Introduction
Although the prognosis of patients with axillary
lymph node-negative (ANN) breast cancer was relatively good,
approximately one-third of them experienced disease recurrence
after treatment (1–3). Among recurrence, distant metastasis
accounted for 90% of the mortality causes in these ANN patients.
Angiogenesis was an essential component of the metastatic pathway,
which was elicited and regulated by a number of factors, such as
the extracellular microenvironment and endothelium-associated small
non-coding RNAs, known as microRNAs.
MicroRNAs (miRNA/miR) are small non-coding RNAs
(18–23 bp in size) generated by the consecutive activity of two
RNAseIII enzymes, DROSHA and DICER (4). They regulate gene activity by sequence
specific binding to messenger RNA (mRNA), triggering either
translational repression or RNA degradation. It has been predicted
that mammalian miRs regulated about 30% of all protein-coding genes
(5). miRs have emerged as key
regulators of several cellular processes, including cell
differentiation, proliferation, tumor invasion, metastasis and
angiogenesis (6,7).
miR-10b was detected to be abnormal in the
progression of many tumor types including nasopharyngeal carcinoma
(8), pancreatic cancer (9), and colorectal cancer (10). In breast cancer cells, miR-10b was
considered to be closely correlated with metastatic behavior, and
stimulated in vitro and in vivo tumor invasion
(11). Although breast carcinoma cell
lines have been explored, tissue examination of miR-10b expression
in breast carcinoma has not been reported yet especially in ANN
patients by in situ hybridization (ISH). Moreover, the role
of miR-10b on angiogenesis was limited in previous studies. In
Plummer's study, targeting miR-10b led to significant defects in
angiogenesis-mediated tumor growth in mice. Suppression of miR-10b
led to significant defects in tube number, length and mobilization
in human and murine endothelial cells (7). Accordingly, we sought to determine the
relationship between miR-10b and microvessel density (MVD) in human
breast cancer, whether miR-10b was differentially expressed between
patients with different characteristics, and whether it could serve
as a metastatic marker in ANN breast cancer.
Patients and methods
Study population
We conducted a retrospective review of all
consecutive ANN breast cancer patients between January 1, 2004 and
December 31, 2006 treated in the Tianjin Medical University Cancer
Institute and Hospital (Tianjin, China). A total of 1,265 cases
were collected with primary, operable, invasive carcinoma, without
axillary lymph node and other sites involvement. Among them, a
total of 893 cases were diagnosed as invasive ductal carcinoma
(IDC) based on paraffin-embedded slices after operation. The
pathological stage of tumor was assessed according to the criteria
established by the 7th edition of the American Joint Committee on
Cancer (AJCC) staging manual. Histological grades of the tumors
were designated as I–III according to Elston and Ellis' criterion
(12). Peritumoural vascular invasion
was assessed following the recommendation by Rosen and Obermann
(13).
The follow-up contacts were applied at 3-month
intervals over the first year, 6–12 months for the following 4
years, and then annually. The medical work-up consisted of regular
physical checkups, imaging tests such as chest X-ray, bone scan
and/or ultrasound, to detect recurrence, second primary tumor, or
metastatic disease. According to 5–10 years follow-up, we found
that 65 ANN cases diagnosed as IDC developed distant metastasis, or
even died of the disease, who were defined as ‘poor group’, with
the median follow-up time of 97.8 months for patients alive.
Meanwhile, 70 patients were observed with local recurrence, and the
other 758 patients were on recurrence-free survival fortunately. A
case-control study was designed by stratified sampling method
(14,15) depending on the patients age
(pre-menopause and post-menopause), and the tumor size of the ‘poor
group’. Each case in the poor group was matched by 2 cases from the
758 progression-free patients. A total of 130 patients were
randomly selected and served as control group (‘good group’), with
the median follow-up time of 98.3 months. We also gathered data on
family history, vascular invasion, tumor grade, treatment
application, and recurrence status by chart review.
ISH assay and evaluation of the
staining
ISH assay was carried out with probes for miR-10b
(5′-CACAAATTCGGTTCTACAGGGTA-3′, probe concentration 80 nM) as well
as positive control (U6, hsa/mmu/rno) and negative control
(scramble-miR) purchasing from Exiqon (Vedbek, Denmark). Four
micron paraffin sections fixed to glass slides were baked, dewaxed,
hydrated, washed and then incubated in pepsin solution for 15 min
at 37°C, fixed in 4% paraformaldehyde for 10 min and washed in PBS
buffer twice for 10 min. Prehybridization solution was dropped onto
the slides and reacted (42°C) in a hygro-cabinet to keep them
moist. After 2 h, superfluous prehybridization solution was
discarded and digoxigenin-labeled miR-10b probediluted with
hybridization solution was denatured for 5 min at 95°C.
Hybridization was performed in a humid chamber for 16 h at 45°C.
After hybridization, the parafilms were removed by stringency
washes twice with 2X SSC at 37°C for 5 min, and then the sections
were in 0.5X SSC at 37°C for 15 min, 0.2X SSC at 37°C for 15 min.
Then, the slides were placed in a blocking solution for 30 min at
37°C, and incubated for 1 h at room temperature with monoclonal
anti-Digoxin Biotin Conjugate (Boster Bio, Co., Ltd., Wuhan,
China). After washing in PBS, they were incubated for 20 min with
strept-avidin biotin complex (SABC) at 37°C. After washing again
with PBS, biotinylation peroxydase was added for 20 min at 37°C.
Afterwards, the assay was colored by diaminobenzidine,
counterstained with hematoxylin, dehydrated in a gradient of
alcohols, and mounted.
The positive signal of miR-10b was a brown
precipitate at sites of hybridization, which was evaluated by the
German semiquantitative scoring system combining intensity of
cytoplasmic staining and number of positive cells (16). For each sample, a score was given with
regard to the percentage of positive cells as follows: None (0
point), 1 to 24% of the cells (1 point), 25 to 49% of the cells (2
points), 50 to 74% of the cells (3 points), and 75 to 100% of the
cells (4 points). Another score was given for the intensity of
staining cells as follows: None (0 point), weak staining (1 point),
intermediate staining (2 points), and strong staining (3 points). A
final score was then calculated by multiplying the above two
scores. If the final score was 4 or more, expression was considered
to be positive.
Immunohistochemical (IHC) assay and
evaluation of the staining
IHC stainings were performed on formalin-fixed,
paraffin-embedded samples obtained from the pathology registry.
Primary antibody used in this study included estrogen-receptor (ER;
SP1, 1:200 dilution; Zeta Corp., Sierra Madre, CA, USA),
progesterone-receptor (PR; SP2, 1:200 dilution; Zeta Corp.), human
epidermal growth factor receptor 2 (HER2; CB11, 1:100 dilution;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), Ki67
(K-2, 1:100 dilution; Invitrogen; Thermo Fisher Scientific, Inc.),
and CD34 (EP88, 1:150 dilution; Abcam, Cambridge, MA, USA). The
immunostaining was scored double blindly by two pathologists, who
were blinded to patients' clinicopathologic characteristics and
outcomes. For each antibody, the location of immunoreactivity,
percentage of stained cells, and intensity were determined. ER and
PR were categorized as negative (<1%) and positive (≥1%), in
accordance with the guidelines (17).
For HER2, the IHC score was assigned according to the American
Society of Clinical Oncology/College of American Pathologists
(ASCO/CAP) guideline (18).
HER2-positive cases were defined as IHC score of 3+ or IHC score of
2+ plus fluorescent ISH with amplification ratio ≥2.0. Ki67
status was expressed in terms of percentage of positive cells, with
a threshold of 14% of positive cells (19). The criteria for subtype classification
were as follows (20): ‘luminal
A-like’ (ER positive and PR ≥20% and HER2 negative and Ki-67
<14%), ‘luminal B-like (HER2 negative)’ (ER positive and HER2
negative and at least one of: Ki-67 ≥14% or PR <20%), ‘luminal
B-like (HER2 positive)’ (ER and HER2 positive, with any Ki67 and
PR), ‘HER2 positive’ (ER and PR negative and HER2 positive),
‘triple negative’ (ER and PR negative and HER2 negative).
MVD was determined by the number of microvessels
positive for CD34, whose label could be observed in the cytoplasm
of endothelial cells. MVD scoring was based on a modification of
the method described by Weidner et al (21) in which large microvessels and any
single brown-staining endothelial cell clearly separated from
adjacent microvessels, tumor cells, and other connective tissue
elements were considered a single and countable microvessel. The
entire tumor section was scanned at low magnification (×40) to
identify the area of highest vessel density (hot spot). The five
most prominent vascular areas within the tumor mass were chosen.
Microvessels in each hot spot were counted in a single ×200 field
and the average counts of the 5 fields were recorded and defined.
Vessel lumen was not required for identification of a microvessel.
Single cell or cell clusters were counted. Branching structures
were counted as single vessel. Large vessels with thick muscular
walls or with lumina greater than 50 µm were excluded from the
count. Discrepant cases were reviewed by the pathologists group,
and the consensus results were used for the analysis.
Statistical analysis
The SPSS software (version 17.0 for Windows) was
used to carry out the statistical analyses. Normal distribution of
the age and MVD total scores was assessed using the
Kolmogorov-Smirnov test. Comparison of MVD between different groups
was performed by ANOVA test. Correlations between two variables
were evaluated by Spearman's rank-correlation test. The correlation
analyses between groups were examined by the χ2 test.
The multivariate analysis used a logistic multiple regression
model. A two-sided P<0.05 was considered statistically
significant in all of the analyses except subdividing RxC table in
χ2 test.
Results
Patient cohort
For 65 cases with ‘poor group’, all of them
developed distant metastases (including bone, lung, liver, brain,
bone marrow, or other organs). These recurrent sites were detected
and proven through roentgenography, sonography, computed
tomography, radioisotope scanning, magnetic resonance imaging, or
puncture biopsy. A total of 45 cases among them developed
cancer-specific death. Clinicopathologic features were presented in
Table I. Among all patients,
significant differences were found in peritumoural vascular
invasion, grade and molecular subtype between ‘poor’ and ‘good’
groups (P<0.05). Invasion of peritumoural vascular vessels and
poor differentiation were strictly correlated with recurrence
(P=0.017 and P=0.031, respectively). ‘Luminal A-like’ subtype
accounted for about 36.9% in the ‘good group’, but only 16.9% in
the ‘poor group’. Among the ‘good group’, 21.5% were ‘luminal
B-like (HER2 negative)’, 10.0% ‘luminal B-like (HER2 positive)’,
8.5% ‘HER2 positive’, and 23.1% ‘triple negative’. While among the
‘poor group’, the percentage of the five subtypes were 16.9, 20.0,
12.3, 18.5, and 32.3%, respectively. Distribution of subtypes
between groups was different with statistical significance
(P=0.026). As mentioned in the ‘Patients and methods’, stratified
sampling method was applied, so no significant difference was found
in age, tumor size, menopausal status (P>0.05). Treatment
including chemotherapy, endocrine therapy and radiotherapy didn't
show statistical differences between the two groups
(P>0.05).
 | Table I.Characteristic of the ANN patients
with poor and good prognosis. |
Table I.
Characteristic of the ANN patients
with poor and good prognosis.
| Characteristic | Total | Poor prognosis | % | Good prognosis | % | P-value |
|---|
| All | 195 | 65 |
| 130 |
|
|
| Age at diagnosis,
years |
|
|
|
|
|
|
|
<35 | 5 | 2 | 3.1 | 3 | 2.3 |
|
| 35 to
<49 | 94 | 31 | 47.7 | 63 | 48.5 |
|
|
>50 | 96 | 32 | 33.3 | 64 | 49.2 | >0.05 |
| Mean ± SD |
| 54.0±10.2 |
| 54.1±10.1 |
| >0.05 |
| Menopausal
status |
|
|
|
|
|
|
|
Premenopausal | 92 | 30 | 46.2 | 62 | 48.0 |
|
|
Postmenopausal | 103 | 35 | 53.8 | 68 | 52.0 | >0.05 |
| Tumor size |
|
|
|
|
|
|
|
T1a+T1b | 6 | 2 | 3.1 | 4 | 3.1 |
|
|
T1c | 40 | 14 | 21.5 | 26 | 20.0 |
|
| T2 | 137 | 45 | 69.2 | 92 | 70.8 |
|
| T3 | 12 | 4 | 6.2 | 8 | 6.1 | >0.05 |
| Family history |
|
|
|
|
|
|
|
Yes | 29 | 9 | 13.8 | 20 | 15.4 |
|
| No | 166 | 56 | 86.2 | 110 | 84.6 | >0.05 |
| Peritumoural
vascular invasion |
|
|
|
|
|
|
|
Absent | 176 | 54 | 83.1 | 123 | 94.6 |
|
|
Present | 19 | 11 | 16.9 | 7 | 5.4 | 0.009 |
| Tumor grade |
|
|
|
|
|
|
|
Low | 26 | 5 | 7.7 | 21 | 16.2 |
|
|
Intermediate | 128 | 40 | 61.5 | 88 | 67.7 |
|
|
High | 41 | 20 | 30.8 | 21 | 16.2 | 0.031 |
| Molecular
subtype |
|
|
|
|
|
|
|
‘Luminal A-like’ | 59 | 11 | 16.9 | 48 | 36.9 |
|
|
‘Luminal B-like (HER2
negative)’ | 41 | 13 | 20.0 | 28 | 21.5 |
|
|
‘Luminal B-like (HER2
positive)’ | 21 | 8 | 12.3 | 13 | 10.0 |
|
| ‘HER2
positive’ | 23 | 12 | 18.5 | 11 | 8.5 |
|
| ‘Triple
negative’ | 51 | 21 | 32.3 | 30 | 23.1 | 0.026 |
| Chemotherapy |
|
|
|
|
|
|
|
Yes | 152 | 52 | 80.0 | 100 | 76.9 |
|
| No | 43 | 13 | 20.0 | 30 | 23.1 | >0.05 |
| Radiotherapy |
|
|
|
|
|
|
|
Yes | 48 | 15 | 23.1 | 33 | 25.4 |
|
| No | 147 | 50 | 76.9 | 97 | 74.6 | >0.05 |
| Endocrine
therapy |
|
|
|
|
|
|
| No. of
ER positive subtype | 121 | 32 | 49.2 | 89 | 68.5 |
|
|
Yes | 108 | 28 | 43.1 | 80 | 61.5 |
|
| No | 13 | 4 | 6.1 | 9 | 7.0 | >0.05 |
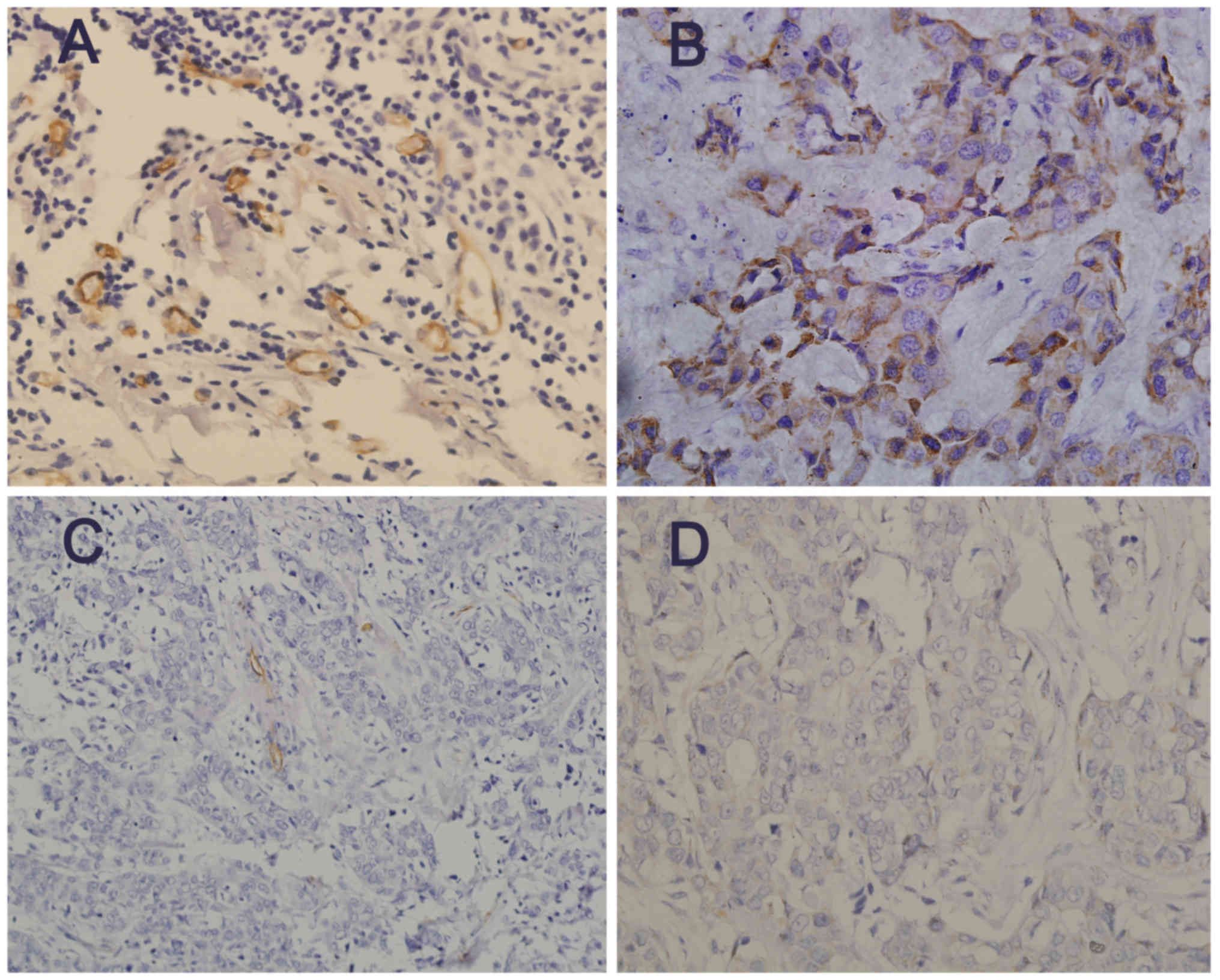
IHC staining of CD34 and ISH of miR-10b in breast
cancer tissues were illustrated in Fig.
1. Microvessels were located predominantly in the stroma
surrounding the tumor nests. Among all cases, greater percentage of
cases displayed cytoplasm staining for miR-10b in ‘poor group’
(73.8%) than in ‘good group’ (51.5%, P=0.003), and MVD count was
higher in ‘poor’ cases than in ‘good’ cases (P<0.001; Table II).
 | Table II.MVD (detected by IHC of CD34) and
miR-10b expression (detected by ISH) in ANN breast cancer with poor
and good prognosis. |
Table II.
MVD (detected by IHC of CD34) and
miR-10b expression (detected by ISH) in ANN breast cancer with poor
and good prognosis.
|
|
| MVD | miR-10b |
|---|
|
|
|
|
|
|---|
| Groups | Number | Mean ± SD |
P-valuea | Positive
number | Positive rate
(%) | P-value |
|---|
| Good prognosis | 130 | 30.81±10.68 | <0.001 | 67 | 51.5 | 0.003 |
| Poor prognosis | 65 | 47.82±16.30 |
| 48 | 73.8 |
|
Univariate analysis showed that clinicopathologic
indicators such as tumor grade, peritumoural vascular invasion and
molecular subtype were correlated with distant metastasis
(P<0.05). To further assess the independence of the prognostic
value of miR-10b expression, these indicators were analyzed with a
multivariate logistic regression model. As shown in Table III, miR-10b retained independent
prognostic significance (OR 2.575, 95% CI, 1.323–5.012; P=0.005)
along with MVD (OR 3.067, 95% CI, 1.605–5.861; P=0.001) which were
adjusted by peritumoural vascular invasion. The significant
influence on distant metastasis by histological grade and subtypes
was not confirmed in the logistic analysis.
 | Table III.Logistic multiple regression analysis
comparing patients with poor and good prognosis. |
Table III.
Logistic multiple regression analysis
comparing patients with poor and good prognosis.
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|---|
| Variables | β | SE | Sig (P) | Expβ | Lower | Lower |
|---|
| MVDa | 1.121 | 0.330 | 0.001 | 3.067 | 1.605 | 5.861 |
|
miR-10ba | 0.946 | 0.340 | 0.005 | 2.575 | 1.323 | 5.012 |
Among all of the 195 patients with ANN disease, high
MVD was associated closely with poor histological grade, existence
of vascular invasion, as well as ‘triple negative’ subtype
(P<0.05), but did not show any relationship with age and tumor
size (Table IV). Meanwhile, we found
that the expression of miR-10b was significantly associated with
tumor grade, tumor size and subtypes (P<0.05). The subdividing
RxC table in χ2 test was used to evaluate the
association between two indicators. miR-10b positive rate was
upregulated from grade 1 (53.8%) and grade 2 (53.9%) to grade 3
(78.0%, χ2=7.517, P=0.006), and its expression between
T2 and T1a+1b (χ2=5.702, P=0.017) remained significant
difference. Meanwhile, among patients with ‘HER2 positive’ subtype,
miR-10b expression rate was 78.3% (18 of 23 cases), whereas only 16
of 59 cases (27.1%) with ‘luminal A-like’ subtype. Expression of
miR-10b rose from ‘luminal A-like’ to ‘luminal B-like (HER2
negative)’ to ‘triple negative’ to ‘luminal B-like (HER2 positive)’
to ‘HER2 positive’, and the differences between ‘luminal A-like’
and ‘luminal B-like (HER2 negative)’ (χ2=14.81,
P<0.001), ‘luminal A-like’ and ‘triple negative’
(χ2=24.58, P<0.001), ‘luminal A-like’ and ‘luminal
B-like (HER2 positive)’ (χ2=15.53, P<0.001), ‘luminal
A-like’ and ‘HER2 positive’ (χ2=17.84, P<0.001) were
significant. However, the expression of miR-10b between other
subtypes did not demonstrate statistical difference
(P>0.005).
 | Table IV.MVD (detected by IHC of CD34) and
miR-10b expression (detected by ISH) with clinicopathologic
features of 195 cases. |
Table IV.
MVD (detected by IHC of CD34) and
miR-10b expression (detected by ISH) with clinicopathologic
features of 195 cases.
|
|
| MVD | miR-10b |
|---|
|
|
|
|
|
|---|
| Indicator | Number | Mean ± SD | P-value | Positive
number | Positive rate |
P-valuec | r |
P-valued |
|---|
| Age |
|
|
|
|
|
|
|
|
<35 | 5 | 37.60±8.26 |
| 3 | 60.0 |
|
|
|
| 35 to
<49 | 94 | 39.2±14.50 |
| 63 | 67.0 |
|
|
|
|
>50 | 96 | 37.1±14.61 | 0.796a | 49 | 51.0 | 0.057 | −0.146 | 0.042 |
| Grade |
|
|
|
|
|
|
|
|
| Grade
1 | 26 | 35.4±12.32 |
| 14 | 53.8 |
|
|
|
| Grade
2 | 128 | 37.3±14.58 |
| 69 | 53.9 |
|
|
|
| Grade
3 | 41 | 43.8±14.00 |
0.023a | 32 | 78.0 | 0.020 | 0.168 | 0.019 |
| Tumor size |
|
|
|
|
|
|
|
|
|
T1a+T1b | 5 | 31.20±13.52 |
| 0 | 0 |
|
|
|
|
T1c | 51 | 37.67±12.94 |
| 26 | 51.0 |
|
|
|
| T2 | 126 | 38.44±15.2 |
| 80 | 63.5 |
|
|
|
| T3 | 13 | 44.77±10.22 | 0.271a | 9 | 69.2 | 0.015 | 0.175 | 0.014 |
| Peritumoural
vascular invasion |
|
|
|
|
|
|
|
|
|
Absent | 175 | 36.92±13.37 |
| 101 | 57.7 |
|
|
|
|
Present | 20 | 52.10±16.20 |
<0.001a | 14 | 70.0 | 0.290 | 0.076 | 0.292 |
| Molecular
subtype |
|
|
|
|
|
|
|
|
|
‘Luminal A-like’ | 59 | 32.69±10.86 |
| 16 | 27.1 |
|
|
|
|
‘Luminal B-like (HER2
negative)’ | 41 | 36.78±13.03 |
| 27 | 65.9 |
|
|
|
|
‘Luminal B-like (HER2
positive)’ | 21 | 38.29±15.00 |
| 16 | 76.2 |
|
|
|
| ‘HER2
positive’ | 23 | 38.74±11.78 |
| 18 | 78.3 |
|
|
|
| ‘Triple
negative’ | 51 | 46.49±16.52 |
<0.001b | 38 | 74.5 |
<0.001 | 0.011 | 0.875 |
| miR-10b
expression |
|
|
|
|
|
|
|
|
|
Positive | 115 | 42.40±14.05 |
|
|
|
|
|
|
|
Negative | 80 | 32.84±13.02 |
<0.001a |
|
| MVD |
0.370 |
<0.001 |
miR-10b expression was positively associated with
the MVD count (r=0.370, P<0.001), tumor grade
(r=0.168, P=0.019) and tumor size (r=0.175, P=0.014),
and negatively associated with age (r=−0.146, P=0.042). No
significant associations were identified between the expression of
miR-10b and vascular invasion (r=0.076, P=0.292), or
intrinsic subtypes (r=0.011, P=0.875; Table IV).
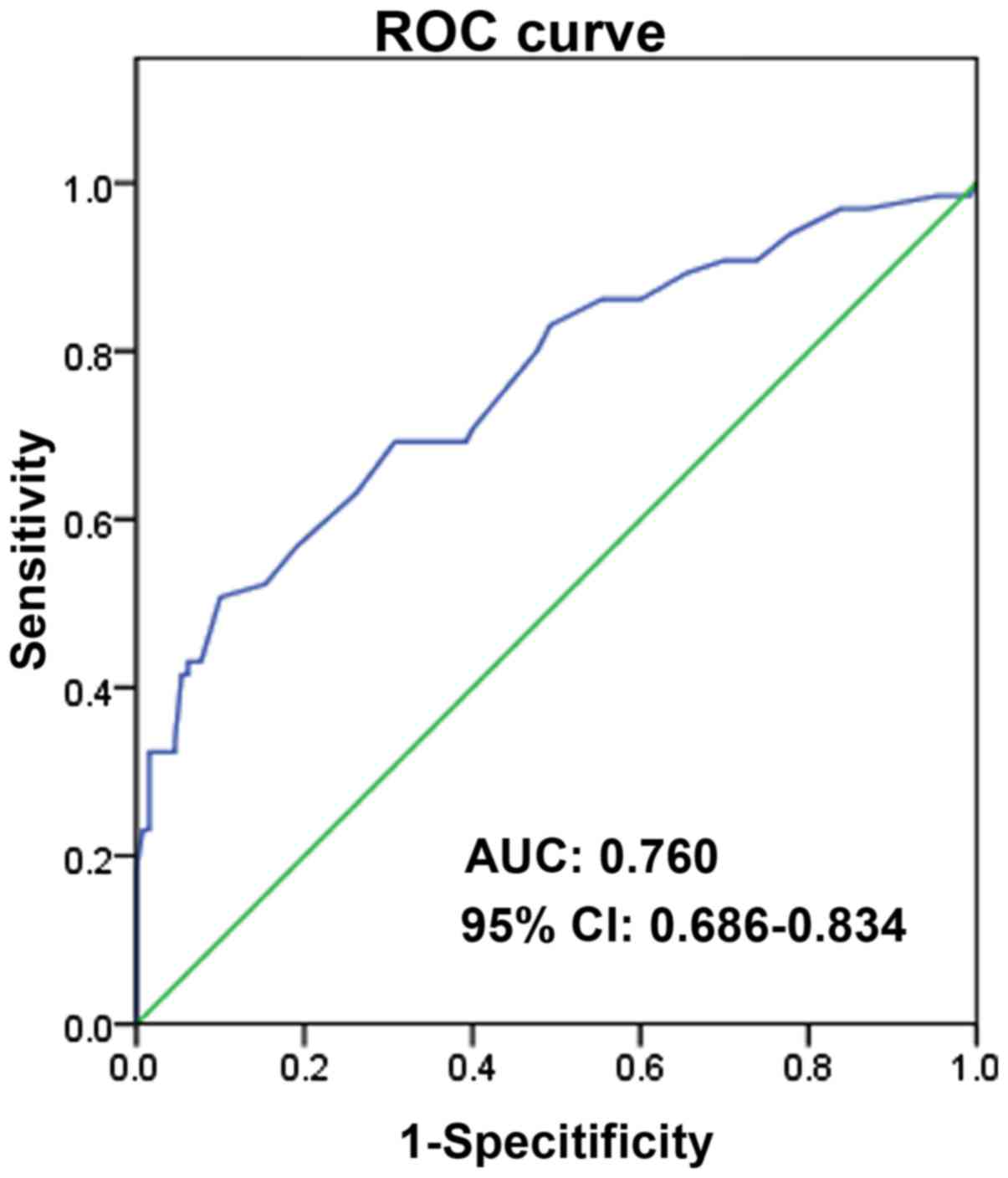
In addition, ROC analysis was performed. As
illustrated in Fig. 2, the area under
the curve (AUC) is 0.760, 95% CI, 0.686–0.834, P<0.0001. And the
sensitivity and specificity of MVD for recurrence of breast cancer
is 0.508 and 0.900, respectively.
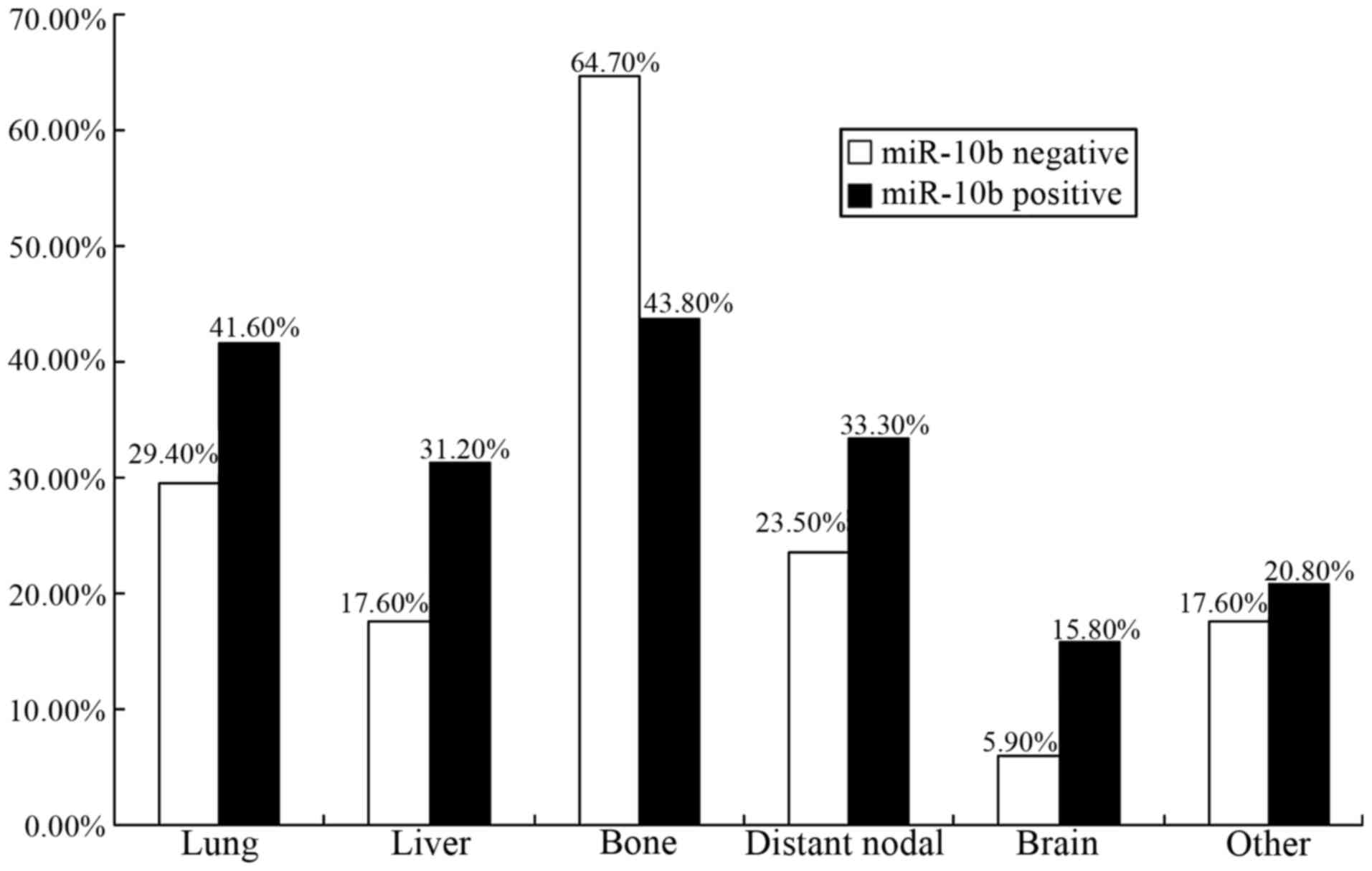
In the 97.8-month interval, patterns of distant
metastasis divided by expression status of miR-10b for cases in the
‘poor group’ were demonstrated in Fig.
3. Bone was the predominant site of metastasis for the two
groups (64.7% in miR-10b-negative tumors and 43.8% in
miR-10b-positive tumors, respectively). Meanwhile, occurrence rate
of distant metastasis was 29.4% (5/17) in lung, 17.6% (3/17) in
liver, 23.5% (4/17) in distant lymph node, 5.9% (1/17) in brain,
and 17.6% (3/17) in other sites for those miR-10b-negative tumors.
Among the patients with miR-10b-positive tumors, the rates of
metastasis in lung (41.6%), liver (31.2%), distant lymph node
(33.3%), brain (15.8%), and other sites (20.8%) were higher to some
extent. It seemed that visceral metastasis was more frequently
found in the miR-10b positive group.
Discussion
miR-10b is known to function as an oncogene in
various kinds of cancers. It has been reported that miR-10b is
highly expressed in metastatic cancer cells propagated as cell
lines as well as in metastatic breast cancer patients (11). One of the representative targets of
miR-10b was HOXD10, whose inhibition led to activation of the
pro-metastatic gene RHOC (22).
Recently, in addition to its metastasis-promoting role, the
angiogenesis effect of miR-10b has been reported. Shen et al
demonstrated a role of miR-10b in regulating angiogenesis
specifically in response to thrombin through down regulation of
HOXD10 (23).
We undertook the study to explore the role of
miR-10b in tumor prognosis, its significance in different subtypes,
and the relationship between miR-10b and MVD in ANN breast cancer.
Our data clearly showed that miR-10b expression was a common
feature of invasive breast carcinoma in a Chinese population. Among
195 interpretable cases with IDC, 59.0% were positive for miR-10b
expression. Besides, miR-10b was found in 51.5% patients with
recurrence-free and 73.8% with metastasis breast cancer cases
(P=0.003; Table II). The presented
data showed that patients whose tumors did not express miR-10b had
more favorable prognosis than those with positive expression.
Accordingly, the miR-10b may play a critical role in breast cancer
metastasis.
This study further indicated that positive miR-10b
expression contributed to poorer differentiation and larger tumor
size. miR-10b expression was gradually upregulated from grade 1
(53.8%) and grade 2 (53.9%), to grade 3 (78.0%), and from T1c
(51.0%) to T2 (63.5%) and T3 (69.2%). In addition, miR-10b
expression was decreased in ‘luminal A-like’ subtype, which was
confirmed to represent excellent prognosis (24). A logistic multiple regression analysis
was also carried out and indicated miR-10b could predict prognosis
independently. These potentially suggested the promotion effect of
miR-10b on breast tumor growth and metastasis. It has been reported
that miR-10b expression was correlated with high-grade malignancy
in various cancer types (25). Zhao
et al (26) measured serum
miR-10b in 122 breast cancer patients with or without bone
metastases. The results showed that serum miR-10b concentrations
were significantly higher in patients with bone metastases than
those without metastasis. This finding was more or less consistent
with our research. Furthermore, in a study by Ma et al
(11), to determine whether miR-10b
expression correlated with clinical outcome in patients, they
measured its levels in primary tumor samples from 23 breast cancer
patients by RT-PCR. When compared with normal breast tissue,
miR-10b expression level was lower in all of the breast carcinomas
from metastasis-free patients (5/5). In contrast, 50% of the
metastasis-positive patients (9/18) had elevated miR-10b levels in
their primary tumors (P=0.03), which were also in consonance with
our study. In research of breast cancer cells, the mechanism of
metastasis seemed to be associated with expression levels of the
epithelial-mesenchymal transition (EMT)-inducing and
metastasis-promoting transcription factor Twist. In mammary
epithelial cells and breast carcinoma cells, miR-10b could directly
suppress the translation of HOXD10, an mRNA encoding a
transcriptional repressor that inhibited expression of several
genes such as RHOC, urokinase plasminogen activator receptor,
α3-integrin, and MT1-MMP, which were involved in cell migration and
extracellular matrix remodeling (11).
Besides the expression of miR-10b, we also measured
MVD count labeled by CD34 of all patients. MVD represented the
degree of tumor neovascularization, and it had been found to be an
important indicator of malignant behavior in breast cancer
(27). Among all the patients, the
mean value of MVD was 38.48±14.40, which was different with
statistical significance between patients in ‘good group’
(30.81±10.68) and patients in ‘poor group’ (47.82±16.30,
P<0.001). Furthermore, results indicated that the patients with
good differentiation, absence of vascular invasion or ‘luminal
A-like’ subtype were correlated with low MVD count, consistent with
the recent studies (28,29). The logistic multiple regression
analysis also detected MVD as an independent prognostic marker for
metastasis.
Moreover, the presented data showed a significantly
positive correlation between miR-10b and MVD count (r=0.370,
P<0.001), indicating that miR-10b expression may be a potential
marker in predicting angiogenesis. In the study by Plummer et
al (30), genome-wide deep
sequencing of small RNAs revealed miR-10b was significantly
upregulated in tumor vasculature. Moreover, suppression of miR-10b
led to significant defects in tube number and length, as well as
mobilization in human and murine endothelial cells, which was in
agreement with an observation showing that miR-10b regulated HOXD10
in microvessels (23). In a study by
Myers et al, HOXD10 was demonstrated to maintain a
nonangiogenic state in the endothelium. Sustained expression of
HOXD10 impaired endothelial cell migration and blocked angiogenesis
induced by basic fibroblast growth factor and vascular endothelial
growth factor in the chick chorioallantoic membrane in vivo.
HOXD10-overexpressing human endothelial cells also failed to form
new vessels when implanted into immunocompromised mice (31). These findings strongly argued for
miR-10b's role of promoting angiogenesis. In the future we plan to
look into the role of angiogenesis of miR-10b in breast cancer
cells and animals, and to explore the possibility of miR-10b as a
new target of anti-angiogenesis therapeutic intervention for breast
cancer.
In this study, histological grade and subtype were
associated with metastasis in univariate analysis. The association,
however, was no longer statistically significant after adjusting
for other variables. It is probably due to a small sample size and
some factors covering and concealing the function of others as a
result of a complex relationship.
In conclusion, the study suggested that miR-10b
expression was associated with breast cancer aggressive behavior,
distant metastasis, angiogenesis and poor prognosis. Further
exploration of the clinical significance of miR-10b expression in
breast cancer requires larger sample size and prospective studies
to confirm the findings of this study. The development of new
strategies which can suppress miR-10b will probably lead to
clinical benefits not only through anti-metastasis but also through
anti-angiogenesis.
Acknowledgements
This study was financially supported by National
Science Foundation of China (81172532, 81470119). The authors
acknowledge the technological assistance of Mrs. Xiumin Ding and
Mrs. Ying Wang.
Glossary
Abbreviations
Abbreviations:
|
ANN
|
axillary lymph node-negative
|
|
MVD
|
microvessel density
|
|
ISH
|
in situ hybridization
|
|
IDC
|
invasive ductal carcinoma
|
|
IHC
|
immunohistochemical
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
AJCC
|
American Joint Committee on Cancer
|
|
ER
|
estrogen-receptor
|
|
PR
|
progesterone-receptor
|
References
|
1
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Metzger-Filho O, Sun Z, Viale G, Price KN,
Crivellari D, Snyder RD, Gelber RD, Castiglione-Gertsch M, Coates
AS, Goldhirsch A and Cardoso F: Patterns of recurrence and outcome
according to breast cancer subtypes in lymph node-negative disease:
Results from international breast cancer study group trials VIII
and IX. J Clin Oncol. 31:3083–3090. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fisher CS, Cole DJ, Mitas M, Garrett-Meyer
E, Metcalf JS, Gillanders WE, Mikhitarian K, Urist MM, Mann GB,
Doherty G, et al: Molecular detection of micrometastatic breast
cancer in histopathology-negative axillary lymph nodes fails to
predict breast cancer recurrence: A final analysis of a prospective
multi-institutional cohort study. Ann Surg Oncol. 17 Suppl
3:S312–S320. 2010. View Article : Google Scholar
|
|
4
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010.PubMed/NCBI
|
|
5
|
Taft RJ, Glazov EA, Cloonan N, Simons C,
Stephen S, Faulkner GJ, Lassmann T, Forrest AR, Grimmond SM,
Schroder K, et al: Tiny RNAs associated with transcription start
sites in animals. Nat Genet. 41:572–578. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goswami RS, Waldron L, Machado J, Cervigne
NK, Xu W, Reis PP, Bailey DJ, Jurisica I, Crump MR and Kamel-Reid
S: Optimization and analysis of a quantitative real-time PCR-based
technique to determine microRNA expression in formalin-fixed
paraffin-embedded samples. BMC Biotechnol. 10:472010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Plummer PN, Freeman R, Taft RJ, Vider J,
Sax M, Umer BA, Gao D, Johns C, Mattick JS, Wilton SD, et al:
MicroRNAs regulate tumor angiogenesis modulated by endothelial
progenitor cells. Cancer Res. 73:341–352. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li G, Wu Z, Peng Y, Liu X, Lu J, Wang L,
Pan Q, He ML and Li XP: MicroRNA-10b induced by Epstein-Barr
virus-encoded latent membrane protein-1 promotes the metastasis of
human nasopharyngeal carcinoma cells. Cancer Lett. 299:29–36. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Setoyama T, Zhang X, Natsugoe S and Calin
GA: microRNA-10b: A new marker or the marker of pancreatic ductal
adenocarcinoma? Clin Cancer Res. 17:5527–5529. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang H, Liu J, Chen Y, Ma C, Li B and Hao
T: Up-regulation of mir-10b predicate advanced clinicopathological
features and liver metastasis in colorectal cancer. Cancer Med.
5:2932–2941. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rosen PP and Oberman HA: Tumors of the
mammary gland. In: Atlas of Tumor Pathology. 3rd series, fascicle
7Armed Forces Institute of Pathology. Washington, DC: 1993
|
|
14
|
Niu Y, Fu X, Lv A, Fan Y and Wang Y:
Potential markers predicting distant metastasis in axillary
node-negative breast carcinoma. Int J Cancer. 98:754–760. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Wang F, Wang L, Wang W, Liu B,
Liu J, Chen M, He Q, Liao Y, Yu X, et al: Prevalence of chronic
kidney disease in China: A cross-sectional survey. Lancet.
379:815–822. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koo CL, Kok LF, Lee MY, Wu TS, Cheng YW,
Hsu JD, Ruan A, Chao KC and Han CP: Scoring mechanisms of p16INK4a
immunohistochemistry based on either independent nucleic stain or
mixed cytoplasmic with nucleic expression can significantly signal
to distinguish between endocervical and endometrial adenocarcinomas
in a tissue microarray study. J Transl Med. 7:252009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hammond ME, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American society of clinical oncology/college of
American pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. J Clin Oncol. 28:2784–2795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wolff AC, Hammond ME, Schwartz JN, Hagerty
KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer
A, et al: American society of clinical oncology/college of American
pathologists guideline recommendations for human epidermal growth
factor receptor 2 testing in breast cancer. J Clin Oncol.
25:118–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheang MC, Chia SK, Voduc D, Gao D, Leung
S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al: Ki67
index, HER2 status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ: Panel members:
Personalizing the treatment of women with early breast cancer:
Highlights of the st Gallen International Expert consensus on the
primary therapy of early breast cancer. Ann Oncol. 24:2206–2223.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weidner N, Folkman J, Pozza F, Bevilacqua
P, Allred EN, Moore DH, Meli S and Gasparini G: Tumor angiogenesis:
A new significant and independent prognostic indicator in
early-stage breast carcinoma. J Natl Cancer Inst. 84:1875–1887.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma L: Role of miR-10b in breast cancer
metastasis. Breast Cancer Res. 12:2102010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen X, Fang J, Lv X, Pei Z, Wang Y, Jiang
S and Ding K: Heparin impairs angiogenesis through inhibition of
microRNA-10b. J Biol Chem. 286:26616–26627. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van der Hage JA, Mieog JS, van de Velde
CJ, Putter H, Bartelink H and van de Vijver MJ: Impact of
established prognostic factors and molecular subtype in very young
breast cancer patients: Pooled analysis of four EORTC randomized
controlled trials. Breast Cancer Res. 13:R682011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baffa R, Fassan M, Volinia S, O'Hara B,
Liu CG, Palazzo JP, Gardiman M, Rugge M, Gomella LG, Croce CM and
Rosenberg A: MicroRNA expression profiling of human metastatic
cancers identifies cancer gene targets. J Pathol. 219:214–221.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao FL, Hu GD, Wang XF, Zhang XH, Zhang
YK and Yu ZS: Serum overexpression of microRNA-10b in patients with
bone metastatic primary breast cancer. J Int Med Res. 40:859–866.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Keyhani E, Muhammadnejad A, Behjati F,
Sirati F, Khodadadi F, Karimlou M, Moghaddam FA and Pazhoomand R:
Angiogenesis markers in breast cancer-potentially useful tools for
priority setting of anti-angiogenic agents. Asian Pac J Cancer
Prev. 14:7651–7656. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Niemiec J, Adamczyk A, Ambicka A,
Mucha-Małecka A, Wysocki W, Mituś J and Ryś J: Lymphangiogenesis
assessed using three methods is related to tumour grade, breast
cancer subtype and expression of basal marker. Pol J Pathol.
63:165–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muhammadnejad S, Muhammadnejad A, Haddadi
M, Oghabian MA, Mohagheghi MA, Tirgari F, Sadeghi-Fazel F and
Amanpour S: Correlation of microvessel density with nuclear
pleomorphism, mitotic count and vascular invasion in breast and
prostate cancers at preclinical and clinical levels. Asian Pac J
Cancer Prev. 14:63–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Plummer PN, Freeman R, Taft RJ, Vider J,
Sax M, Umer BA, Gao D, Johns C, Mattick JS, Wilton SD, et al:
MicroRNAs regulate tumor angiogenesis modulated by endothelial
progenitor cells. Cancer Res. 73:341–352. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Myers C, Charboneau A, Cheung I, Hanks D
and Boudreau N: Sustained expression of homeobox D10 inhibits
angiogenesis. Am J Pathol. 161:2099–2109. 2002. View Article : Google Scholar : PubMed/NCBI
|