Introduction
Hepatocellular carcinoma (HCC) is the most common
type of malignancy worldwide and has been a serious worldwide
public health problem (1). In China,
the mortality rate induced by HCC ranks highest, particularly in
economically underdeveloped areas (2–4).
Metastasis remains the primary cause of HCC-associated mortality.
It was reported that >60% of HCC cases had encountered
metastasis at the time of diagnosis (5–7). The
five-year survival rate of patients with HCC and metastasis is
markedly higher compared with patients with HCC without metastasis
(8). Investigations into the
molecular mechanism underlying metastasis are important for
understanding HCC.
Epithelial-mesenchymal transition (EMT), a
biological process during whereby epithelial cells transform into
mesenchymal cells under a specific program, serves an important
role in numerous physiological and pathological processes,
including embryogenesis, organ development, tissue repair, organ
fibrosis, and tumor metastasis (9,10). In
epithelial malignancies, tumor cells acquire potent migratory and
invasive abilities, transfer to a different site via the blood, and
form further tumor metastasis through mesenchymal-epithelial
transition (MET) (11–13). The mechanism underlying EMT in a
number of solid tumors has been investigated extensively, including
HCC (14,15). However, the prevention and control of
HCC metastasis in clinics requires further study. Further
investigations targeting the regulation of the mechanism underlying
EMT progression of HCC are required.
T-cell immunoglobulin and mucin domain-containing
molecule 3 (TIM-3), a novel participant in HCC progression, has
recently been reported to regulate the biological behaviors of HCC.
Tumor-derived TIM-3+ cluster of differentiation (CD)4 T
cells were revealed to suppress the proliferation of autologous
CD8+ T cells in vitro significantly, compared
with tumor-derived TIM-3− CD4 T cells, suggesting the
regulatory role of TIM-3 for T cells in human hepatocellular,
cervical, colorectal and ovarian carcinomas (16). Furthermore, the impact of TIM-3 on
hepatitis B virus (HBV) infection progression has been assessed,
and genetic variants of TIM-3 were demonstrated to serve important
roles in the disease progression of HBV infection (17,18). TIM-3
is also involved in the pathogenesis of human osteosarcoma, and
TIM-3-triggered tumor cells have been observed to acquire the
characteristics of aggressive EMT, indicating the possible role of
TIM-3 in EMT occurrence (19).
However, to the best of our knowledge, no study has been performed
regarding the function of TIM-3 in the EMT progression of HCC.
The present study focused on the role of TIM-3 in
EMT progression of HCC based on cultured SMMC-7721 cells. TIM-3
short interfering (si) RNA and TIM-3 lentiviral activation
particles were applied to alter the TIM-3 expression level, and EMT
biomarkers, including epithelial (E)-cadherin, neuronal
(N)-cadherin, matrix metallopeptidase-9 (MMP-9), Twist 1, Slug,
Snail and Smad were analyzed. The results of the present study
revealed that the migratory and invasive ability of SMMC-7721 cells
was positively associated with the expression level of TIM-3. The
EMT biomarkers all changed accordingly with the TIM-3 expression
level, trending to EMT occurrence in TIM-3 upregulated cells. It
was concluded that TIM-3 serves an important role in the metastasis
of HCC and the underlying mechanism is associated with EMT
occurrence. The results of the present study suggest that TIM-3 may
be a potential inducer of EMT and promote the metastasis of
HCC.
Materials and methods
Materials
The human HCC SMMC-7721 cell line was obtained from
the American Type Culture Collection (Manassas, VA, USA). Cell
culture medium Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS) and 0.02% EDTA-trypsin digestion were all
obtained from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The primary antibodies anti-TIM-3 and anti-β-actin supplied
by OriGene Technologies, Inc. (Rockville, MD, USA); TIM-3 siRNA
(human; h), TIM-3 lentiviral activation particles (h), primary
antibodies anti-E-cadherin, anti-N-cadherin, anti-MMP-9, anti-Twist
1, anti-Slug, anti-Snail and anti-Smad were all purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Anti-β-actin
antibody was obtained from OriGene Technologies, Inc. All primers
were designed by the PrimerBank (http://pga.mgh.harvard.edu/primerbank/) and
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China).
Quantitative polymerase chain reaction (qPCR) reagents were
supplied by Thermo Fisher Scientific, Inc. All others reagents,
mainly including Chemiluminescence Western Blotting kit, crystal
violet dye, QuantiPro™ BCA Assay Kit and SYBR-Green, were purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Cell culture
The SMMC-7721 human HCC cells were cultured in DMEM
supplemented with 10% FBS, 100 U/ml penicillin and 100 mg
streptomycin. The conditions of the incubator were 37°C and
saturated humidity with 5% CO2. Cells passage was
performed in 0.02% EDTA-trypsin digestion when fully
integrated.
Interference or activation of TIM-3
expression
Interference or activation of TIM-3 expression were
performed according to the manufacturer's instructions (Santa Cruz
Biotechnology, Inc.). In brief, for TIM-3 interference, TIM-3 siRNA
(10 µM, 3 µl) was added to confluent cells for 6–8 h at 37°C. For
activation of TIM-3, 5 µl TIM-3 lentiviral activation particles (h)
was added to the medium of confluent cells for 6–8 h at 37°C.
Subsequently, cells were further cultured in updated growth medium
(10% FBS-DMEM) for 48 h for further detections at 37°C.
Migration assay
Transwell™ chambers were used to analyze the
migration of SMMC-7721 cells. In brief, 600–700 µl DMEM without FBS
was added to the lower chamber of each well at room temperature and
then co-cultured with inoculated cells at 37°C for 24 h.
Subsequently, cells were seeded in the upper chamber at an
appropriate density (5.0×104/ml) in 100 µl 10% FBS-DMEM.
Following 24 h incubation at 37°C, the chamber was removed and
fixed with 95% alcohol for 15 min at room temperature following
cleaning cells of the upper chamber using a cotton swab.
Subsequently, the chamber was immersed in crystal violet dye for
staining for 15 min at room temperature. After being thoroughly
washed in PBS, migrated cells were observed under a phase contrast
microscope. Analyses of each group were repeated in three
replicates and all experiments were repeated three times. Cell
numbers from five random fields was determined to represent the
migration ability of each group.
The invasion ability detection
To assess invasion, each well was first coated with
80 µg Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) overnight
at 4°C. Subsequently, protocols similar to the migration assay were
performed under the same conditions. The cell density was
5.0×103 cells/well. A total of five random fields were
selected and cell numbers was determined to represent the invasion
ability of each group. All experiments were repeated three
times.
qPCR and western blotting
Proteins were extracted using RIPA lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China) in ice for
30–45 min, during which, tubes were agitated on an oscillator for 1
min every 5–10 min. The proteins were quantified using a BCA kit
(Sigma-Aldrich; Merck KGaA). Total mRNA was extracted from the
control, TIM-3 activation and TIM-3 siRNA groups respectively,
using TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.). qPCR and western blotting were performed as common (20,21). For
qPCR detection, cDNA was firstly obtained based on reverse
transcription from total mRNA. SYBR Green (1X; Sigma-Aldrich; Merck
KGaA) was used as fluorophore. The qPCR reaction was performed
under the following conditions: 95°C denaturing for 5 min, followed
by 36 cycles each of 95°C 40 sec, 58°C 30 sec and 72°C 60 sec. For
Western blotting, 40 µg of total protein was loaded in each lane,
12.5% SDS-PAGE gels were used to detect each protein, and 5%
skimmed milk was used as blocking reagent to block the unspecific
proteins in each membrane for 1 h at room temperature.
Polyvinylidene difluoride (PVDF) membranes (0.45 µm) were applied.
The dilutions of the primary antibodies for TIM-3 (TA-807034;
OriGene Technologies, Inc.; 1:1,000) and β-actin (TA-09; OriGene
Technologies, Inc.; 1:2,500) were used. Primary antibodies from
Santa Cruz Biotechnology, Inc. for E-cadherin (sc-71008),
N-cadherin (sc-59987), MMP-9 (sc-21733), Twist 1 (sc-81417), Slug
(sc-166476), Snail (sc-271977), Smad (sc-7965) were all 1:200. They
were incubated with PVDF membrane for 30–60 min at room temperature
and then incubated overnight at 4°C. The secondary goat-anti-mouse
horseradish peroxidase (HRP)-conjugated antibody and goat-anti-rat
HRP-conjugated antibody were both from OriGene Technologies, Inc.,
incubated with the PVDF membrane at room temperature for 1 h at
1:5,000 dilutions. Enhanced chemiluminescence (EMD Millipore,
Billerica, MA, USA) was used as visualization reagent, by mixing by
equal solution A and B and incubating for 2–3 min in the dark. The
target bands of TIM-3, E-cadherin, N-cadherin, MMP-9, Twist 1,
Slug, Snail, Smad and β-actin were visualized at apparent molecular
weights of 33, 120, 130, 92, 28, 30, 29, 52–56 and 43 kDa,
respectively. Semi-quantitative analysis was performed by ImageJ
software 1.41o (National Institutes of Health, Bethesda, MD, USA).
β-actin was used as the control. For qPCR, Ct value was compared to
represent the transcription level of each molecule, using the
2−ΔΔCq method (22). Each
experiment was repeated three times. The primers are presented in
Table I.
 | Table I.Sequence information of the primers
used for quantitative polymerase chain reaction. |
Table I.
Sequence information of the primers
used for quantitative polymerase chain reaction.
| Genes | Sequences | Product size
(bp) | Gene Bank |
|---|
| E-cadherin | Forward:
5′-CGAGAGCTACACGTTCACGG-3′ | 119 | NM 004360.3 |
|
| Reverse:
5′-GGGTGTCGAGGGAAAAATAGG-3′ |
|
|
| N-cadherin | Forward:
5′-AGCCAACCTTAACTGAGGAGT-3′ | 136 | NM_001792.3 |
|
| Reverse:
5′-GGCAAGTTGATTGGAGGGATG-3′ |
|
|
| MMP-9 | Forward:
5′-GGGACGCAGACATCGTCATC-3′ | 139 | NM_004994.2 |
|
| Reverse:
5′-TCGTCATCGTCGAAATGGGC-3′ |
|
|
| Twist 1 | Forward:
5′-GAGACTCTGGAGCTGGATAACT-3′ | 100 | NM_000474.3 |
|
| Reverse:
5′-CGTCTGGGAATCACTGTCCA-3′ |
|
|
| Slug | Forward:
5′-TGTGACAAGGAATATGTGAGCC-3′ | 203 | NM_003068.4 |
|
| Reverse:
5′-TGAGCCCTCAGATTTGACCTG-3 |
|
|
| Snail | Forward:
5′-TCGGAAGCCTAACTACAGCGA-3′ | 140 | NM_005985.3 |
|
| Reverse:
5′-AGATGAGCATTGGCAGCGAG-3′ |
|
|
| Smad | Forward:
5′-AGAGACTTCTTGGGTGGAAACA-3′ | 157 | NM_001003688.1 and
NM_005900.2 |
|
| Reverse:
5′-ATGGTGACACAGTTACTCGGT-3′ |
|
|
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using SPSS software package, version 16.0 (SPSS,
Inc., Chicago, IL, USA). Comparisons between two groups were
analyzed using a t-test. Comparisons of datasets containing
multiple groups (three or more) were performed using analysis of
variance and Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Monitoring of TIM-3 expression level
under TIM-3 siRNA and TIM-3 lentiviral activation particles in
SMMC-7721 cells
To investigate the role of TIM-3 in the metastasis
of HCC, TIM-3 siRNA and TIM-3 lentiviral activation particles were
applied in cultured SMMC-7721 cells to regulate the expression
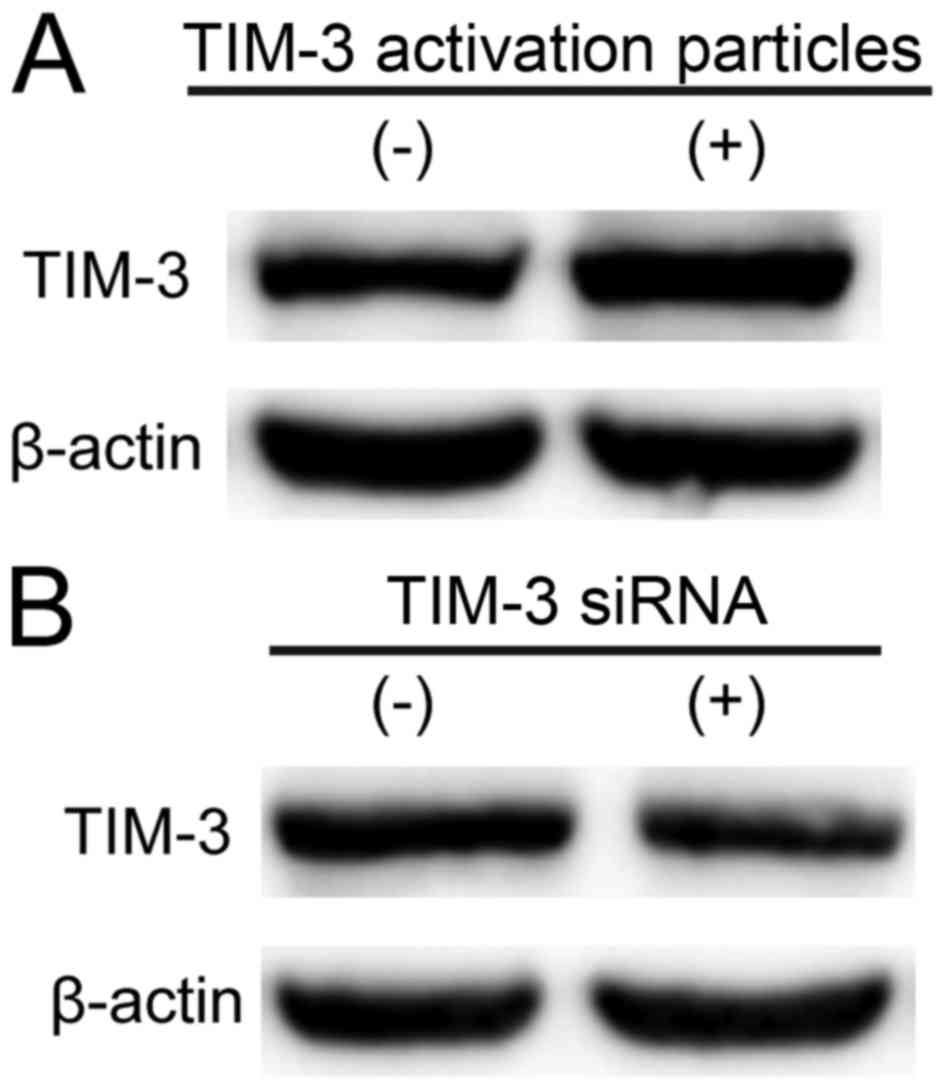
level of TIM-3. As presented in Fig.
1, TIM-3 was upregulated markedly by TIM-3 lentiviral
activation particles (5 µl; Fig. 1A)
and downregulated markedly in SMMC-7721 cells transfected with
TIM-3 siRNA (10 µM, 3 µl; Fig. 1B).
This result suggested that TIM-3 lentiviral activation particles (5
µl) and TIM-3 siRNA (10 µM, 3 µl) upregulated, and downregulated
TIM-3 expression levels, respectively.
Alteration of TIM-3 expression level
affects the migration and invasion of SMMC-7721 cells
As presented in Fig.
2A, in the migration and invasion assays, more TIM-3
overexpressed cells and fewer cells transfected with TIM-3 siRNA
passed through the filter compared with the control group. A total
of five random fields were selected and the number of cells in each
group was determined. Taking the relative number of migrated and
invaded cells in control group as 100, the migrated and invaded
number of cells in the TIM-3 upregulated group was 157±10 and
179±11, and the number in the TIM-3 interference group was 61±8 and
34±7, respectively (Fig. 2B).
Significant differences were revealed in the migration and invasion
abilities of the cells compared with the control (P<0.05).
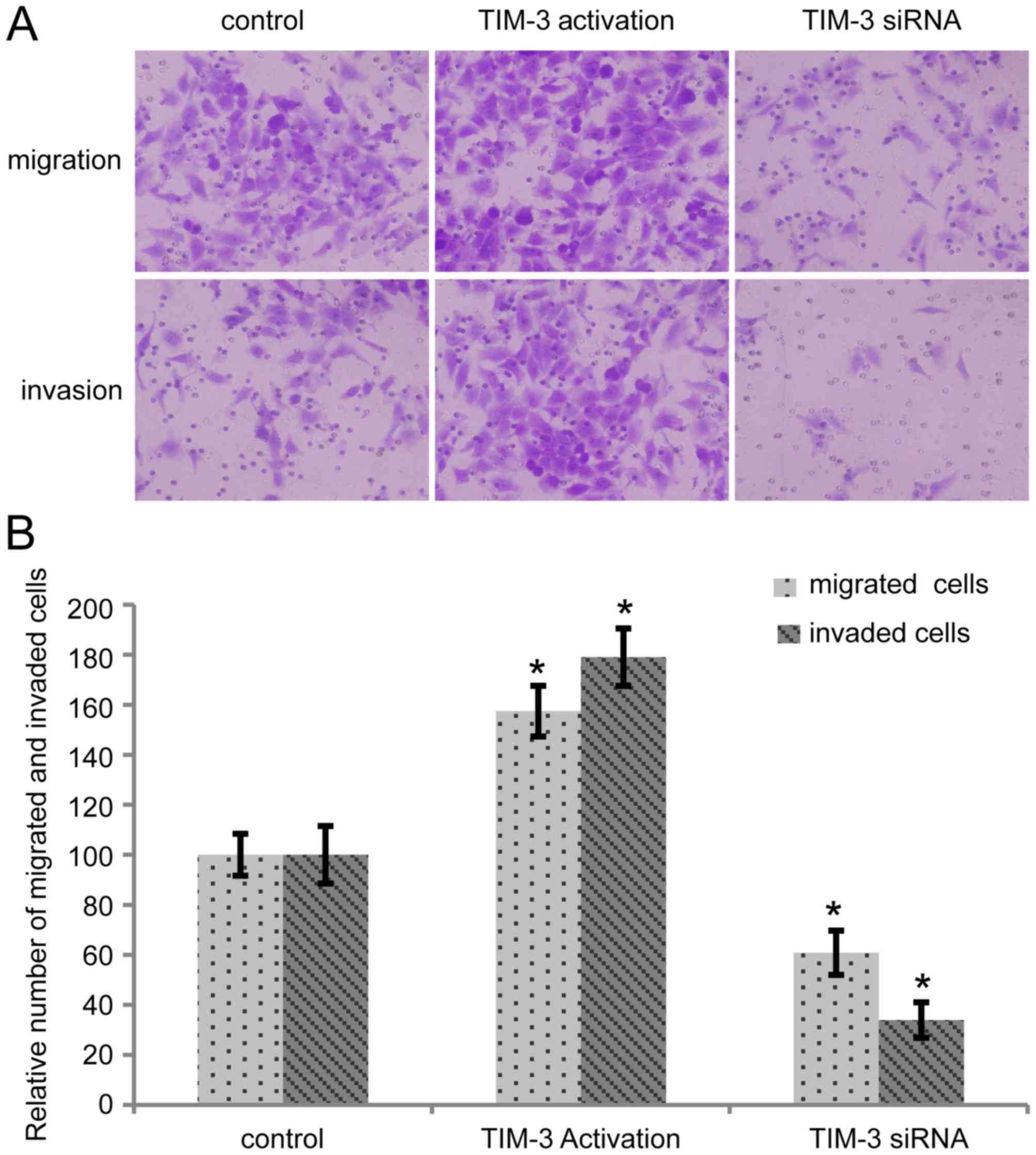 | Figure 2.Alteration in TIM-3 expression level
affects migration and invasion of SMMC-7721 cells (magnification,
×200). (A) Migration and invasion abilities were detected by
Transwell and Matrigel assays in control cells, cells transfected
with TIM-3 activation particles (5 µl), and cells transfected with
TIM-3 siRNA (10 µM, 3 µl). (B) Five random fields were selected,
and the number of cells in each group was evaluated. Compared with
the control, TIM-3-overexpressed cells revealed significantly
higher migration and invasion abilities, which were reversed in
TIM-3 downregulated cells (*P<0.05). TIM-3, T-cell
immunoglobulin mucin-3; siRNA, short interfering RNA; MMP-9, matrix
metallopeptidase-9. |
Alteration of TIM-3 expression level
influences cell morphology of SMMC-7721 cells
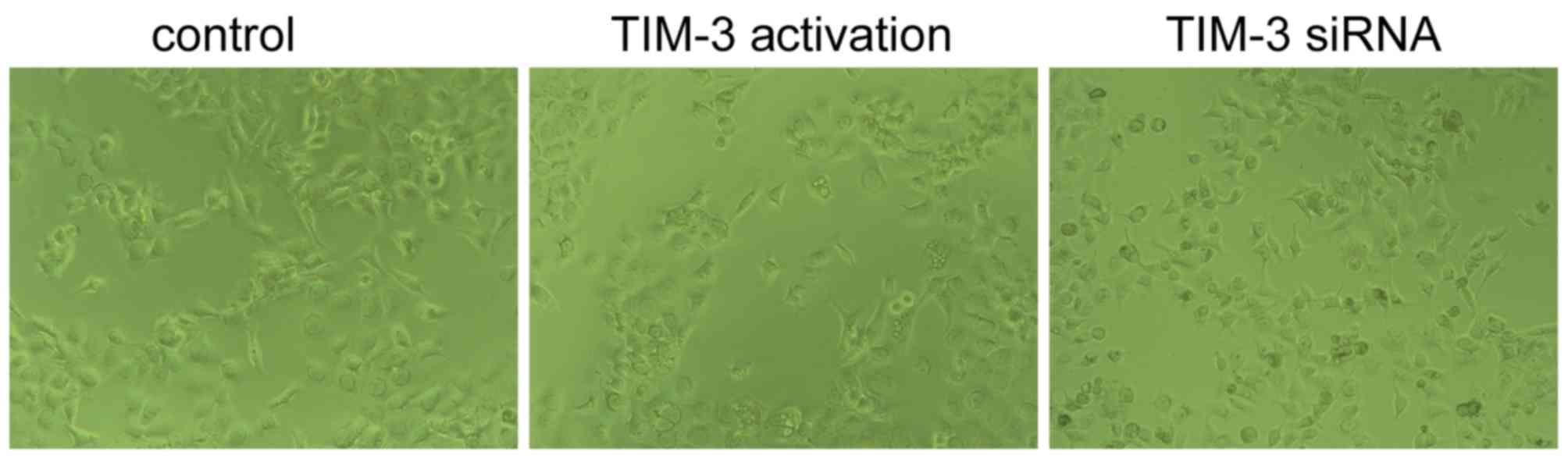
As presented in Fig.
3, cells transfected with TIM-3 activation particles (5 µl) a
more spindle-like morphology and connections between cells were
fewer, which was previously revealed to be more conducive of
migration and invasion (23).
Simultaneously, SMMC-7721 cells transfected with TIM-3 siRNA (10
µM, 3 µl) demonstrated epithelial and the adhesion showed stronger,
with more antennas, indicating a lower aggressive type of
cancer.
Alteration in TIM-3 expression levels
affects the transcription of EMT biomarkers in SMMC-7721 cells
EMT occurrence was indicated by the reduced
expression level of epithelial cell markers, with E-cadherin being
the most apparent, and increased expression levels of mesenchymal
cell markers, including N-cadherin, MMP, Twist 1, Snail, Slug and
Smad. In order to investigate the effect of TIM-3 in EMT occurrence
of HCC, the present study designed numerous specific primers
targeted to E-cadherin, N-cadherin, MMP, Twist 1, Snail, Slug and
Smad. qPCR was performed. Fig. 4
presents the results, with reduced E-cadherin expression levels and
increased expression levels of N-cadherin, MMP, Twist 1, Snail,
Slug and Smad in SMMC-7721 cells transfected with TIM-3 activation
particles (5 µl; Fig. 4A).
Downregulation of TIM-3 based on TIM-3 siRNA (10 µM, 3 µl) revealed
the opposite changes for all the aforementioned molecules (Fig. 4B). Each molecular was analyzed more
than three times. The differences between control and TIM-3
activation (Fig. 4A) or between
control and TIM-3 siRNA (Fig. 4B)
were all significant for the expression of E-cadherin, N-cadherin,
MMP, Twist 1, Snail, Slug and Smad, respectively (*P<0.05).
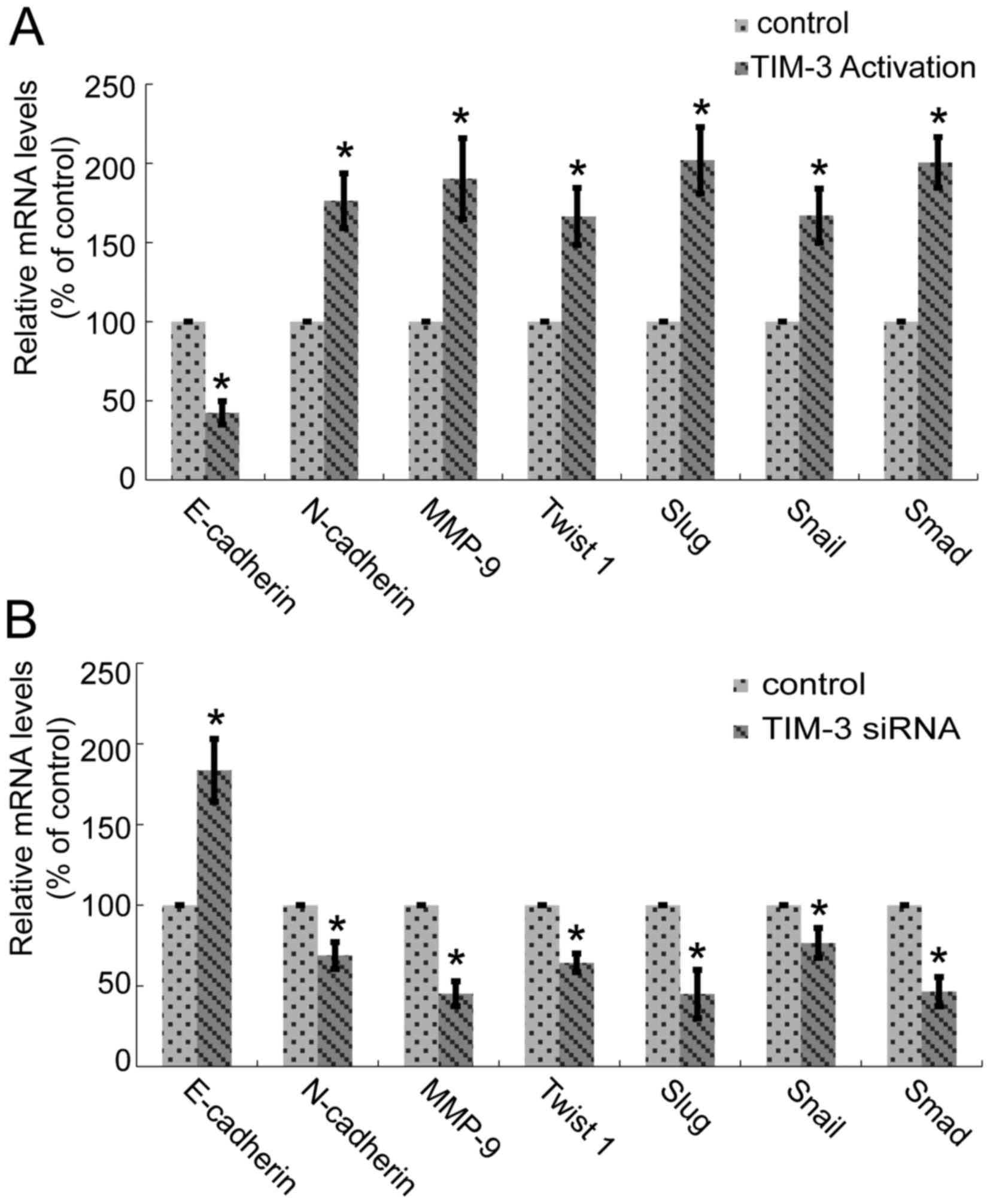 | Figure 4.Alteration in TIM-3 expression level
affects the transcription of epithelial-mesenchymal transition
biomarkers of SMMC-7721 cells. (A) In TIM-3-overexpressed cells,
the expression levels of E-cadherin were reduced, and N-cadherin,
MMP-9, Twist 1, Snail, Slug and Smad were increased (*P<0.05).
(B) Downregulation of TIM-3 increased the mRNA expression levels of
E-cadherin, and decreased the N-cadherin, MMP-9, Twist 1, Snail,
Slug and Smad mRNA expression levels (*P<0.05). TIM-3, T-cell
immunoglobulin mucin-3; MMP-9, matrix metallopeptidase-9; E,
epithelial; N, neuronal. |
Expressions of EMT biomarkers are
regulated by TIM-3 in SMMC-7721 cells
As presented in Fig.
5, the results demonstrated were consistent with the results of
mRNA detections in Fig. 4.
Overexpression of TIM-3 reduced E-cadherin expression levels, and
increased the expression levels of N-cadherin, MMP, Twist 1, Snail,
Slug and Smad (Fig. 5A).
Downregulation of TIM-3 revealed overexpression of E-cadherin and
downregulation of N-cadherin, MMP, Twist 1, Snail, Slug and Smad
(Fig. 5B). Each experiment was
repeated three times. Semi-quantitative analysis using ImageJ
software revealed significance in the TIM-3-upregulated (Fig. 5C) and TIM-3-interference groups,
compared with the control (Fig. 5D;
P<0.05).
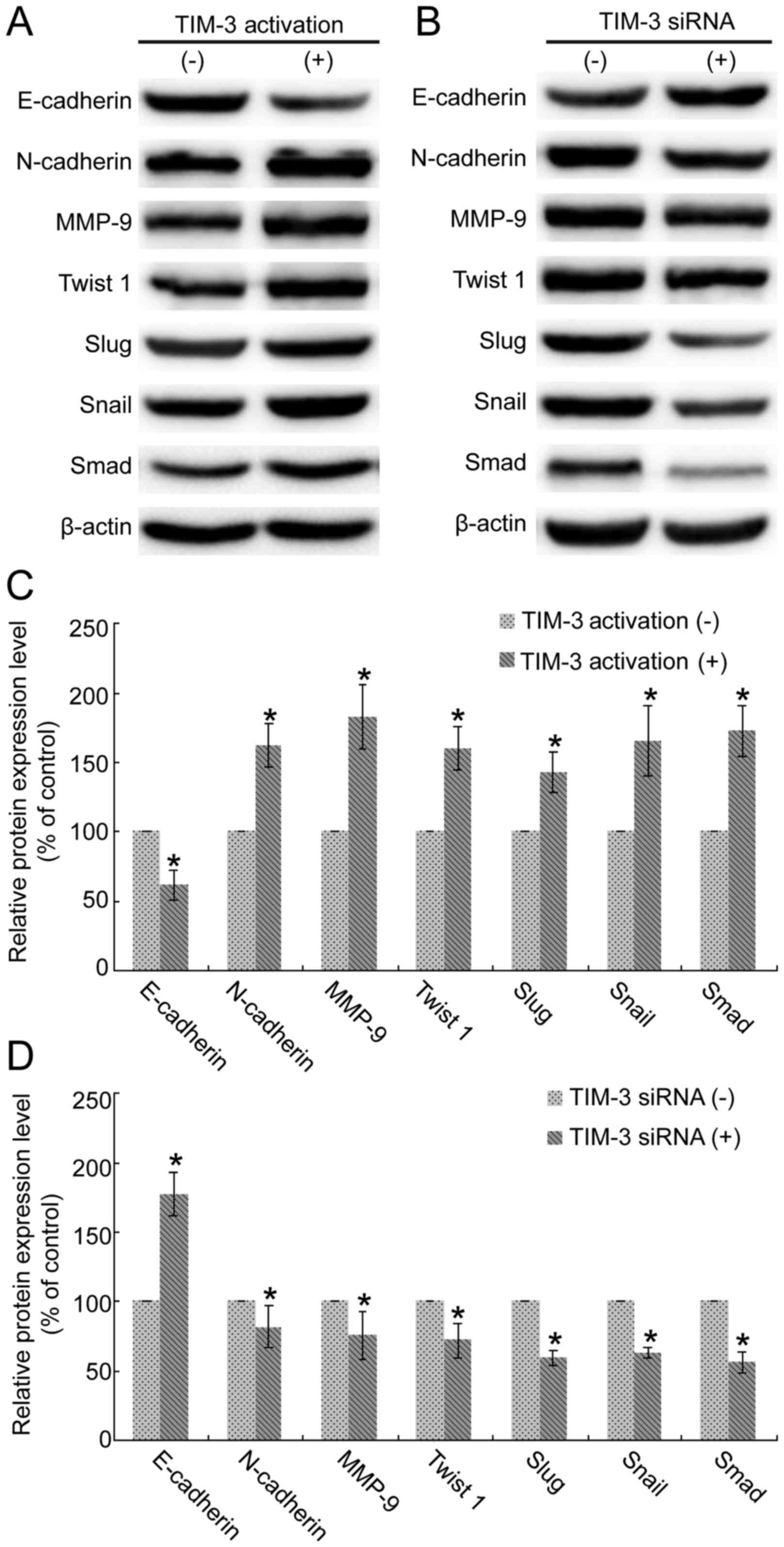 | Figure 5.Alteration in TIM-3 expression level
affects the translation of epithelial-mesenchymal transition
biomarkers in SMMC-7721 cells. (A) Overexpression of TIM-3 reduced
E-cadherin expression level and downregulated expression levels of
N-cadherin, MMP-9, Twist 1, Snail, Slug and Smad. (B)
Downregulation of TIM-3 increased E-cadherin and reduced
N-cadherin, MMP-9, Twist 1, Snail, Slug and Smad expression levels.
(C) Quantitative analysis based on ImageJ software revealed the
significant downregulation of E-cadherin and upregulations of
N-cadherin, MMP-9, Twist 1, Snail, Slug and Smad under TIM-3
overexpression (*P<0.05). (D) Quantitative analysis based on
ImageJ software demonstrated the significant upregulation of
E-cadherin and downregulations of N-cadherin, MMP-9, Twist 1,
Snail, Slug and Smad under TIM-3 siRNA (*P<0.05). TIM-3, T-cell
immunoglobulin mucin-3; MMP-9, matrix metallopeptidase-9; siRNA,
short interfering RNA; E, epithelial; N, neuronal. |
Discussion
Although certain previous studies have investigated
the regulation of HCC metastasis (24–26), the
prevention and control of HCC metastasis in clinics requires
further study. Further studies investigating the regulation of HCC
metastasis are required. The present study explored the role of
TIM-3 in EMT the occurrence of HCC and aimed to provide specific
signs for HCC metastasis. The results demonstrated that alteration
of TIM-3 expression levels correlated positively with EMT
occurrence, and the migration and invasion ability of SMMC-7721
cells, suggesting that TIM-3 may be a potential inducer of EMT and
further promote the metastasis of HCC.
Certain regulating factors in HCC metastasis have
been previously reported, including microRNA, oncogenes and tumor
suppressor genes (27). The
inactivation of tumor suppressor genes is considered to be
essential in HCC progression. TIM-3 is a type of suppressive
molecule located at the T cell surface, and can induce T cell
depletion in cancer and chronic viral infections. TIM-3 has been
studied mostly as an immunotherapeutic target in various types of
cancer, including HCC. Cooperation or interaction of TIM-3 and
programmed cell death-1 has been suggested to be more relevant than
either molecule alone to immune dysfunction in HCC in chronic HBV
infections (28). A previous study
investigating the role of TIM-3 in patients with HCC with chronic
hepatitis B virus infection also supports the role of TIM-3 in
persistent HBV infections and HCC development (18). The present study demonstrated the
essential role of TIM-3 in the migration and invasion of HCC by
performing a Transwell assay. Overexpression of TIM-3 promoted the
migration and invasion of SMMC-7721 cells, which was reduced in
TIM-3 interference cells. Previous studies and the present study
indicated the potential function of TIM-3 in HCC metastasis
(29,30); however, the underling molecular
mechanism remains unclear.
EMT is an iconic phenomenon in cancer metastasis,
particularly in epithelial cell-derived cancer, including HCC. A
number of studies have investigated the EMT regulation of HCC
(31,32). For example, transforming growth
factor-β was revealed to be involved in regulating EMT of HCC, and
is the primary inducer of EMT (31).
However, a large number of other factors limit the prevention and
control of EMT, and further metastasis of HCC in the clinic, such
as small nucleolar RNA ACA11 (ACA11) (33), vitamin C (34), free fatty acid (35) and membrane-associated heparan sulfate
proteoglycan Glypican-3 (GPC3) (36).
The present study focused on the possible role of TIM-3 in EMT
occurrence of HCC, by monitoring alterations of cell morphology and
biomarkers of EMT. From the phase contrast microscope, it was
observed that normal HCC cells showed regular polygon and were
connected closely and arranged regularly. When cells encountered
EMT, they were arranged in a loose fashion and intercellular
adhesion among cells was weakened (32). The present study observed the
morphological changes under TIM-3 alteration. Cells with TIM-3
overexpression changed to a spindle-like morphology and connections
between cells were fewer, which contrasted with the effects in
TIM-3 downregulated cells. This morphological change indicated that
alteration of TIM-3 influenced the morphology, and upregulation of
TIM-3 promoted EMT occurrence of SMMC-7721 cells.
A variety of biomarkers have been used to represent
the EMT progress, primarily cell surface markers, cytoskeletal
markers, extracellular matrix proteins and transcriptional factors
(37). The present study investigated
the changes of specific EMT biomarkers, including E-cadherin,
N-cadherin, MMP-9, Twist 1, Slug, Snail and Smad. E-cadherin, a
classic marker of epithelial cells is expressed at low levels in
EMT of numerous types of cancer (38,39).
N-cadherin, a mesenchymal cell marker, has an increased expression
level in EMT. The conversion of E-cadherin to N-cadherin was
previously used to monitor EMT process (40). In the present study, overexpression of
TIM-3 reduced E-cadherin and upregulated N-cadherin, which was in
contrast to the TIM-3 interference group at the transcription, and
translation levels. This result revealed the function of TIM-3 in
HCC EMT.
Snail, Slug, Twist 1 and MMP-9 are also primary
regulators of EMT. Snail is the most well-known E-cadherin
suppressor gene, and a significant expression level of Snail was
reported in HCC, and was associated with occurrence and metastasis
(41). Slug belongs to the same gene
family as Snail and is expressed in the membrane and cytoplasm of
HCC cells. Twist 1, a basic helix-loop-helix protein, may inhibit
the expression of E-cadherin independent of Snail, and enhanced the
expression levels of fibronectin and N-cadherin (42,43). In
the present study, in the TIM-3 upregulated group of SMMC-7721,
Snail, Slug, Twist 1 and MMP-9 had high expression levels,
suggesting TIM-3 may be an inducer of EMT in HCC and serve an
essential role in the induction of EMT in HCC.
Taken together, the results of the present study
provided evidence suggesting the role of TIM-3 expression in
regulating EMT occurrence and further metastasis of HCC. To
substantiate our findings, samples from patients with HCC samples
have been collected. Our future experiments will focus on the
association between TIM-3 expression level and HCC clinical stages.
This will further the knowledge of the mechanisms underlying TIM-3
in HCC metastasis.
References
|
1
|
Zhu RX, Seto WK, Lai CL and Yuen MF:
Epidemiology of hepatocellular carcinoma in the Asia-Pacific
region. Gut Liver. 10:332–339. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ge S and Huang D: Systemic therapies for
hepatocellular carcinoma. Drug Discov Ther. 9:352–362. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Waller LP, Deshpande V and Pyrsopoulos N:
Hepatocellular carcinoma: A comprehensive review. World J Hepatol.
7:2648–2663. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Toy M, Salomon JA, Jiang H, Gui H, Wang H,
Wang J, Richardus JH and Xie Q: Population health impact and
cost-effectiveness of monitoring inactive chronic hepatitis B and
treating eligible patients in Shanghai, China. Hepatology.
60:46–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Wang Y, Zhang D, Liu B and Ou Q:
Comparison of survival and quality of life of hepatectomy and
thrombectomy using total hepatic vascular exclusion and
chemotherapy alone in patients with hepatocellular carcinoma and
tumor thrombi in the inferior vena cava and hepatic vein. Eur J
Gastroenterol Hepatol. 24:186–194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo G, Chao YL, Tang B, Li BS, Xiao YF,
Xie R, Wang SM, Wu YY, Dong H, Liu XD, et al: miR-149 represses
metastasis of hepatocellular carcinoma by targeting
actin-regulatory proteins PPM1F. Oncotarget. 6:37808–37823. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li T, Xie J, Shen C, Cheng D, Shi Y, Wu Z,
Deng X, Chen H, Shen B, Peng C, et al: Amplification of long
noncoding RNA ZFAS1 promotes metastasis in hepatocellular
carcinoma. Cancer Res. 75:3181–3191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimada S, Kamiyama T, Yokoo H, Wakayama
K, Tsuruga Y, Kakisaka T, Kamachi H and Taketomi A:
Clinicopathological characteristics and prognostic factors in young
patients after hepatectomy for hepatocellular carcinoma. World J
Surg Oncol. 11:522013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guan X: Cancer metastases: Challenges and
opportunities. Acta Pharm Sin B. 5:402–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mitra A, Mishra L and Li S: EMT, CTCs and
CSCs in tumor relapse and drug-resistance. Oncotarget.
6:10697–10711. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Su Z, Yang Z, Xu Y, Chen Y and Yu Q:
Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol
Cancer. 14:482015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bao YX, Cao Q, Yang Y, Mao R, Xiao L,
Zhang H, Zhao HR and Wen H: Expression and prognostic significance
of golgiglycoprotein73 (GP73) with epithelial-mesenchymal
transition (EMT) related molecules in hepatocellular carcinoma
(HCC). Diagn Pathol. 8:1972013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang PF, Li KS, Shen YH, Gao PT, Dong ZR,
Cai JB, Zhang C, Huang XY, Tian MX, Hu ZQ, et al: Galectin-1
induces hepatocellular carcinoma EMT and sorafenib resistance by
activating FAK/PI3K/AKT signaling. Cell Death Dis. 7:e22012016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan J, Zhang Y, Zhang JP, Liang J, Li L
and Zheng L: Tim-3 expression defines regulatory T cells in human
tumors. PLoS One. 8:e580062013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao J, Zhang Q, Liao Y, Cai B, Chen J, Li
L and Wang L: Association of T-cell immunoglobulin and mucin
domain-containing molecule 3 (Tim-3) polymorphisms with
susceptibility and disease progression of HBV infection. PLoS One.
9:e982802014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Z, Liu Z, Zhang G, Han Q, Li N, Zhu Q,
Lv Y, Chen J, Xing F, Wang Y, et al: TIM3 gene polymorphisms in
patients with chronic hepatitis B virus infection: Impact on
disease susceptibility and hepatocellular carcinoma traits. Tissue
Antigens. 80:151–157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shang Y, Li Z, Li H, Xia H and Lin Z:
TIM-3 expression in human osteosarcoma: Correlation with the
expression of epithelial-mesenchymal transition-specific
biomarkers. Oncol Lett. 6:490–494. 2013.PubMed/NCBI
|
|
20
|
Wang JC, Wang Z, Fan YX, Si YQ and Wang
JX: DNA methyltransferase 3b silencing affects locus-specific DNA
methylation and inhibits proliferation, migration and invasion in
human hepatocellular carcinoma SMMC-7721 and BEL-7402 cells. Oncol
Lett. 9:2499–2506. 2015.PubMed/NCBI
|
|
21
|
Gui R, Li D, Qi G, Suhad A and Nie X:
Inhibition of Grb2-mediated activation of MAPK signal transduction
suppresses NOR1/CB1954-induced cytotoxicity in the HepG2 cell line.
Oncol Lett. 4:566–570. 2012.PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shan B, Man H, Liu J, Wang L, Zhu T, Ma M,
Xv Z, Chen X, Yang X and Li P: TIM-3 promotes the metastasis of
esophageal squamous cell carcinoma by targeting
epithelial-mesenchymal transition via the Akt/GSK-3β/Snail
signaling pathway. Oncol Rep. 36:1551–1561. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song G, Cao HX, Yao SX and Li CT: Abnormal
expression of WIF1 in hepatocellular carcinoma cells and its
regulating effect on invasion and metastasis factors of TIMP-3 and
caveolin-1 of hepatocellular carcinoma. Asian Pac J Trop Med.
8:958–963. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li WX, Chen LP, Sun MY, Li JT, Liu HZ and
Zhu W: 3′3-Diindolylmethane inhibits migration, invasion and
metastasis of hepatocellular carcinoma by suppressing FAK
signaling. Oncotarget. 6:23776–23792. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao Y, Ruan B, Liu W, Wang J, Yang X,
Zhang Z, Li X, Duan J, Zhang F, Ding R, et al: Knockdown of CD44
inhibits the invasion and metastasis of hepatocellular carcinoma
both in vitro and in vivo by reversing epithelial-mesenchymal
transition. Oncotarget. 6:7828–7837. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feitelson MA, Sun B, Tufan Satiroglu NL,
Liu J, Pan J and Lian Z: Genetic mechanisms of
hepatocarcinogenesis. Oncogene. 21:2593–2604. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Z, Li N, Zhu Q, Zhang G, Han Q, Zhang
P, Xun M, Wang Y, Zeng X, Yang C, et al: Genetic variations of PD1
and TIM3 are differentially and interactively associated with the
development of cirrhosis and HCC in patients with chronic HBV
infection. Infect Genet Evol. 14:240–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Z, Li N, Li F, Zhou Z, Sang J, Chen Y,
Han Q, Lv Y and Liu Z: Immune checkpoint proteins PD-1 and TIM-3
are both highly expressed in liver tissues and correlate with their
gene polymorphisms in patients with HBV-related hepatocellular
carcinoma. Medicine (Baltimore). 95:e57492016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Z, Li N, Li F, Zhou Z, Sang J, Jin Z,
Liu H, Han Q, Lv Y and Liu Z: Genetic polymorphisms of immune
checkpoint proteins PD-1 and TIM-3 are associated with survival of
patients with hepatitis B virus-related hepatocellular carcinoma.
Oncotarget. 7:26168–26180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wittmann P, Grubinger M, Gröger C, Huber
H, Sieghart W, Peck-Radosavljevic M and Mikulits W: Neuropilin-2
induced by transforming growth factor-β augments migration of
hepatocellular carcinoma cells. BMC Cancer. 15:9092015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y and Shang Y: Epigenetic control of
epithelial-to-mesenchymal transition and cancer metastasis. Exp
Cell Res. 319:160–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu L, Zheng J, Chen P, Liu Q and Yuan Y:
Small nucleolar RNA ACA11 promotes proliferation, migration and
invasion in hepatocellular carcinoma by targeting the PI3K/AKT
signaling pathway. Biomed Pharmacother. 90:705–712. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sajadian SO, Tripura C, Samani FS, Ruoss
M, Dooley S, Baharvand H and Nussler AK: Vitamin C enhances
epigenetic modifications induced by 5-azacytidine and cell cycle
arrest in the hepatocellular carcinoma cell lines HLE and Huh7.
Clin Epigenetics. 8:462016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nath A, Li I, Roberts LR and Chan C:
Elevated free fatty acid uptake via CD36 promotes
epithelial-mesenchymal transition in hepatocellular carcinoma. Sci
Rep. 5:147522015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu Y, Liu H, Weng H, Zhang X, Li P, Fan
CL, Li B, Dong PL, Li L, Dooley S, et al: Glypican-3 promotes
epithelial-mesenchymal transition of hepatocellular carcinoma cells
through ERK signaling pathway. Int J Oncol. 46:1275–1285.
2015.PubMed/NCBI
|
|
37
|
Yun SJ and Kim WJ: Role of the
epithelial-mesenchymal transition in bladder cancer: From prognosis
to therapeutic target. Korean J Urol. 54:645–650. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Z, Bu X, Chen H, Wang Q and Sha W:
Bmi-1 promotes the invasion and migration of colon cancer stem
cells through the downregulation of E-cadherin. Int J Mol Med.
38:1199–1207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu CL, Ho JY, Chou SC and Yu DS: miR-429
reverses epithelial-mesenchymal transition by restoring E-cadherin
expression in bladder cancer. Oncotarget. 7:26593–26603. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Grant CM and Kyprianou N: Epithelial
mesenchymal transition (EMT) in prostate growth and tumor
progression. Transl Androl Urol. 2:202–211. 2013.PubMed/NCBI
|
|
41
|
Miyoshi A, Kitajima Y, Kido S, Shimonishi
T, Matsuyama S, Kitahara K and Miyazaki K: Snail accelerates cancer
invasion by upregulating MMP expression and is associated with poor
prognosis of hepatocellular carcinoma. Br J Cancer. 92:252–258.
2005.PubMed/NCBI
|
|
42
|
Behnsawy HM, Miyake H, Harada K and
Fujisawa M: Expression patterns of epithelial-mesenchymal
transition markers in localized prostate cancer: Significance in
clinicopathological outcomes following radical prostatectomy. BJU
Int. 111:30–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
McConkey DJ, Choi W, Marquis L, Martin F,
Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, et al: Role
of epithelial-to-mesenchymal transition (EMT) in drug sensitivity
and metastasis in bladder cancer. Cancer Metastasis Rev.
28:335–344. 2009. View Article : Google Scholar : PubMed/NCBI
|