increase therapeutic efficacy. Although PKN3 siRNA
may induce antitumor effects in metastases by suppression of PKN3
mRNA in stroma cells, such as endothelial cells, rather than tumor
cells, PKN3 may be a promising therapeutic target for the treatment
of lung and liver metastases.
Acknowledgements
The authors thank Ms. Chiho Amiya and Ms. Natsumi
Yamamoto (Department of Drug Delivery Research, Hoshi University,
Tokyo, Japan) for assistance with experimental work. This study was
supported in part by a Grant-in-Aid for Scientific Research (C)
from the Japan Society for the Promotion of Science (grant no.
26460046).
References
|
1
|
Aagaard L and Rossi JJ: RNAi therapeutics:
Principles, prospects and challenges. Adv Drug Deliv Rev. 59:75–86.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aigner A: Applications of RNA
interference: Current state and prospects for siRNA-based
strategies in vivo. Appl Microbiol Biotechnol. 76:9–21. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uprichard SL: The therapeutic potential of
RNA interference. FEBS Lett. 579:5996–6007. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leenders F, Möpert K, Schmiedeknecht A,
Santel A, Czauderna F, Aleku M, Penschuck S, Dames S, Sternberger
M, Röhl T, et al: PKN3 is required for malignant prostate cell
growth downstream of activated PI 3-kinase. EMBO J. 23:3303–3313.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aleku M, Schulz P, Keil O, Santel A,
Schaeper U, Dieckhoff B, Janke O, Endruschat J, Durieux B, Röder N,
et al: Atu027, a liposomal small interfering RNA formulation
targeting protein kinase N3, inhibits cancer progression. Cancer
Res. 68:9788–9798. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santel A, Aleku M, Röder N, Möpert K,
Durieux B, Janke O, Keil O, Endruschat J, Dames S, Lange C, et al:
Atu027 prevents pulmonary metastasis in experimental and
spontaneous mouse metastasis models. Clin Cancer Res. 16:5469–5480.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Strumberg D, Schultheis B, Traugott U,
Vank C, Santel A, Keil O, Giese K, Kaufmann J and Drevs J: Phase I
clinical development of Atu027, a siRNA formulation targeting PKN3
in patients with advanced solid tumors. Int J Clin Pharmacol Ther.
50:76–78. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taetz S, Bochot A, Surace C, Arpicco S,
Renoir JM, Schaefer UF, Marsaud V, Kerdine-Roemer S, Lehr CM and
Fattal E: Hyaluronic acid-modified DOTAP/DOPE liposomes for the
targeted delivery of anti-telomerase siRNA to CD44-expressing lung
cancer cells. Oligonucleotides. 19:103–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Wolf HK, Snel CJ, Verbaan FJ,
Schiffelers RM, Hennink WE and Storm G: Effect of cationic carriers
on the pharmacokinetics and tumor localization of nucleic acids
after intravenous administration. Int J Pharm. 331:167–175. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cardoso AL, Simões S, de Almeida LP,
Plesnila N, de Lima Pedroso MC, Wagner E and Culmsee C:
Tf-lipoplexes for neuronal siRNA delivery: A promising system to
mediate gene silencing in the CNS. J Control Release. 132:113–123.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hattori Y, Nakamura A, Arai S, Kawano K,
Maitani Y and Yonemochi E: siRNA delivery to lung-metastasized
tumor by systemic injection with cationic liposomes. J Liposome
Res. 25:279–286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eliyahu H, Servel N, Domb AJ and Barenholz
Y: Lipoplex-induced hemagglutination: Potential involvement in
intravenous gene delivery. Gene Ther. 9:850–858. 2002.PubMed/NCBI
|
|
13
|
Simberg D, Weisman S, Talmon Y, Faerman A,
Shoshani T and Barenholz Y: The role of organ vascularization and
lipoplex-serum initial contact in intravenous murine lipofection. J
Biol Chem. 278:39858–39865. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hattori Y, Arai S, Kikuchi T, Ozaki K,
Kawano K and Yonemochi E: Therapeutic effect for liver-metastasized
tumor by sequential intravenous injection of anionic polymer and
cationic lipoplex of siRNA. J Drug Target. 24:309–317. 2016.
View Article : Google Scholar
|
|
15
|
Hattori Y, Arai S, Okamoto R, Hamada M,
Kawano K and Yonemochi E: Sequential intravenous injection of
anionic polymer and cationic lipoplex of siRNA could effectively
deliver siRNA to the liver. Int J Pharm. 476:289–298. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kato M, Hattori Y, Kubo M and Maitani Y:
Collagenase-1 injection improved tumor distribution and gene
expression of cationic lipoplex. Int J Pharm. 423:428–434. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taniguchi Y, Kawano K, Minowa T, Sugino T,
Shimojo Y and Maitani Y: Enhanced antitumor efficacy of
folate-linked liposomal doxorubicin with TGF-β type I receptor
inhibitor. Cancer Sci. 101:2207–2213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Unsal-Kacmaz K, Ragunathan S, Rosfjord E,
Dann S, Upeslacis E, Grillo M, Hernandez R, Mack F and Klippel A:
The interaction of PKN3 with RhoC promotes malignant growth. Mol
Oncol. 6:284–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Palmer RH, Ridden J and Parker PJ: Cloning
and expression patterns of two members of a novel
protein-kinase-C-related kinase family. Eur J Biochem. 227:344–351.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lachmann S, Jevons A, De Rycker M,
Casamassima A, Radtke S, Collazos A and Parker PJ: Regulatory
domain selectivity in the cell-type specific PKN-dependence of cell
migration. PLoS One. 6:e217322011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goel S, Duda DG, Xu L, Munn LL, Boucher Y,
Fukumura D and Jain RK: Normalization of the vasculature for
treatment of cancer and other diseases. Physiol Rev. 91:1071–1121.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weis SM and Cheresh DA: Pathophysiological
consequences of VEGF-induced vascular permeability. Nature.
437:497–504. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pinon P and Wehrle-Haller B: Integrins:
Versatile receptors controlling melanocyte adhesion, migration and
proliferation. Pigment Cell Melanoma Res. 24:282–294. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Laurent VM, Duperray A, Rajan Sundar V and
Verdier C: Atomic force microscopy reveals a role for endothelial
cell ICAM-1 expression in bladder cancer cell adherence. PLoS One.
9:e980342014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jimenez D, Roda-Navarro P, Springer TA and
Casasnovas JM: Contribution of N-linked glycans to the conformation
and function of intercellular adhesion molecules (ICAMs). J Biol
Chem. 280:5854–5861. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mukai H, Muramatsu A, Mashud R, Kubouchi
K, Tsujimoto S, Hongu T, Kanaho Y, Tsubaki M, Nishida S, Shioi G,
et al: PKN3 is the major regulator of angiogenesis and tumor
metastasis in mice. Sci Rep. 6:189792016. View Article : Google Scholar : PubMed/NCBI
|