Introduction
Renal cell carcinoma (RCC) accounts for 2–3% of all
cases of malignancy in adults globally in 2014 (1). Although associated diagnostic techniques
and targeted therapies have greatly improved in recent decades, in
2004, 20–30% of RCC patients were diagnosed with metastatic
disease, and a further one-third of patients with localized disease
that undergo curative surgery subsequently experience recurrence or
metastasis in North America (2). The
tumor-node-metastasis (TNM) stage (3)
and Fuhrman grade (4) systems remain
the most used outcome predictors; several integrated models have
also been established, including the Mayo Clinic stage, size, grade
and necrosis (SSIGN) score (5) and
the University of California Integrated Staging System (UISS)
(6); however, these parameters are
not entirely reliable (7). In the era
of precision medicine, it is expected that specific molecular
biomarkers will provide supplementary prognostic information that
may be incorporated into existing conventional models for the
improved prediction of patient prognosis (8).
Copper transporter (CTR) 1, encoded by the
SLC31A1 gene, has been characterized as a high-affinity
copper transporter since its identification in 1997 (9). This membrane-bound molecule is the major
driving force in facilitating copper import, and thus may raise the
Cu2+ concentration in expressing cells (10). Copper, as an essential trace mineral,
serves an important role in the regulation of human physiological
functions; its aberrant upregulation may promote tumor angiogenesis
and progression in various types of cancer (11–13). This
oncogenic potential is partially associated with the stimulation of
the hypoxia-inducible factor (HIF) pathway by copper. For example,
a previous study has identified that copper serves an essential
role in the HIF-1α/hypoxia response element-binding process; high
concentrations of Cu2+ may stabilize HIF-1α in the
nucleus and activate downstream signals, including the upregulation
of vascular endothelial growth factor (VEGF), and induce the
epithelial-mesenchymal transition (EMT) (14). CTR1-knockdown, leading to reduced
intracellular copper, has been demonstrated to inhibit angiogenesis
and EMT in multiple types of cell, including human breast cancer
and endothelial cells (15,16). Clinical trials with copper chelation
therapy for metastatic diseases have also generated promising data
(17).
In RCC, the most common histological type is clear
cell renal cell carcinoma (ccRCC; 70–85%) (18), which exhibits a Von Hippel-Lindau
(VHL) mutation, leading to an activation of HIF signaling (19). Since CTR1 may be associated with
cellular copper regulation and interact with the HIF pathway, this
molecule may be associated with the outcome of patients with ccRCC.
In the present study, the association between CTR1 expression, as
determined with IHC, and the survival time of 293 patients with
ccRCC was evaluated. Prognostic improvements using CTR1 data with
several well-established predictive models were also analyzed, and
two nomograms integrating the expression of this molecule with
other clinical parameters were formed to predict the overall
survival (OS) and disease-free survival (DFS) outcomes for
patients.
Materials and methods
Patients and clinical database
A total of 293 patients (age range, 15–86 years;
median age, 55 years; 90 female, 203 male) with ccRCC who underwent
nephrectomy were retrospectively recruited from the Department of
Urology, Zhongshan Hospital, Fudan University (Fudan, China)
between January 2005 and June 2007. All methods in the present
study were approved by the Ethics Committee of Zhongshan Hospital
(approval no. B2015-030) and were performed in accordance with the
committee guidelines. Written informed consent was obtained from
all individual patients included in the present study. The
inclusion criteria were as follows: No history of other malignant
tumors, no history of targeted therapy prior to or following
surgery, and pathologically determined ccRCC. Patients with
mixed-type renal cancer, bilateral renal cancer, tumor necrosis
area >80% or those with perioperative morbidity were
excluded.
The median follow-up for all available patients was
99.10 months (range, 2.63–120.47 months) and the follow-up interval
was 3 months, until January 30, 2015. Metastasis or recurrences
were defined based on imaging tests or histopathology information.
Age, sex, tumor size, TNM stage, Fuhrman grade, tumor necrosis and
Eastern Cooperative Oncology Group performance status (ECOG PS)
(20) information for patients was
obtained and is included in Table I.
Tumor histological type and differentiation were reassessed by two
urological pathologists according to the 2004 World Health
Organization criteria (21). Tumor
stage was reclassified according to chest radiography, abdominal
computerized tomography and pathological reports of patients based
on the 2010 American Joint Committee on Cancer TNM classification
(3). Tumor size was measured as the
longest diameter in the pathological information. Tumor necrosis
was defined as the presence of microscopic coagulative
necrosis.
 | Table I.Clinical characteristics of patients
according to CTR1 expression. |
Table I.
Clinical characteristics of patients
according to CTR1 expression.
|
|
| CTR1 expression,
n |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Patients, n | Low | High | P-value |
|---|
| All patients | 293 | 177 | 116 |
|
| Age, years |
|
|
| 0.077a |
|
≤55 | 145 | 95 | 50 |
|
|
>55 | 148 | 82 | 66 |
|
| Sex |
|
|
| 0.870a |
|
Female | 90 | 55 | 35 |
|
|
Male | 203 | 122 | 81 |
|
| Tumor size, cm |
|
|
| 0.576a |
| ≤4 | 165 | 102 | 63 |
|
|
>4 | 128 | 75 | 53 |
|
| T
classification |
|
|
|
<0.001b |
| T1 | 185 | 132 | 53 |
|
| T2 | 27 | 13 | 14 |
|
| T3 | 77 | 31 | 46 |
|
| T4 |
4 |
1 |
3 |
|
| N
classification |
|
|
| 0.489a |
| N0 | 35 | 17 | 18 |
|
| N1 |
2 |
0 |
2 |
|
| Nx | 256 |
|
|
|
| Distant
metastasis |
|
|
| 0.726a |
| No | 277 | 168 | 109 |
|
|
Yes | 16 |
9 |
7 |
|
| Tumor, node,
metastasis stage |
|
|
|
<0.001b |
| I | 179 | 128 | 51 |
|
| II | 23 | 10 | 13 |
|
|
III | 71 | 29 | 42 |
|
| IV | 20 | 10 | 10 |
|
| Fuhrman grade |
|
|
| 0.003b |
| 1 | 31 | 20 | 11 |
|
| 2 | 217 | 140 | 77 |
|
| 3 | 42 | 17 | 25 |
|
| 4 |
3 |
0 |
3 |
|
| Necrosis |
|
|
| 0.194a |
|
Absent | 252 | 156 | 96 |
|
|
Present | 41 | 21 | 20 |
|
| Eastern Cooperative
Oncology Group performance status |
|
|
| 0.018b |
| 0 | 214 | 137 | 77 |
|
| 1 | 64 | 33 | 31 |
|
| 2 | 11 |
7 |
4 |
|
| 3 |
4 |
0 |
4 |
|
OS time was calculated from the date of nephrectomy
to the date of mortality the last follow-up. DFS time was defined
as the time from nephrectomy to disease recurrence or the last
follow-up time; mortalities occurring without disease recurrence
were considered to be censored. The SSIGN score was applied to
classify patients into three risk levels: 0–3 (low), 4–7
(intermediate) and ≥8 (high) in OS analyses (5,22).
Similarly, patients were classified into three risk levels for DFS
analyses: 0–2 (low), 3–5 (intermediate) and ≥6 (high), based on
SSIGN (localized) score, as previously reported (23).
IHC and evaluation
Immunohistochemical staining was performed on a
tissue microarray, as previously described (24). For each primary tumor block, two
representative cores from areas without necrosis and hemorrhage
were selected for analysis. A rabbit polyclonal antibody against
human CTR1 (cat. no. ab133385; dilution, 1:100; Abcam, Cambridge,
MA, USA) and the EnVision Detection System (Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) were applied in the
procedure. The specificity of the anti-CTR1 antibody was confirmed
by western blot procedure, performed as previously described
(25) (anti-CTR1 antibody dilution,
1:1,000). The RCC cell lines ACHN and 786-O were purchased from the
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). As a negative control, similar IHC procedures
were applied to tissue samples without applying the primary
antibody.
An Olympus camera (Olympus Corporation, Tokyo,
Japan), a Nikon eclipse Ti-s microscope (magnification, ×200; Nikon
Corporation, Tokyo Japan) and NIS-Elements F3.2 software (Nikon
Corporation) were used in recording the staining results. The three
images with the strongest staining were obtained from each core and
the associated integrated optical density (IOD) scores were
calculated with Image-Pro Plus version 6.0 software (Media
Cybernetics Inc., Rockville, MD, USA). The combined IOD mean of the
six images from two cores was regarded as the final staining
intensity for each block used for further statistical analysis.
These slides were evaluated by two experienced pathologists that
were unaware of the clinical features and outcomes of patients.
Statistical analysis
For determining CTR1 high/low expression, the IOD
score cut-point was evaluated by X-tile software (version 3.6.1)
(26) through the minimum P-value
method. χ2 test, Fisher's exact method and
Cochran-Mantel-Haenszel χ2 test were applied for
assessing the association between CTR1 expression and the
clinicopathological parameters of patients. Survival curves for OS
and DFS were estimated using the Kaplan-Meier method and analyzed
by log-rank test. Cox univariate analysis was performed and
parameters with statistical significance were brought into a
multivariate Cox proportional hazards model. Harrell's concordance
index (c-index) and Akaike's information criterion (AIC) were
generated to compare the predictive ability of various models with
or without the addition of CTR1 (27). GraphPad Prism 6 (GraphPad Software,
Inc., La Jolla, CA, USA), SPSS 21.0 (IBM SPSS, Armonk, NY, USA) and
Stata (version 12.1; StataCorp LP, College Station, TX, USA) were
used in these procedures. P<0.05 was considered to indicate a
statistically significant difference. For nomogram formation and
calibrations, R software version 3.1.2 with the ‘rms’ package (R
Foundation for Statistical Computing, Vienna, Austria) was applied
and the c-index was used for measuring its prognostic accuracy.
Results
Immunohistochemical CTR1 intensity and
its association with clinicopathological characteristics
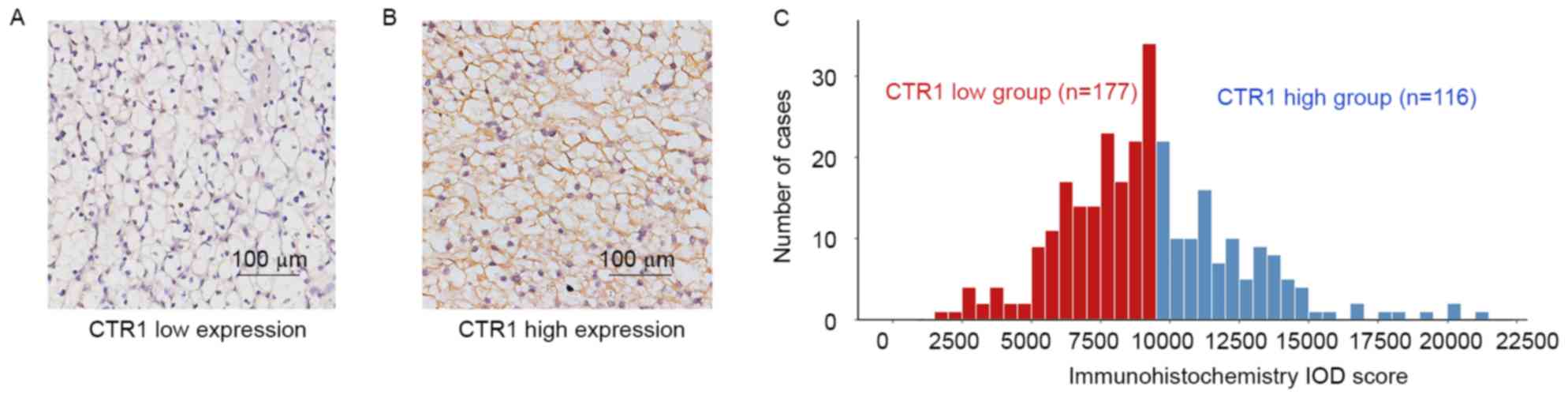
CTR1 expression was predominantly observed in the
cytoplasm and/or membrane in IHC. The staining intensity was
divided into low expression and high expression based on the cutoff
value (9,500) derived from total IOD score (median, 9,040;
interquartile range, 7,291–11,038; mean ± standard deviation,
9,267±3,129) using the ‘minimum P-value’ approach (Fig. 1A and B). Patients were subsequently
separated into CTR1 low (n=177) and CTR1 high (n=116) groups
(Fig. 1C). The detailed
characteristics of patients and the association between CTR1
expression and clinicopathological features are included in
Table I. CTR1 expression was
significantly associated with T classification (P<0.001), TNM
stage (P<0.001), Fuhrman grade (P=0.003) and ECOG PS
(P=0.018).
Kaplan-Meier and subgroup analysis to
assess the prognostic value of CTR1 in patients with ccRCC
Within the cohort, 28.3% (83/293) patients succumbed
to any cause during the follow-up period. Subsequent to excluding
25 patients with pre-operational metastasis or without recurrence
information, 26.5% (71/268) patients developed disease recurrence
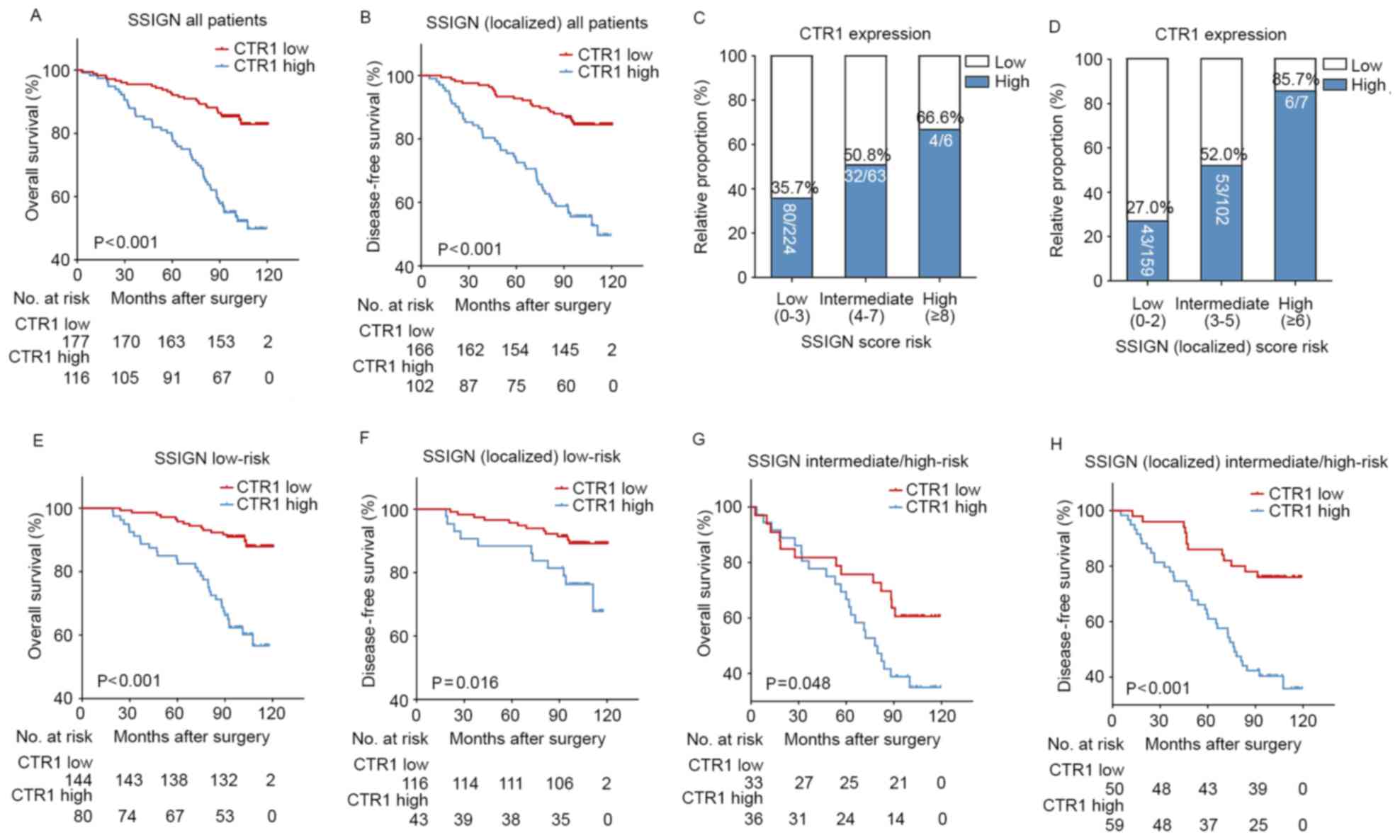
in the present study. Kaplan-Meier curves revealed that patients
with ccRCC with high CTR1 expression had a significantly poorer OS
(P<0.001; Fig. 2A) and DFS
(P<0.001; Fig. 2B) rate compared
with those with low CTR1 expression. To investigate whether this
was dependent on the SSIGN score, a subgroup analysis was performed
on the overall cohort. Fig. 2C and D
demonstrate that the proportion of high CTR1 expression specimens
was elevated with an increasing SSIGN/SSIGN (localized) risk score.
The relatively small number of patients in the high-risk groups
were combined with the intermediate group for subsequent analysis.
The results revealed that high CTR1 expression was associated with
a poor prognosis in the low (OS, P<0.001; DFS, P=0.016; Fig. 2E and F, respectively) and
intermediate/high (OS, P=0.048; DFS, P<0.001; Fig. 2G and H, respectively) risk groups.
Univariate and multivariate
analyses
A univariate analysis highlighted the prognostic
value of high CTR1 expression in the prediction of a poor OS
(P<0.001) and DFS (P<0.001) (Table
II). Due to the small prognostic difference between Fuhrman
grades 1 and 2, they were combined into one category. A
multivariate analysis was performed, including the parameters with
univariate statistical significance, in which tumor size and TNM
stage were ruled out as potential confounding factors for T stage
and distant metastasis. The age of patients was also removed, since
it did not meet statistical significance subsequent to adjusting
for other parameters. The analysis revealed that high expression of
CTR1 in ccRCC was associated with a higher risk of mortality
(hazard ratio, 2.291; 95% CI, 1.389–3.777; P<0.001) and a
shorter DFS time (hazard ratio, 2.210; 95% CI, 1.299–3.759;
P=0.003; Table III). These results
suggested that high tumor CTR1 expression was an independent poor
prognostic marker in ccRCC adjusted with T stage, distant
metastasis, Fuhrman grade, necrosis and ECOG PS.
 | Table II.Univariate analyses of
characteristics associated with OS and DFS. |
Table II.
Univariate analyses of
characteristics associated with OS and DFS.
|
| OS (n=293) | DFS (n=268) |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI |
P-valuea | HR | 95% CI |
P-valuea |
|---|
| Age, years |
|
|
|
|
|
|
| >55
vs. ≤55 | 2.076 | 1.322–3.260 | 0.002 | 1.740 | 1.084–2.794 | 0.022 |
| Sex |
|
|
|
|
|
|
| Male
vs. female | 1.044 | 0.653–1.669 | 0.857 | 0.950 | 0.578–1.562 | 0.841 |
| Tumor size, cm |
|
|
|
|
|
|
| >4
vs. ≤4 | 2.045 | 1.320–3.168 | 0.001 | 2.339 | 1.456–3.755 | <0.001 |
| T
classification |
|
| <0.001 |
|
| <0.001 |
| T1 | RV | – | – | RV | – | – |
| T2 | 3.340 | 2.066–5.402 | <0.001 | 3.626 | 1.769–7.432 | <0.001 |
| T3 | 3.542 | 1.857–6.756 | <0.001 | 3.004 | 1.784–5.057 | <0.001 |
| T4 | 7.946 | 2.420–26.092 | 0.001 | 15.514 | 5.350–44.983 | <0.001 |
| N classification
(n=44) |
|
|
|
|
|
|
| N1 vs.
N0 | 1.145 | 0.150–8.728 | 0.896 | – | – | – |
| Distant
metastasis |
|
|
|
|
|
|
| Yes vs.
no | 5.390 | 2.918–9.956 | <0.001 | – | – | – |
| Tumor, node,
metastasis stage |
|
| <0.001 |
|
| <0.001 |
| I | RV | – | – | RV | – | – |
| II | 3.279 | 1.592–6.756 | 0.001 | 3.875 | 1.938–7.751 | <0.001 |
|
III | 3.403 | 2.031–5.702 | <0.001 | 2.920 | 1.726–4.940 | <0.001 |
| IV | 9.429 | 5.023–17.698 | <0.001 | 15.532 | 5.356–45.039 | <0.001 |
| Fuhrman grade |
|
| <0.001 |
|
| <0.001 |
|
1–2 | RV | – | – | RV | – | – |
| 3 | 3.187 | 1.971–5.152 | <0.001 | 3.478 | 2.061–5.868 | <0.001 |
| 4 | 4.367 | 1.366–13.962 | 0.013 | 4.968 | 1.545–15.977 | 0.007 |
| Necrosis |
|
|
|
|
|
|
| Present
vs. absent | 2.699 | 1.656–4.401 | <0.001 | 3.081 | 1.834–5.175 | <0.001 |
| Eastern Cooperative
Oncology Group performance status |
|
| <0.001 |
|
| <0.001 |
| 0 | RV | – | – | RV | – | – |
| 1 | 3.167 | 2.005–5.003 | <0.001 | 2.706 | 1.629–4.494 | <0.001 |
| 2 | 2.148 | 0.770–5.990 | 0.144 | 2.093 | 0.467–6.768 | 0.217 |
| 3 | 8.667 | 3.078–24.405 | <0.001 | 9.471 | 3.350–26.778 | <0.001 |
| Tumor CTR1
expression |
|
|
|
|
|
|
| High
vs. low | 3.636 | 2.305–5.736 | <0.001 | 3.933 | 2.403–6.436 | <0.001 |
 | Table III.Multivariate analyses of
characteristics associated with OS and DFS. |
Table III.
Multivariate analyses of
characteristics associated with OS and DFS.
|
| OS (n=293) | DFS (n=268) |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI |
P-valuea | HR | 95% CI |
P-valuea |
|---|
| T stage |
|
| 0.008 |
|
| <0.001 |
| T1 | RV | – | – | RV | – | – |
| T2 | 2.356 | 1.380–4.021 | 0.002 | 2.516 | 1.155–5.483 | 0.020 |
| T3 | 2.406 | 1.200–4.824 | 0.013 | 2.735 | 1.542–4.853 | 0.001 |
| T4 | 3.319 | 0.885–12.447 | 0.075 | 8.353 | 2.457–28.393 | 0.001 |
| Distant
metastasis |
|
|
|
|
|
|
| Yes vs.
no | 3.534 | 1.841–6.784 | <0.001 | – | – | – |
| Fuhrman grade |
|
| 0.002 |
|
| <0.001 |
|
1–2 | RV | – | – | RV | – | – |
| 3 | 2.230 | 1.298–3.832 | 0.004 | 2.839 | 1.553–5.190 | 0.001 |
| 4 | 4.125 | 1.182–14.401 | 0.026 | 4.810 | 1.342–17.242 | 0.016 |
| Necrosis |
|
|
|
|
|
|
| Present
vs. absent | 2.010 | 1.151–3.511 | 0.014 | 2.087 | 1.177–3.701 | 0.012 |
| ECOG PS |
|
| 0.005 |
|
| 0.012 |
| 0 | RV | – | – | RV | – | – |
| 1 | 2.130 | 1.301–3.487 | 0.003 | 1.957 | 1.115–3.434 | 0.019 |
| 2 | 2.310 | 0.789–6.760 | 0.126 | 3.233 | 0.980–10.667 | 0.054 |
| 3 | 3.514 | 1.169–10.568 | 0.025 | 3.236 | 1.048–9.993 | 0.041 |
| Tumor CTR1
expression |
|
|
|
|
|
|
| Low vs.
high | 2.291 | 1.389–3.777 | 0.001 | 2.210 | 1.299–3.759 | 0.003 |
Extension of established prognostic
models with CTR1 expression
CTR1 expression was added as a binary variable to
well-established prognostic systems (TNM stage, SSIGN and UISS) and
it was investigated whether this biomarker could improve their
prognostic power. As included in Table
IV, subsequent to being integrated with CTR1 expression, the
c-indexes of all existing models increased markedly for OS (P=0.003
for TNM, P=0.024 for SSIGN, P=0.021 for UISS, respectively) and DFS
analyses (P=0.015 for T stage, P=0.090 for SSIGN, P=0.021 for UISS,
respectively). The AIC was also calculated for each model; models
that included CTR1 status presented a lower score than the models
that did not include CTR1 status, indicating the potential
prognostic value of CTR1.
 | Table IV.Comparison of the predictive accuracy
of the prognostic models. |
Table IV.
Comparison of the predictive accuracy
of the prognostic models.
|
| Overall survival
(n=293) | Disease free
survival (n=268) |
|---|
|
|
|
|
|---|
| Models | C-index |
P-valuea | AIC | C-index |
P-valuea | AIC |
|---|
| CTR1 | 0.654 |
| 878.03 | 0.664 |
| 736.39 |
| TNM | 0.702 |
| 865.51 | 0.653 |
| 746.76 |
| TNM + CTR1 | 0.750 | 0.003 | 849.72 | 0.722 | 0.015 | 726.30 |
| SSIGN | 0.740 |
| 858.94 | 0.725 |
| 722.78 |
| SSIGN + CTR1 | 0.779 | 0.024 | 839.81 | 0.763 | 0.090 | 705.21 |
| UISS | 0.722 |
| 861.26 | 0.713 |
| 726.29 |
| UISS + CTR1 | 0.766 | 0.021 | 842.16 | 0.750 | 0.021 | 711.79 |
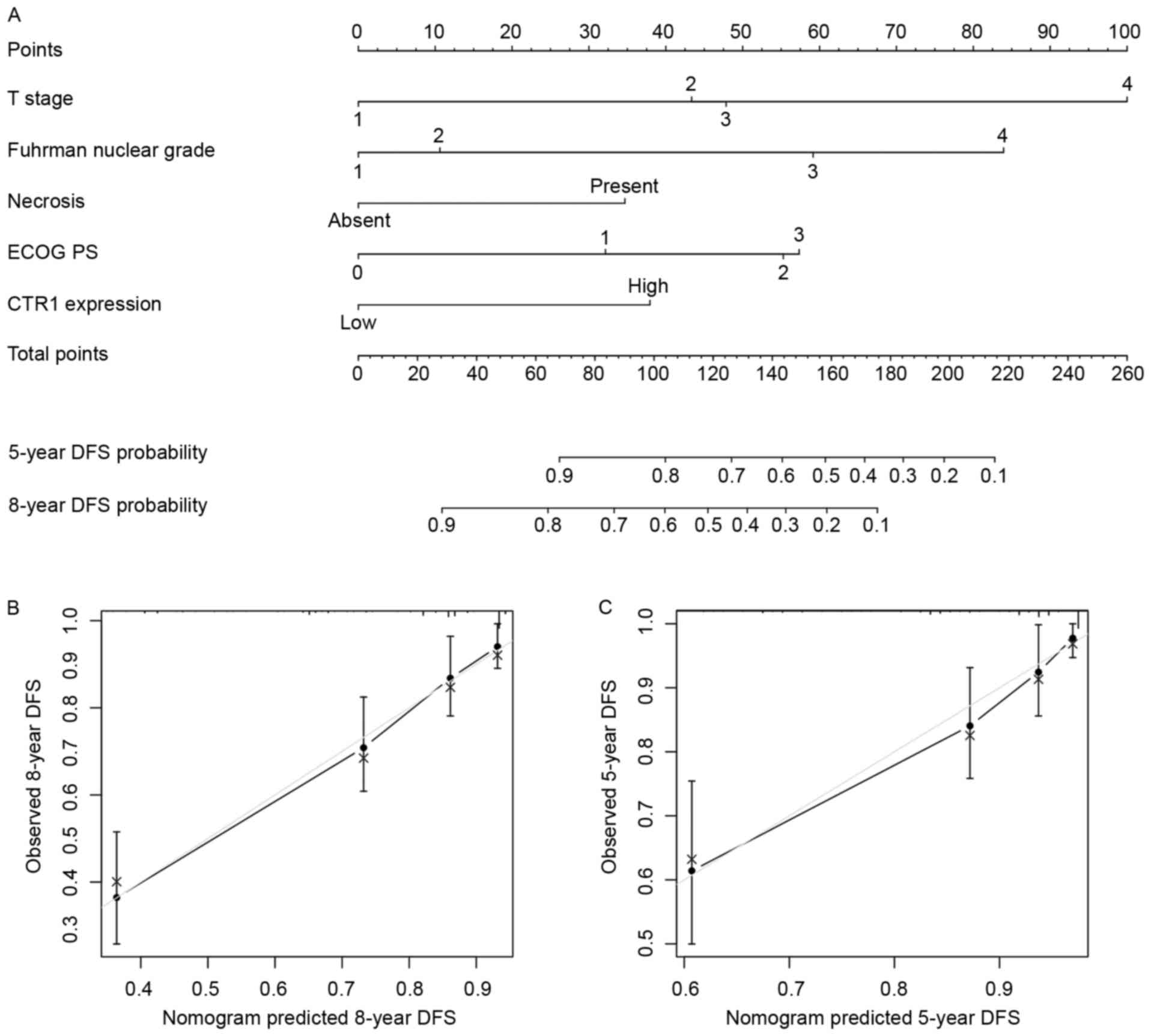
Nomograms for predicting OS and DFS in
patients with ccRCC
Based on the results arising from multivariate
analysis, two nomograms were constructed for predicting the 5- and
8-year OS and DFS rates of patients with ccRCC (Figs. 3A and 4A). The predictors included were T stage,
distant metastasis, Fuhrman grade, necrosis status, ECOG PS and
tumor CTR1 expression. Bootstrap validations were performed for
calibration (Figs. 3B and C; 4B and C). The Harrell's c-indexes were 0.805
(95% CI, 0.764–0.846) and 0.787 (95% CI, 0.736–0.838) for OS and
DFS prediction, respectively.
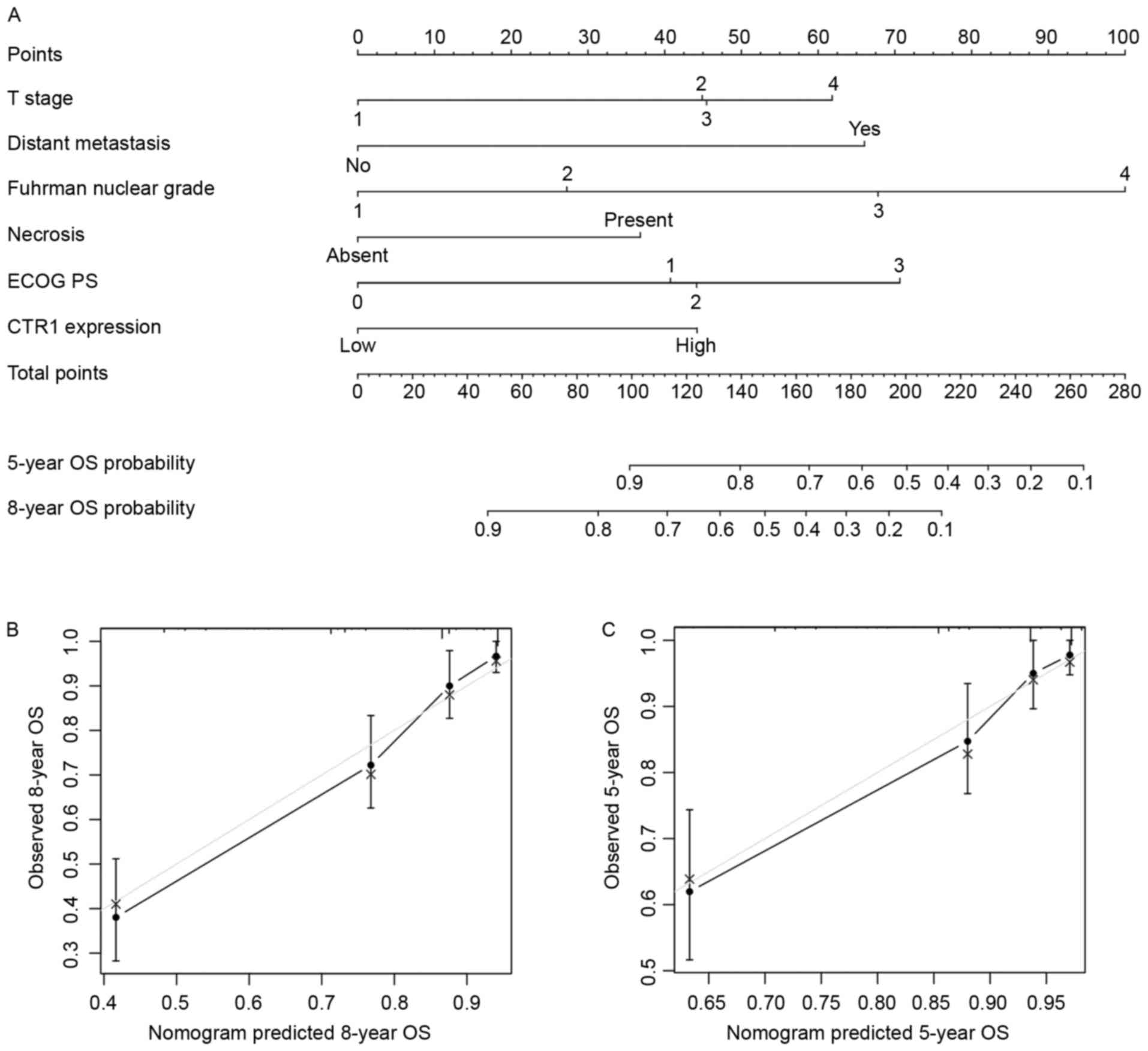 | Figure 3.Nomogram for predicting 8- and 5-year
overall survival in patients with clear cell renal cell carcinoma.
(A) Nomogram for predicting clinical outcomes, integrating T stage,
distant metastasis, Fuhrman nuclear grade, necrosis, ECOG PS and
tumor CTR1 expression. (B) Calibration plot for predicted and
observed 8-year OS rate. (C) Calibration plot for predicted and
observed 5-year OS rate. Grey line, ideal model; vertical bars, 95%
confidence interval. ECOG PS, Eastern Cooperative Oncology Group
performance status; CRT1, copper transporter 1; OS, overall
survival. |
Discussion
ccRCC is well elucidated for its VHL/HIF
dysregulation and downstream signal abnormalities (19). Furthermore, CTR1, as a high-affinity
copper transporter mediating cellular copper upregulation, is
considered to promote tumor angiogenesis and progression indirectly
through the HIF pathway (15).
Therefore, the prognostic role of CTR1 expression was explored in
patients with ccRCC.
Through IHC, CTR1 was identified on the cell plasma
membrane and intracellular area in ccRCC specimens, consistent with
previous findings (28). In addition,
high tumor CTR1 expression was positively associated with various
clinical characteristics and a higher SSIGN score, and could be
used to stratify the outcome of patients in different SSIGN risk
groups. CTR1 also exhibited an independent negative prognostic
effect on the predictions of OS and DFS of patients with ccRCC,
adjusted with other parameters. Adding CTR1 expression information
into existing prognostic models, including TNM, SSIGN and UISS,
noticeably enhanced their prognostic power. Finally, two nomograms
were generated by integrating CTR1 expression with other clinical
parameters to predict the OS and DFS of patients with ccRCC. The
c-indexes were 0.805 and 0.787 for OS and DFS, respectively,
revealing an improved prognostic ability, compared with existing
survival models, for the present cohort.
CTR1 is a transmembrane glycoprotein encoded by gene
hCTR1 (SLC31A1), located on chromosome 9q31/32
together with its homolog hCTR2 (SLC31A2) (9). Although previous study has predominantly
emphasized the platinum uptake capability of CTR1, which may
amplify the therapeutic effects of platinum-based chemotherapies in
ovarian and lung cancer (29), its
primary copper regulation ability and downstream signaling still
serve important roles in tumor progression.
CTR1 functions as a stimulator for tumor
angiogenesis based on its copper intake ability and the activation
of the HIF-1α/VEGF pathway by copper (16). Furthermore, evidence indicates that
CTR1 incorporates with HIF-2α (30),
which may be a more important regulator for ccRCC proliferation
(31). In addition, knockdown of CTR1
in human breast cancer cells was identified to inhibit EMT
formation through HIF1-α inhibition (15). The mitogen-activated protein kinase
(MAPK) kinase 1/extracellular signal-regulated kinase (ERK) pathway
can also be regulated by CTR1 through enhancement of ERK1/2
phosphorylation by copper (32), and
the aberrant activation of MAPKs was regarded as an important
mechanism for RCC mechanistic target of rapamycin (mTOR) inhibitor
resistance (33).
Since dysregulation of the VHL/HIF pathway is a
dominant driving force for ccRCC initiation, and evidence suggests
that copper and ceruloplasmin levels are upregulated in ccRCC
(34), it is tempting to speculate
that in the present study, the poor prognostic effect of high CTR1
expression may be associated with the copper level and the HIF
pathway in ccRCC cells. However, this hypothesis remains to be
validated rigorously through further experiments.
Our previous study revealed that the expression of
another copper transporter, CTR2, was decreased in ccRCC compared
with peritumoral tissue, and low tumor CTR2 expression predicted a
reduced OS and DFS time for patients with ccRCC (35). CTR2 is a low affinity copper
transporter and a previous study has identified its opposite role
against CTR1, based on evidence that CTR2 may aid the degradation
of CTR1 into a cleaved form, which imports copper less efficiently
(36). The opposite prognostic roles
of CTR1 and CTR2 in platinum-based chemotherapy resistance also
support this theory (37). However, a
previous study has also suggested that CTR1 is essential to
maintain the stability of CTR2 (38).
The present study revealed that high CTR1 expression indicated a
poor prognosis for patients with ccRCC, which is in contrary to
CTR2. The underlying mechanisms require further study, and whether
CTR1 expression can provide additional prognosis value when
considered with CTR2 expression is under investigation.
For metastatic RCC, antiangiogenic therapies,
including tyrosine kinase and mTOR inhibitors targeting the HIF
pathway, may prolong the patient survival time. Since copper is
also associated with the promotion of tumor angiogenesis and
progression, a depletion of copper may have therapeutic effects. A
phase II clinical trial was performed with tetrathiomolybdate, an
oral copper chelator, for treating patients with metastatic RCC
(39). However, the results were
limited to stable disease for a median of 34 weeks in one-third of
the patients. As CTR1 exhibited a significant prognostic effect in
patients with ccRCC in the present study, future studies should
investigate whether this molecule can identify the sensitivity of
patients with ccRCC to targeted therapies. In addition, treatments
targeting CTR1 and associated molecules may provide a novel
perspective in ccRCC management.
The major limitations of the present study are the
retrospective nature of the study and the relatively small sample
size. A multicenter prospective study is required to validate the
results in a larger population in the future. SSIGN was applied
rather than UISS in the subgroup OS analysis in Fig. 2, as the former was more sensitive in
risk stratification and prognosis prediction in the database
(c-index 0.740 vs. 0.722; Table IV),
although it was initially designed for predicting cancer-specific
survival, defined as the time from nephrectomy to disease-induced
mortality. In addition, the proportion of patients with advanced
ccRCC was markedly smaller in the present cohort compared with
other clinical databases and there were only 2 patients with
positive lymph node metastasis, which may have influenced the
non-significance of N stage in the univariate analysis. In
addition, several HIF-associated molecules, including HIF-1α,
HIF-2α, VEGF and EMT-associated proteins have not been analyzed and
incorporated with CTR1 in the present study. A number of other
survival predictors, including the positive margin rates,
sarcomatoid differentiation, and the diabetes status and body mass
index of patients have not been involved in the multivariate
analysis and merit further study.
In conclusion, the present study indicated that the
high expression of tumor CTR1 is associated with poor survival in
patients with ccRCC. This novel biomarker may be incorporated with
other clinical parameters to form nomograms for the improved
prediction of prognosis.
Acknowledgements
The authors thank Dr Yuan Ji, Dr Jun Hou and Ms
Haiying Zeng (Department of Pathology, Zhongshan Hospital of Fudan
University, Fudan, China) for diagnosis confirmation and technical
assistance, respectively. The present study was funded by grants
from the National Basic Research Program of China (grant no.
2012CB822104), the National Key Projects for Infectious Diseases of
China (grant nos. 2012ZX10002012-007 and 2016ZX10002018-008), the
National Natural Science Foundation of China (grant nos. 31100629,
31270863, 81372755, 31470794, 81401988, 81402082, 81402085,
81471621, 81472227, 81472376, 31570803, 81501999 and 81572352) and
the Program for New Century Excellent Talents in University (grant
no. NCET-13-0146). All study sponsors had no role in the study
design or the collection, analysis and interpretation of data.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lam JS, Shvarts O, Leppert JT, Figlin RA
and Belldegrun AS: Renal cell carcinoma 2005: New frontiers in
staging, prognostication and targeted molecular therapy. J Urol.
173:1853–1862. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Frank I, Blute ML, Cheville JC, Lohse CM,
Weaver AL and Zincke H: An outcome prediction model for patients
with clear cell renal cell carcinoma treated with radical
nephrectomy based on tumor stage, size, grade and necrosis: The
SSIGN score. J Urol. 168:2395–2400. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun M, Shariat SF, Cheng C, Ficarra V,
Murai M, Oudard S, Pantuck AJ, Zigeuner R and Karakiewicz PI:
Prognostic factors and predictive models in renal cell carcinoma: A
contemporary review. Eur Urol. 60:644–661. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Volpe A and Patard JJ: Prognostic factors
in renal cell carcinoma. World J Urol. 28:319–327. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shariat SF and Xylinas E: Biomarkers in
personalised treatment of renal-cell carcinoma. Lancet Oncol.
13:751–752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou B and Gitschier J: hCTR1: A human
gene for copper uptake identified by complementation in yeast. Proc
Natl Acad Sci USA. 94:7481–7486. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee J, Prohaska JR, Dagenais SL, Glover TW
and Thiele DJ: Isolation of a murine copper transporter gene,
tissue specific expression and functional complementation of a
yeast copper transport mutant. Gene. 254:87–96. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu GF: Copper stimulates proliferation of
human endothelial cells under culture. J Cell Biochem. 69:326–335.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Díez M, Arroyo M, Cerdàn FJ, Muñoz M,
Martin MA and Balibrea JL: Serum and tissue trace metal levels in
lung cancer. Oncology. 46:230–234. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuo HW, Chen SF, Wu CC, Chen DR and Lee
JH: Serum and tissue trace elements in patients with breast cancer
in Taiwan. Biol Trace Elem Res. 89:1–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martin F, Linden T, Katschinski DM, Oehme
F, Flamme I, Mukhopadhyay CK, Eckhardt K, Tröger J, Barth S,
Camenisch G and Wenger RH: Copper-dependent activation of
hypoxia-inducible factor (HIF)-1: Implications for ceruloplasmin
regulation. Blood. 105:4613–4619. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li S, Zhang J, Yang H, Wu C, Dang X and
Liu Y: Copper depletion inhibits CoCl2-induced aggressive phenotype
of MCF-7 cells via downregulation of HIF-1 and inhibition of
Snail/Twist-mediated epithelial-mesenchymal transition. Sci Rep.
5:124102015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Narayanan GRBS, Vuyyuru H, Muthuvel B and
Natrajan Konerirajapuram S: CTR1 silencing inhibits angiogenesis by
limiting copper entry into endothelial cells. PLoS One.
8:e719822013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brewer GJ, Dick RD, Grover DK, LeClaire V,
Tseng M, Wicha M, Pienta K, Redman BG, Jahan T, Sondak VK, et al:
Treatment of metastatic cancer with tetrathiomolybdate, an
anticopper, antiangiogenic agent: Phase I study. Clin Cancer Res.
6:1–10. 2000.PubMed/NCBI
|
|
18
|
Escudier B, Porta C, Schmidinger M,
Rioux-Leclercq N, Bex A, Khoo V, Gruenvald V and Horwich A: ESMO
Guidelines Committee: Renal cell carcinoma: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 27
Suppl 5:v58–v68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cohen HT and McGovern FJ: Renal-cell
carcinoma. N Engl J Med. 353:2477–2490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Buccheri G, Ferrigno D and Tamburini M:
Karnofsky and ECOG performance status scoring in lung cancer: A
prospective, longitudinal study of 536 patients from a single
institution. Eur J Cancer. 32:1135–1141. 1996. View Article : Google Scholar
|
|
21
|
Lopez-Beltran A, Bassi P, Pavone-Macaluso
M and Montironi R: Handling and pathology reporting of specimens
with carcinoma of the urinary bladder, ureter, and renal pelvis.
Eur Urol. 45:257–266. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang Y, An H, Xu L, Zhu Y, Yang Y, Lin Z
and Xu J: Systemic inflammation score predicts postoperative
prognosis of patients with clear-cell renal cell carcinoma. Br J
Cancer. 113:626–633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leibovich BC, Blute ML, Cheville JC, Lohse
CM, Frank I, Kwon ED, Weaver AL, Parker AS and Zincke H: Prediction
of progression after radical nephrectomy for patients with clear
cell renal cell carcinoma: A stratification tool for prospective
clinical trials. Cancer. 97:1663–1671. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan D, Xu L, Liu H, Zhang W, Zhu Y, Xu J
and Gu J: Interleukin-11 receptor predicts post-operative clinical
outcome in patients with early-stage clear-cell renal cell
carcinoma. Jpn J Clin Oncol. 45:202–209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu K, Xu L, Zhang L, Lin Z and Hou J: High
Jagged1 expression predicts poor outcome in clear cell renal cell
carcinoma. Jpn J Clin Oncol. 41:411–416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harrell FE Jr, Califf RM, Pryor DB, Lee KL
and Rosati RA: Evaluating the yield of medical tests. JAMA.
247:2543–2546. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Klomp AE, Tops BB, Van Denberg IE, Berger
R and Klomp LW: Biochemical characterization and subcellular
localization of human copper transporter 1 (hCTR1). Biochem J.
364:497–505. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Howell SB, Safaei R, Larson CA and Sailor
MJ: Copper transporters and the cellular pharmacology of the
platinum-containing cancer drugs. Mol Pharmacol. 77:887–894. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pourvali K, Matak P, Latunde-Dada GO,
Solomou S, Mastrogiannaki M, Peyssonnaux C and Sharp PA: Basal
expression of copper transporter 1 in intestinal epithelial cells
is regulated by hypoxia-inducible factor 2α. FEBS Lett.
586:2423–2427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gordan JD, Bertout JA, Hu CJ, Diehl JA and
Simon MC: HIF-2alpha promotes hypoxic cell proliferation by
enhancing c-myc transcriptional activity. Cancer Cell. 11:335–347.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brady DC, Crowe MS, Turski ML, Hobbs GA,
Yao X, Chaikuad A, Knapp S, Xiao K, Campbell SL, Thiele DJ and
Counter CM: Copper is required for oncogenic BRAF signalling and
tumorigenesis. Nature. 509:492–496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bailey ST, Zhou B, Damrauer JS, Krishnan
B, Wilson HL, Smith AM, Li M, Yeh JJ and Kim WY: mTOR inhibition
induces compensatory, therapeutically targetable MEK activation in
renal cell carcinoma. PLoS One. 9:e1044132014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stassar MJ, Devitt G, Brosius M, Rinnab L,
Prang J, Schradin T, Simon J, Petersen S, Kopp-Schneider A and
Zöller M: Identification of human renal cell carcinoma associated
genes by suppression subtractive hybridization. Br J Cancer.
85:1372–1382. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xia Y, Liu L, Long Q, Bai Q, Wang J, Xu J
and Guo J: Decreased expression of CTR2 predicts poor prognosis of
patients with clear cell renal cell carcinoma. Urol Oncol.
34:5.e1–e9. 2016. View Article : Google Scholar
|
|
36
|
Ohrvik H, Nose Y, Wood LK, Kim BE, Gleber
SC, Ralle M and Thiele DJ: Ctr2 regulates biogenesis of a cleaved
form of mammalian Ctr1 metal transporter lacking the copper- and
cisplatin-binding ecto-domain. Proc Natl Acad Sci USA.
110:E4279–E4288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee YY, Choi CH, Do IG, Song SY, Lee W,
Park HS, Song TJ, Kim MK, Kim TJ, Lee JW, et al: Prognostic value
of the copper transporters, CTR1 and CTR2, in patients with ovarian
carcinoma receiving platinum-based chemotherapy. Gynecol Oncol.
122:361–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tsai CY, Liebig JK, Tsigelny IF and Howell
SB: The copper transporter 1 (CTR1) is required to maintain the
stability of copper transporter 2 (CTR2). Metallomics. 7:1477–1487.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Redman BG, Esper P, Pan Q, Dunn RL,
Hussain HK, Chenevert T, Brewer GJ and Merajver SD: Phase II trial
of tetrathiomolybdate in patients with advanced kidney cancer. Clin
Cancer Res. 9:1666–1672. 2003.PubMed/NCBI
|