Introduction
Ovarian cancer (OC) is the sixth most common
malignant gynecological tumor and accounts for 190,000 novel cases
every year in the USA (1). Owing to
the lack of explicit early symptoms and effective biomarkers for OC
monitoring and diagnosis, the overall 5-year survival rate for
patients with OC is <30% (2,3).
Therefore, elucidation of the molecular mechanisms underlying the
progression and metastasis of OC is critical to improve therapeutic
effects for patients with OC.
MicroRNAs (miRNAs/miRs) are a class of
single-stranded small non-coding RNAs 22 nucleotides in length,
which are capable of regulating the expression levels of a number
of genes at the transcriptional and post-transcriptional level
(4,5).
Multiple miRNAs function as potential tumor suppressors or
oncogenes due to their fundamental roles in diverse cellular
processes of various types of human cancer, including
proliferation, migration, invasion and apoptosis (6–11).
Currently, numerous miRNAs including miR-126 (12), miR-137 (13), miR-145 (14), miR-148a (15) and miR-498 (16) have been demonstrated as tumor
suppressor genes in OC. Conversely, miR-196a (17), miR-200a (18), miR-205 (19), miR-543 (20) and miR-661 (21) have been revealed to function as
oncogenes in OC. All these results highlight the involvement of
miRNAs in the development and progression of OC.
miR-18b is located on the X chromosome in the human
genome (22). Previous studies have
demonstrated that the aberrant expression level of miR-18b may be
present in diverse human pathologies, including multiple sclerosis,
cardiac hypertrophy and chronic hepatitis B virus infection
(22,23). miR-18b has attracted much attention as
it is frequently upregulated and acts as a oncogene in human
gastric cancer (24), colonic cancer
(25), breast cancer (22) and hepatocellular carcinoma (26). However, whether miR-18b is involved in
the development and progression of OC remains unclear.
The results of the present study revealed that
miR-18b was significantly upregulated in OC tissues and cell lines
in comparison with the normal ovarian counterparts. Furthermore,
miR-18b may promote OC cell migration and invasion by directly
targeting phosphatase and tensin homolog (PTEN). The result of the
present study suggested that miR-18b may act as an oncogene and
support the utility of miR-18b and PTEN as potential biomarkers
that may be promising targets for the treatment of OC.
Materials and methods
Clinical samples and cell lines
A total of 60 fresh OC tissue samples and 60 matched
normal ovarian tissues were collected from 60 patients with OC
between December 2008 and December 2014 (mean age, 54.3±3.7 years)
from the Navy General Hospital (Beijing, China). Written informed
consent was obtained from all patients for use of tissue samples in
the present study. The present study approved by the Ethics
Committee of Navy General Hospital. All specimens were frozen
immediately in liquid nitrogen until use. Tissue sample
characteristics and information are presented in Table I. ES-2 and HO8910 human OC cells, and
HOSE human normal ovarian epithelial cells were obtained from the
American Type Culture Collection (Manassas, VA, USA).
 | Table I.Association of miR-18b expression
level with clinicopathological features in patients with ovarian
cancer. |
Table I.
Association of miR-18b expression
level with clinicopathological features in patients with ovarian
cancer.
|
|
| miR-18b expression
level |
|
|
|---|
|
|
|
|
|
|
|---|
| Group | n | Low | High | Pearson's
χ2 | P-value |
|---|
| Age, years |
|
|
| 0.031 | 0.861 |
|
<50 | 25 | 12 | 13 |
|
|
| ≥50 | 35 | 16 | 19 |
|
|
| Tumor grade |
|
|
| 7.890 | 0.005 |
|
G1-G2 | 27 | 18 | 9 |
|
|
| G3 | 33 | 10 | 23 |
|
|
| Lymph node
metastasis |
|
|
| 15.654 | <0.001 |
|
Negative | 14 | 13 | 1 |
|
|
|
Positive | 46 | 15 | 31 |
|
|
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA of OC tissues and cells were extracted
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's instructions. The cDNA was synthesized by Oligo-dT
(Takara Bio, Inc., Otsu, Japan) or specific miRNA stem-loop RT
primers (Invitrogen; Thermo Fisher Scientific, Inc.) with 1 µg
total RNA, according to the manufacturer's protocols. RT-qPCR was
performed using an ABI PRISM 7000 Sequence Detection System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
temperature protocol of RT was as follows: 65°C for 5 min, 4°C for
1 min, 50°C for 50 min and 85°C for 5 min. To assess miR-18b
expression levels, RT-qPCR was performed using an NCode™ EXPRESS
SYBR-Green ER™ miRNA qRT-PCR kit (Invitrogen; Thermo Fisher
Scientific, Inc.) with U6 as an internal control. For determination
of the PTEN expression level, RT-qPCR was performed using the
EmeraldAmp PCR Master mix (Takara Bio, Inc.). The conditions for
qPCR were as follows: 98°C for 10 min, followed by 45 cycles of
98°C for 10 sec, 60°C for 30 sec and 72°C for 30 sec. The specific
primers are presented in Table II.
Relative expression levels of miR-18b and PTEN were normalized to
those of U6 and GAPDH, respectively, and quantified using the
comparative Cq method with the formula 2−ΔΔCq (27).
 | Table II.Primers used in reverse
transcription-quantitative polymerase chain reaction analysis. |
Table II.
Primers used in reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene symbol | Forward sequence
(5′-3′) | Reverse sequence
(5′-3′) |
|---|
| miR-18b |
GCAGTTAGTGAAGCAGCTTAGA | Universal
primer |
| U6 |
CTCGCTTCGGCAGCACA |
ACGCTTCACGAATTTGCGT |
| PTEN |
GCGTGCAGATAATGACAAGG |
GGATTTGACGGCTCCTCTAC |
| GAPDH |
CAAGGTCATCCATGACAACTTTG |
GTCCACCACCCTGTTGCTGTAG |
Cell culture and transfection
All cells were maintained in Dulbecco's modified
Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% (v/v) fetal bovine serum (FBS) at 37°C in a
humidified chamber containing 5% CO2. miR-18b mimic,
corresponding mimic negative control, miR-18b inhibitor and
corresponding inhibitor negative control were obtained from
GenePharm, Inc. (Sunnyvale, CA, USA). Lipofectamine
2000® transfection reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to transfect either miR-18b mimic or
mimic negative control, and either miR-18b inhibitor or inhibitor
negative control into ES-2 and HO8910 cells, according to the
manufacturer's protocol. After 48 h of transfection, the efficiency
of miR-18b overexpression or knockdown in OC cells was quantified
by RT-qPCR analysis prior to in vitro cellular functional
experiments.
Protein extraction and western blot
analysis
Total proteins of OC cells were collected using
radioimmunoprecipitation assay (RIPA) reagents (Beyotime Institute
of Biotechnology, Haimen, China), according to the manufacturer's
protocol. Cell lysates were washed with PBS three times and
incubated with ice-cold RIPA buffer for 30 min at 4°C.
Subsequently, the cell lysates were centrifuged at 13,000 × g for
15 min at 4°C. Subsequently, SDS-PAGE (10% gel; Beyotime Institute
of Biotechnology) was used for separating total proteins into equal
amounts (50 µg), which were transferred onto polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA). Following
blocking with 5% non-fat milk (diluted in TBST) for 1 h at 37°C,
the membranes were incubated with anti-PTEN antibodies (#9552;
1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight
at 4°C, followed by horseradish peroxidase-conjugated corresponding
secondary antibodies (#7074; 1:2,000; Santa Cruz Biotechnology,
Inc.).
Cell migration and invasion
assays
Wound healing assays were performed to determine the
migratory ability of miR-18b-transfected OC cells. ES-2 and HO8910
cells were seeded at a density of ~8×105 cells/well in
6-well plates and transfected as aforementioned. Following a 6-h
transfection period, artificial wounds were created and cell
migration images were captured from three randomly selected fields
at 0, 12 and 16 h using a Leica DM4000B microscope (magnification,
×200; Leica Microsystems, Inc., Buffalo Grove, IL, USA). OC cell
invasion assays were performed in triplicate using Transwell
assays. Briefly, 5×104 cells were plated in Dulbecco's
modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc.) without serum, and DMEM supplemented with 10% FBS
was added as a chemoattractant to the lower chamber. Following
incubation for 48 h, cells on top of the filter were removed, and
those that migrated to the underside of the filter were stained
with Hema-Diff solution (Thermo Fisher Scientific, Inc.) and
counted for five random fields/well using a Leica DM4000B
microscope (magnification, ×200; Leica Microsystems, Inc.).
Luciferase reporter assay
Target sequences were inserted into psiCHECK-2
luciferase reporter vectors (Promega Corporation, Madison, WI, USA)
to obtain psiCHECK-2-PTEN-WT recombinant plasmid, which contained
the miR-18b-binding sequences at the PTEN mRNA 3′-untranslated
region (UTR) region. psiCHECK-2-PTEN-MUT contained sequences with
mutations in the putative binding site of PTEN 3′-UTRs and was
chemically synthesized by GenePharm, Inc. ES-2 cells
(~4×104) were seeded in 24-well plates and cultured for
24 h at 37°C. Subsequently, cells were co-transfected with 400 ng
psiCHECK-2-PTEN (WT) or psiCHECK-2-PTEN mutant (MUT), and 20 pmol
miR-18b mimic or miR-18b inhibitor for 6 h at 37°C. Firefly and
Renilla luciferase activities were determined 48 h after
transfection at 37°C using the Dual-Luciferase Reporter Assay kit
(Promega Corporation).
Statistical analysis
All statistical analyses were performed using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). The data are
presented as the mean ± standard deviation. The differences between
groups were analyzed using two-sided Student's t-tests. P<0.05
was considered to indicate a statistically significant difference.
All experiments were performed ≥3 times.
Results
miR-18b is upregulated in human OC
tissues and cell lines
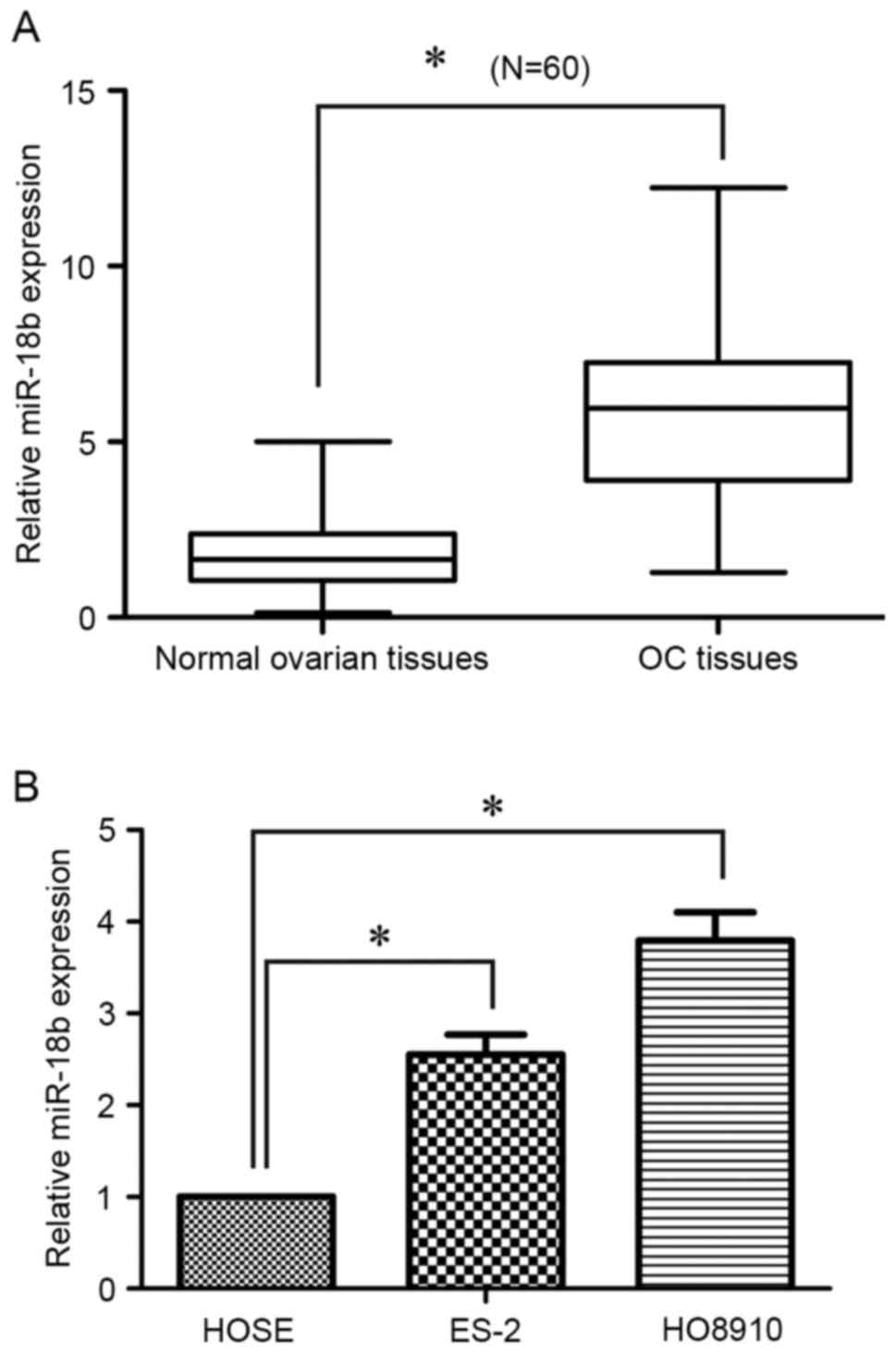
To determine the miR-18b expression level in OC, 60
pairs of human OC tissues and matched normal ovarian tissues were
analyzed using RT-qPCR. As presented in Fig. 1A, the expression level of miR-18b was
significantly increased in OC tissues, compared with the normal
ovarian tissues (P<0.05). In addition, for the
clinicopathological correlation analysis, the relative higher
expression level of miR-18b was positively associated with advanced
tumor grade and lymph node metastasis, but not with age (Table I). Furthermore, the miR-18b expression
level in OC cells was also detected using RT-qPCR. Of note, its
expression level was also upregulated in ES-2 (P<0.05) and
HO8910 (P<0.05) cells, in comparison with in the HOSE human
normal ovarian epithelial cells (Fig.
1B). These results suggested that miR-18b may act as an
oncogene in OC progression.
Upregulation of miR-18b promotes OC
cell migration
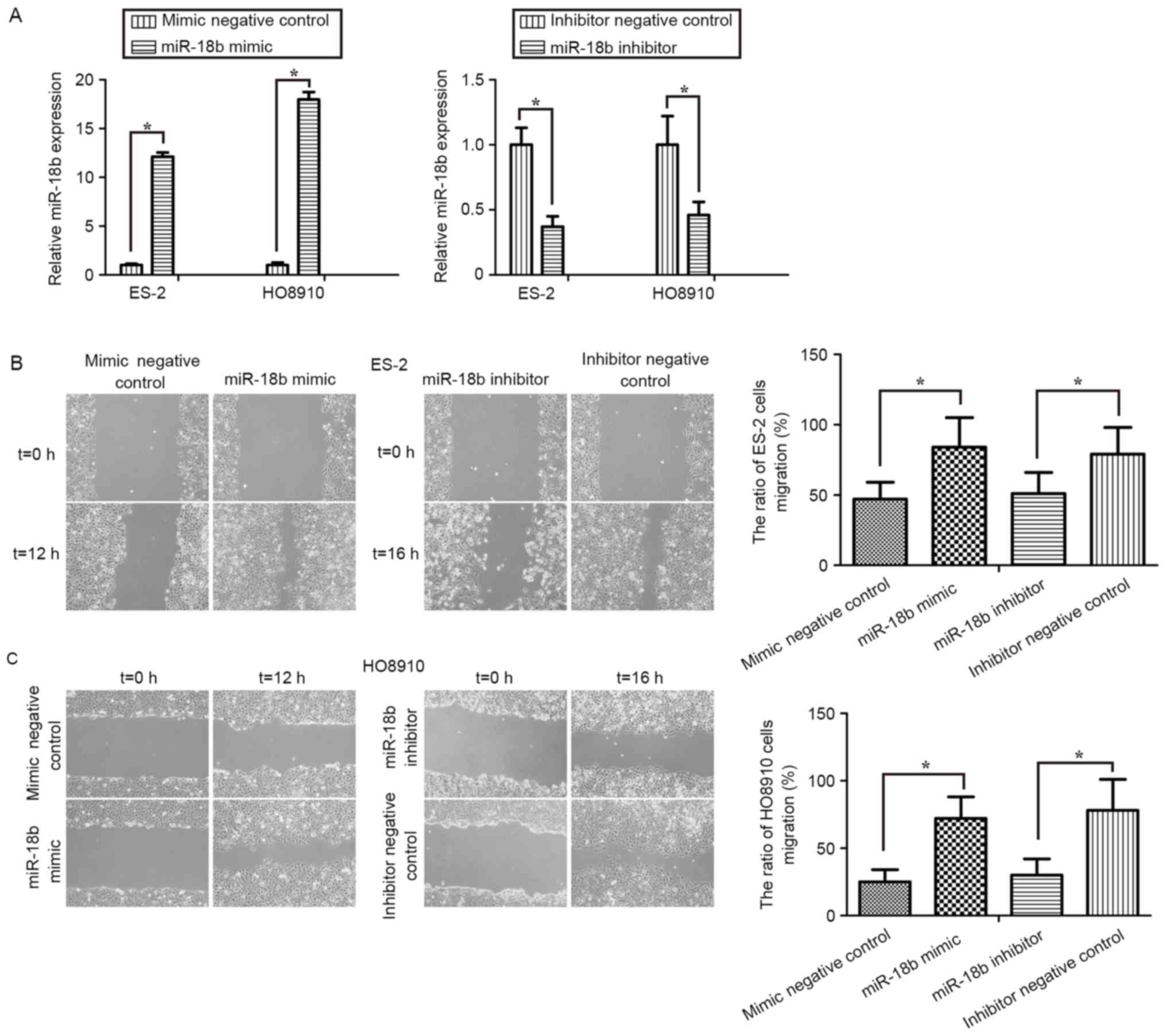
In order to identify the role of miR-18b in OC,
miR-18b mimic or mimic negative control, and miR-18b inhibitor or
inhibitor negative control were transfected into ES-2 and HO8910
cells. First, RT-qPCR was performed to assess the transfection
efficiency in OC cells. The expression levels of miR-18b in the
ES-2 and HO8910 cells treated with miR-18b mimic were significantly
increased, whereas its expression level was markedly decreased
following treatment with the miR-18b inhibitor (P<0.05; Fig. 2A).
The effect of miR-18b on OC cell migration was
assessed by performing wound healing assays. As presented in
Fig. 2B and C, treatment with miR-18b
mimic induced a significant increase in the migratory activity of
ES-2 (P<0.05) and HO8910 (P<0.05) cells, whereas transfection
with miR-18b inhibitor exhibited a marked decrease in cell
migratory levels (P<0.05). These results clearly suggested that
miR-18b may activate OC cell migration.
Upregulation of miR-18b promotes OC
cells invasion
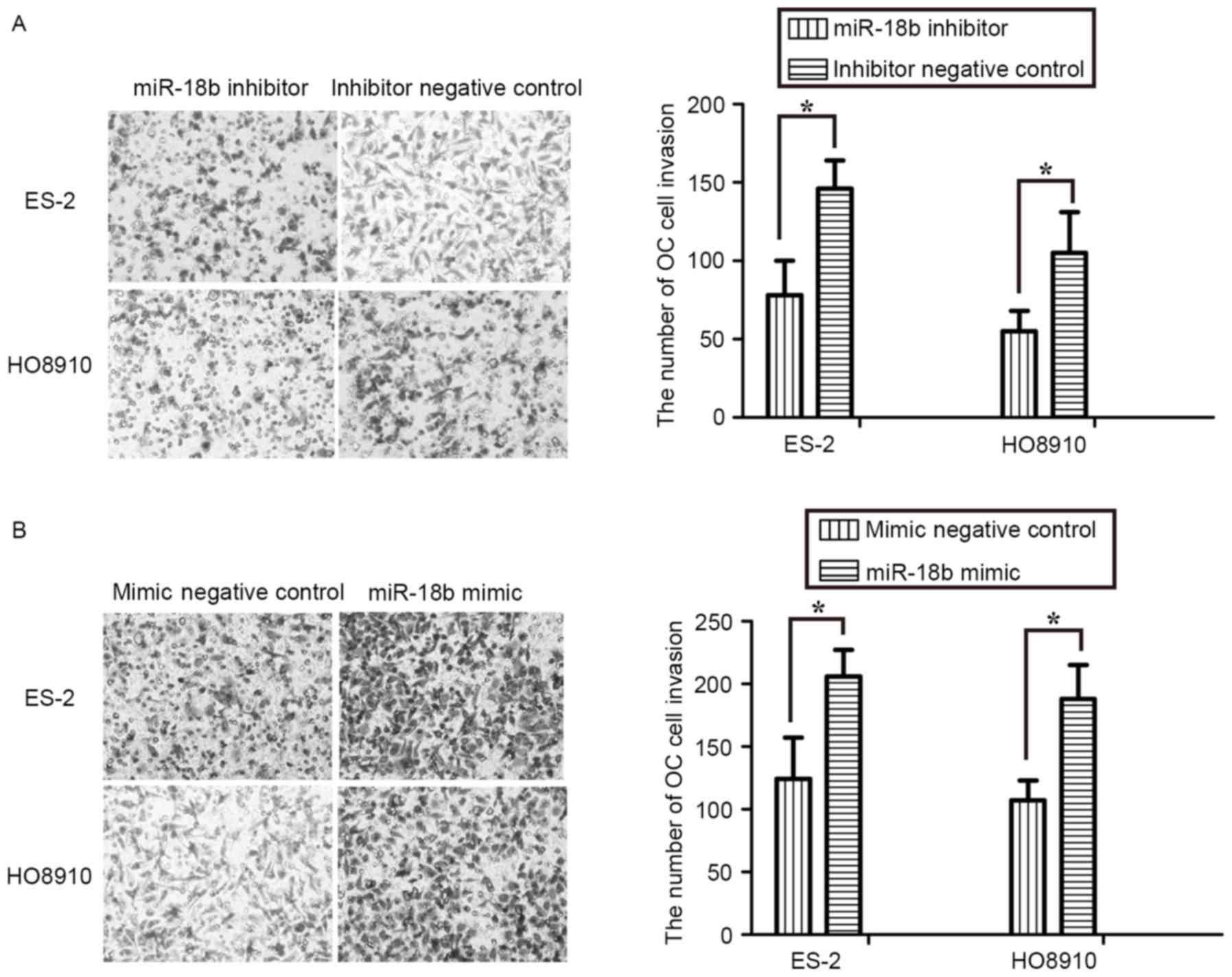
The effect of miR-18b on OC cell invasion was
assessed using Transwell assays. The results revealed that
inhibition of miR-18b expression by an inhibitor repressed invasion
of ES-2 and HO8910 cells (P<0.05; Fig.
3A). Conversely, overexpression of miR-18b markedly promoted
the invasive activity of OC cells (P<0.05; Fig. 3B). These results further suggested
that miR-18b acted as an oncogene in OC progression and
development.
PTEN is a direct target of miR-18b in
OC cells
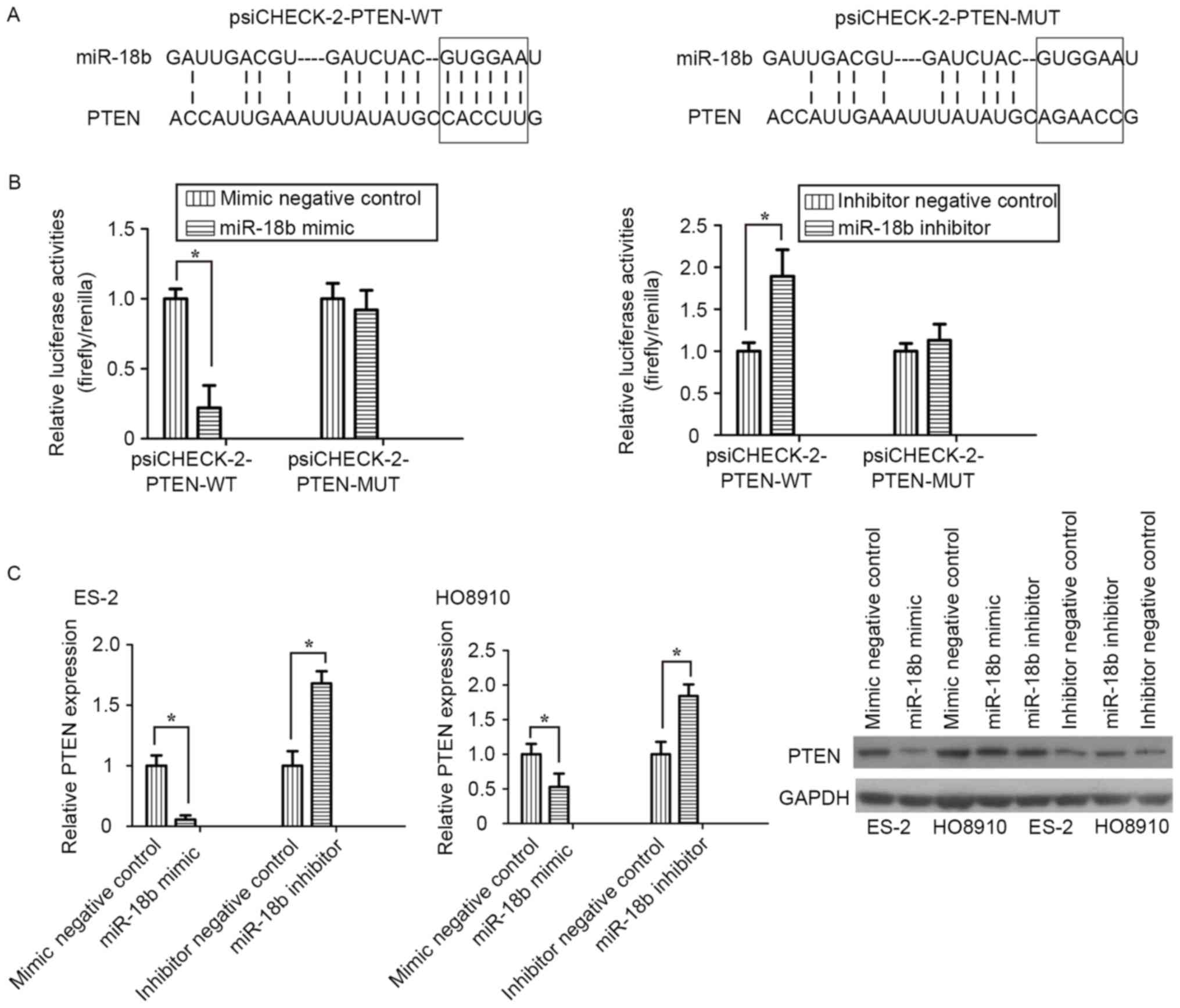
To clarify the molecular mechanism underlying
miR-18b activity in OC cell migration and invasion, bioinformatics
analysis was performed to identify potential targets of miR-18b.
Among those targets predicted, PTEN was selected for further
research. Two luciferase reporters, one containing wild-type PTEN
3′-UTR (psiCHECK-2-PTEN-WT) with the miR-18b-binding sites and
another containing mutant PTEN 3′-UTR (psiCHECK-2-PTEN-MUT), were
constructed and used to explore whether PTEN is a direct target of
miR-18b (Fig. 4A). In luciferase
reporter assay, transfection of miR-18b mimic significantly
suppressed the luciferase activities, as compared with the mimic
control, but did not alter the luciferase activities of the mutant
reporter plasmid. Conversely, inhibition of miR-18b endogenous
expression induced a marked increase in luciferase activities,
compared with the inhibitor negative control (P<0.05; Fig. 4B).
RT-qPCR and western blot analyses demonstrated that
knockdown of miR-18b induced a significant decrease in PTEN mRNA
and protein expression levels in ES-2 and HO8910 cells, whereas
silencing of miR-18b increased the PTEN expression level
(P<0.05; Fig. 4C). The results of
the present study demonstrated that PTEN was a direct target of
miR-18b in OC cells.
Discussion
Recent studies have revealed that miRNAs serve key
roles in cancer progression and potentially serve as novel
biomarkers for the prediction and treatment in various types of
human cancer (28–30). The aim of the present study was to
clarify the biological function of miR-18b in human OC. The
preliminary experiments demonstrated that miR-18b was highly
expressed in OC tissues and cell lines, compared with in normal
ovarian tissues and ovarian epithelial cells. The relatively
increased expression level of miR-18b was positively associated
with advanced tumor grade and lymph node metastasis, which
suggested that upregulation of miR-18b may be involved in OC
metastasis. Exogenous knockdown of miR-18b expression markedly
inhibited OC cell migration and invasion activities, whereas
overexpression of miR-18b promoted the migration and invasion of OC
cells. Furthermore, miR-18b inhibited PTEN expression by directly
targeting its 3′-UTR.
Recent studies have provided abundant evidence that
miR-18b functions as onco-miRNAs in numerous types of human cancer.
For instance, upregulation of miR-18b identified patients with
mantle cell lymphoma with poor outcome and improved the mantle cell
lymphoma prognostic indicator-B by decreasing the proliferation
rate of mantle cell lymphoma cells (31). miRNA-18b was highly expressed in
breast cancer and modulated gene expression levels involved in cell
migration (22). The expression level
of miR-18b in hepatocellular carcinoma was associated with the
grade of malignancy and prognosis (26). The results of the present study have
revealed that miR-18b was significantly upregulated and promoted
human OC cell migration and invasion. Therefore, the results of the
present study also support the view that miR-18b acts as an
oncogenic miRNA in OC.
miRNAs typically exert their functions by inhibiting
the expression levels of target gene mRNA, therefore the further
aim of the present study was to identify miR-18b target genes in
OC. PTEN was predicted as a critical downstream target gene using
various prediction algorithms (32,33). PTEN
was identified as a tumor suppressor gene located on chromosome 10
(34). In recent years, PTEN has
gained particular attention for its critical role in multiple types
of cancer. Numerous previous studies have demonstrated that low
expression levels of PTEN were associated with poor prognosis and
chemoresistance in OC (35,36).
In the present study, luciferase reporter assays
revealed that miR-18b induced a significant decrease in the
luciferase activities of the psiCHECK-2-PTEN-WT reporter, but did
not affect the mutant reporter activities. Of note, transfection of
miR-18b mimic decreased the PTEN expression level in OC cells. In
view of these results, it is proposed that miR-18b downregulates
PTEN gene expression by directly binding to its 3′-UTR. The results
of the present study revealed for the first time, to the best of
our knowledge, that miR-18b-induced cell migration and invasion may
be mediated by targeting PTEN.
In conclusion, the results of the present study
provided novel evidence that miR-18b promoted the migration and
invasion of OC cells by directly targeting PTEN, which suggested
that miR-18b may be a novel promising biomarker for OC prognosis
and treatment.
Acknowledgements
The present study was supported by the National
Science and Technology Pillar Program during the Twelfth Five-Year
Plan Period (grant no. 2012BAI32B05) and the National Natural
Science Foundation of China (grant nos. 81000245 and 81370703).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Legge F, Ferrandina G, Salutari V and
Scambia G: Biological characterization of ovarian cancer:
Prognostic and therapeutic implications. Ann Oncol. 16 Suppl
4:iv95–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pasquinelli AE: MicroRNAs and their
targets: Recognition, regulation and an emerging reciprocal
relationship. Nat Rev Genet. 13:271–282. 2012.PubMed/NCBI
|
|
5
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang H, Cao F, Li X, Miao HEJ, Xing J and
Fu CG: miR-320b suppresses cell proliferation by targeting c-Myc in
human colorectal cancer cells. BMC Cancer. 15:7482015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tai MC, Kajino T, Nakatochi M, Arima C,
Shimada Y, Suzuki M, Miyoshi H2, Yatabe Y3, Yanagisawa K and
Takahashi T: miR-342-3p regulates MYC transcriptional activity via
direct repression of E2F1 in human lung cancer. Carcinogenesis.
36:1464–1473. 2015.PubMed/NCBI
|
|
8
|
Matsuyama R, Okuzaki D, Okada M and
Oneyama C: MicroRNA-27b suppresses tumor progression by regulating
ARFGEF1 and focal adhesion signaling. Cancer Sci. 107:28–35. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu W, An J, Li K and Hou H: MiR-429
regulates gastric cancer cell invasiveness through ZEB proteins.
Tumour Biol. Oct 15–2015.(Epub ahead of print).
|
|
10
|
Li HT, Zhang H, Chen Y, Liu XF and Qian J:
MiR-423-3p enhances cell growth through inhibition of p21Cip1/Waf1
in colorectal cancer. Cell Physiol Biochem. 37:1044–1054. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu T, Liu L, Li J, Yan M, Lin H, Liu Y,
Chu D, Tu H, Gu A and Yao M: MiRNA-10a is upregulated in NSCLC and
may promote cancer by targeting PTEN. Oncotarget. 6:30239–30250.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo P, Fei J, Zhou J and Zhang W:
microRNA-126 suppresses PAK4 expression in ovarian cancer SKOV3
cells. Oncol Lett. 9:2225–2229. 2015.PubMed/NCBI
|
|
13
|
Guo J, Xia B, Meng F and Lou G: miR-137
suppresses cell growth in ovarian cancer by targeting AEG-1.
Biochem Biophys Res Commun. 441:357–363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong R, Liu X, Zhang Q, Jiang Z, Li Y, Wei
Y, Li Y, Yang Q, Liu J, Wei JJ, et al: miR-145 inhibits tumor
growth and metastasis by targeting metadherin in high-grade serous
ovarian carcinoma. Oncotarget. 5:10816–10829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wen Z, Zhao S, Liu S, Liu Y, Li X and Li
S: MicroRNA-148a inhibits migration and invasion of ovarian cancer
cells via targeting sphingosine-1-phosphate receptor 1. Mol Med
Rep. 12:3775–3780. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu R, Liu F, Li L, Sun M and Chen K:
MiR-498 regulated FOXO3 expression and inhibited the proliferation
of human ovarian cancer cells. Biomed Pharmacother. 72:52–57. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan Y, Fan J, Huang L, Ye M, Huang Z, Wang
Y, Li Q and Huang J: Increased expression of microRNA-196a predicts
poor prognosis in human ovarian carcinoma. Int J Clin Exp Pathol.
8:4132–4137. 2015.PubMed/NCBI
|
|
18
|
Liu N, Zhong L, Zeng J, Zhang X, Yang Q,
Liao D and Wang Y, Chen G and Wang Y: Upregulation of microRNA-200a
associates with tumor proliferation, CSCs phenotype and
chemosensitivity in ovarian cancer. Neoplasma. 62:550–559. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Niu K, Shen W, Zhang Y, Zhao Y and Lu Y:
MiR-205 promotes motility of ovarian cancer cells via targeting
ZEB1. Gene. 574:330–336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song N, Liu H, Ma X and Zhang S: Placental
growth factor promotes metastases of ovarian cancer through
MiR-543-regulated MMP7. Cell Physiol Biochem. 37:1104–1112. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu T, Yuan J, Wang Y, Gong C, Xie Y and
Li H: MiR-661 contributed to cell proliferation of human ovarian
cancer cells by repressing INPP5J expression. Biomed Pharmacother.
75:123–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fonseca-Sanchéz MA, Pérez-Plasencia C,
Fernández-Retana J, Arechaga-Ocampo E, Marchat LA, Rodríguez-Cuevas
S, Bautista-Piña V, Arellano-Anaya ZE, Flores-Pérez A, Diaz-Chávez
J and López-Camarillo C: microRNA-18b is upregulated in breast
cancer and modulates genes involved in cell migration. Oncol Rep.
30:2399–2410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang ZZ, Liu X, Wang DQ, Teng MK, Niu LW,
Huang AL and Liang Z: Hepatitis B virus and hepatocellular
carcinoma at the miRNA level. World J Gastroenterol. 17:3353–3358.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo J, Miao Y, Xiao B, Huan R, Jiang Z,
Meng D and Wang Y: Differential expression of microRNA species in
human gastric cancer versus non-tumorous tissues. J Gastroenterol
Hepatol. 24:652–657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang YX, Zhang XY, Zhang BF, Yang CQ, Chen
XM and Gao HJ: Initial study of microRNA expression profiles of
colonic cancer without lymph node metastasis. J Dig Dis. 11:50–54.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Murakami Y, Tamori A, Itami S, Tanahashi
T, Toyoda H, Tanaka M, Wu W, Brojigin N, Kaneoka Y, Maeda A, et al:
The expression level of miR-18b in hepatocellular carcinoma is
associated with the grade of malignancy and prognosis. BMC Cancer.
13:992013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guan Y, Chen L, Bao Y, Li Z, Cui R, Li G
and Wang Y: Identification of low miR-105 expression as a novel
poor prognostic predictor for human glioma. Int J Clin Exp Med.
8:10855–10864. 2015.PubMed/NCBI
|
|
29
|
Dou H, Wang Y, Su G and Zhao S: Decreased
plasma let-7c and miR-152 as noninvasive biomarker for
non-small-cell lung cancer. Int J Clin Exp Med. 8:9291–9298.
2015.PubMed/NCBI
|
|
30
|
Cai K, Shen F, Cui JH, Yu Y and Pan HQ:
Expression of miR-221 in colon cancer correlates with prognosis.
Int J Clin Exp Med. 8:2794–2798. 2015.PubMed/NCBI
|
|
31
|
Husby S, Ralfkiaer U, Garde C, Zandi R, Ek
S, Kolstad A, Jerkeman M, Laurell A, Räty R, Pedersen LB, et al:
miR-18b overexpression identifies mantle cell lymphoma patients
with poor outcome and improves the MIPI-B prognosticator. Blood.
125:2669–2677. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Betel D, Wilson M, Gabow A, Marks DS and
Sander C: The microRNA.org resource: Targets and expression.
Nucleic Acids Res. 36:(Database issue). D149–D153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015. View Article : Google Scholar
|
|
34
|
Ying H, Qu D, Liu C, Ying T, Lv J, Jin S
and Xu H: Chemoresistance is associated with Beclin-1 and PTEN
expression in epithelial ovarian cancers. Oncol Lett. 9:1759–1763.
2015.PubMed/NCBI
|
|
35
|
Cheaib B, Auguste A and Leary A: The
PI3K/Akt/mTOR pathway in ovarian cancer: Therapeutic opportunities
and challenges. Chin J Cancer. 34:4–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu H, Wang K, Liu W and Hao Q: PTEN
overexpression improves cisplatin-resistance of human ovarian
cancer cells through upregulating KRT10 expression. Biochem Biophys
Res Commun. 444:141–146. 2014. View Article : Google Scholar : PubMed/NCBI
|