Introduction
Reactive oxygen species (ROS) include oxygen
molecules (O2), superoxide anion radicals
(O2−), hydroxyl free radicals
(HO−) and hydrogen peroxide
(H2O2). ROS are generated as a result of
single- or multi-electron reductions of oxygen by cellular enzymes
or in the mitochondrial respiratory pathway (1–5). Although
an increase in the level of intracellular ROS leads to oxidative
stress and DNA damage, the effects of ROS are normally balanced by
antioxidants, such as reduced glutathione (GSH), ascorbic acid, and
ureic acid (6). Disruption of the
oxidant-antioxidant balance through alterations to cellular
homeostasis or defective repair of ROS-induced damage is involved
in the pathogenesis of several diseases (7). In particular, it can be the primary
trigger and/or mediator of carcinogenesis by contributing to the
initiation of cellular malignancy and the progression of cancer
(8–10).
Furthermore, it is known that anticancer drugs
induce oxidative stress in patients with cancer being treated with
chemotherapy. Elevated levels of oxidants in the circulation have
been reported in patients with cancer following administration of
epirubicin (11,12). Epirubicin and doxorubicin possess an
anthracycline skeleton, and generate ROS that lead to DNA damage
and subsequently antitumor activity (13,14).
Vinblastine and vinorelbine belong to the class of vinca alkaloids.
It has been demonstrated that vinorelbine depletes intracellular
GSH and increases intracellular ROS production (15). However, few studies have investigated
the association between oxidative stress and the effects of
anticancer drugs.
Available methods for measuring oxidative stress
inducibility include direct measurement of intracellular ROS,
indirect measurement of the resulting damage to biomolecules,
including proteins, DNA, RNA and lipids, and the detection of
antioxidant levels. In the present study, cell staining with
CellROX® Green reagent was performed in order to
directly measure the intracellular ROS formation (16–18).
CellROX® reagent is a fluorogenic probe used for
measuring oxidative stress in cells and exhibits bright-green
photostable fluorescence upon oxidation by ROS. The oxygen radical
absorbance capacity (ORAC) assay is a method for estimating
antioxidant activity (19–23) and is often used to determine
antioxidant levels in foods. Previous studies have demonstrated
that copper (Cu2+) undergoes redox cycling reactions and
possesses the ability to produce reactive radicals in normal and
cancer cells (24,25). Therefore, in the present study the
ORAC assay in the presence of Cu2+ [ORAC
(Cu2+) assay] was used for quantification of the oxidant
activity of anticancer drugs. In the ORAC (Cu2+) assay,
the reduction in the fluorescence intensity of fluorescein was
calculated as a measure of the oxidant activity of the anticancer
drug. In the current study, the ability of 20 anticancer drugs to
induce oxidative stress was measured using the methods described
above in vitro and in the absence of cells.
Materials and methods
Cell culture
The DLD-1 human colorectal cancer cell line was
purchased from the Cell Resource Center for Biomedical Research at
Tohoku University (Sendai, Japan) and maintained in RPMI media
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented
with 10% heat-inactivated fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.). DLD-1 cells were maintained under standard
culture conditions in a 5% CO2 incubator at 37°C until
they reached 80–90% confluence.
Treatment with anticancer drugs
DLD-1 cells were treated for 24 h in a 5%
CO2 incubator at 37°C with 10 µM of the following
anticancer drugs: Cyclophosphamide, dacarbazine, nimustine,
temozolomide, actinomycin D, doxorubicin, mitomycin C,
mitoxantrone, carmofur, cytarabine, fluorouracil, gemcitabine,
mercaptopurine, carboplatin, oxaliplatin, camptothecin, etoposide,
paclitaxel, vinblastine and vinorelbine. Positive control cells
were treated with 10 µM 2,2′-azobis (2-amidinopropane)
dihydrochloride (AAPH), which is a free radical generator. All
anticancer drugs and AAPH were obtained from Wako Pure Chemical
Industries, Ltd. (Osaka, Japan).
Cell staining with CellROX®
ROS detection reagent
Following incubation with the anticancer drugs or
AAPH for 24 h, 5 µM CellROX® Green reagent (Thermo
Fisher Scientific, Inc.) was added to DLD-1 cells and incubated for
30 min in a 5% CO2 incubator at 37°C. The cells were
washed with PBS, fixed with 3.7% formaldehyde in PBS for 15 min and
permeabilized with 0.5% Triton X-100 in PBS for 5 min, at room
temperature. Glass coverslips were mounted using
SlowFade® Diamond Antifade mountant (Thermo Fisher
Scientific, Inc.). The samples were examined using a Leica DM IL
LED fluorescence microscope (Leica Microsystems GmbH, Wetzlar,
Germany). Cells treated with each anticancer drug were examined at
three or more fields of view, and the ratings of -, none or weak;
+, slight; ++, moderate and +++, severe were manually assigned to
the stained cell slides.
ORAC and ORAC (Cu2+)
assay
Application of the ORAC and ORAC (Cu2+)
assay determined the fluorescence intensity in the presence of the
drug relative to that in the presence of dimethyl sulfoxide (DMSO;
Wako Pure Chemical Industries, Ltd.). The ORAC assays contained 50
µM of an anticancer drug or 5 mM AAPH, with 10 mM fluorescein (Wako
Pure Chemical Industries, Ltd.). The ORAC (Cu2+) assay
additionally contained 10 µM CuSO4 (Wako Pure Chemical
Industries, Ltd.). The assay mixtures were incubated for 1.5 h at
room temperature, in the dark. The fluorescence intensity was
subsequently measured using a Flex station 3 fluorescence plate
reader (Molecular Devices, LLC, Sunnyvale, CA, USA) with filters
for excitation wavelength of 494 nm and emission wavelength of 523
nm. The result was described as the percentage of fluorescence
intensity relative to DMSO treatment. The ORAC and ORAC
(Cu2+) assay was tested once.
Cell staining with Annexin V-Cy3
apoptosis detection reagent
Following incubation with the anticancer drugs for
24 h, Annexin V-Cy3 reagent (dilution, 1:200; BioVision, Inc.,
Milpitas, CA, USA) was added to the DLD-1 cells and incubated for 5
min at room temperature, in the dark. The cells were examined using
the previously described Leica DM IL LED fluorescence
microscope.
Calculation of radical structural
energy of anticancer drugs
To calculate the radical structural energy of the
previously described anticancer drugs, modeling with the
B3LYP/6-31G(d) method was performed in Gaussian version 09 D.01
(Gaussian Inc., Wallingford, CT, USA). The radical structural
energies were calculated as Δ (E+ZPE) or ΔE (26).
Results
Detection of ROS in DLD-1 cells with
CellROX® reagent
The investigated anticancer drugs are classified as
follows: i) Alkylating agents-cyclophosphamide, dacarbazine,
nimustine and temozolomide; ii) antibiotics-actinomycin D,
doxorubicin, mitomycin C and mitoxantrone; iii) antineoplastic
agents-carmofur, cytarabine, fluorouracil, gemcitabine and
mercaptopurine; iv) platinating agents-carboplatin and oxaliplatin;
and v) vinca alkaloids-camptothecin, etoposide, paclitaxel,
vinblastine and vinorelbine. To investigate whether these
anticancer drugs induce intracellular ROS formation, DLD-1 cells
treated with each anticancer drug were stained with
CellROX® Green reagent. As demonstrated in Fig. 1, intracellular ROS formation was
induced in DLD-1 cells treated with actinomycin D, carmofur and
vinorelbine. DLD-1 cells treated with vinorelbine exhibited the
strongest CellROX® Green signal among all anticancer
drugs. The results of CellROX® staining are summarized
in Table I. Furthermore, certain
anticancer drugs, including vinblastine and vinorelbine, markedly
decreased the number of cells. It was initially assumed that the
anticancer drugs had induced growth inhibition or apoptotic cell
death. Annexin V-Cy3 staining indicated that the cells treated with
actinomycin D, doxorubicin, mitomycin C, vinblastine or vinorelbine
had undergone apoptotic cell death (data not shown).
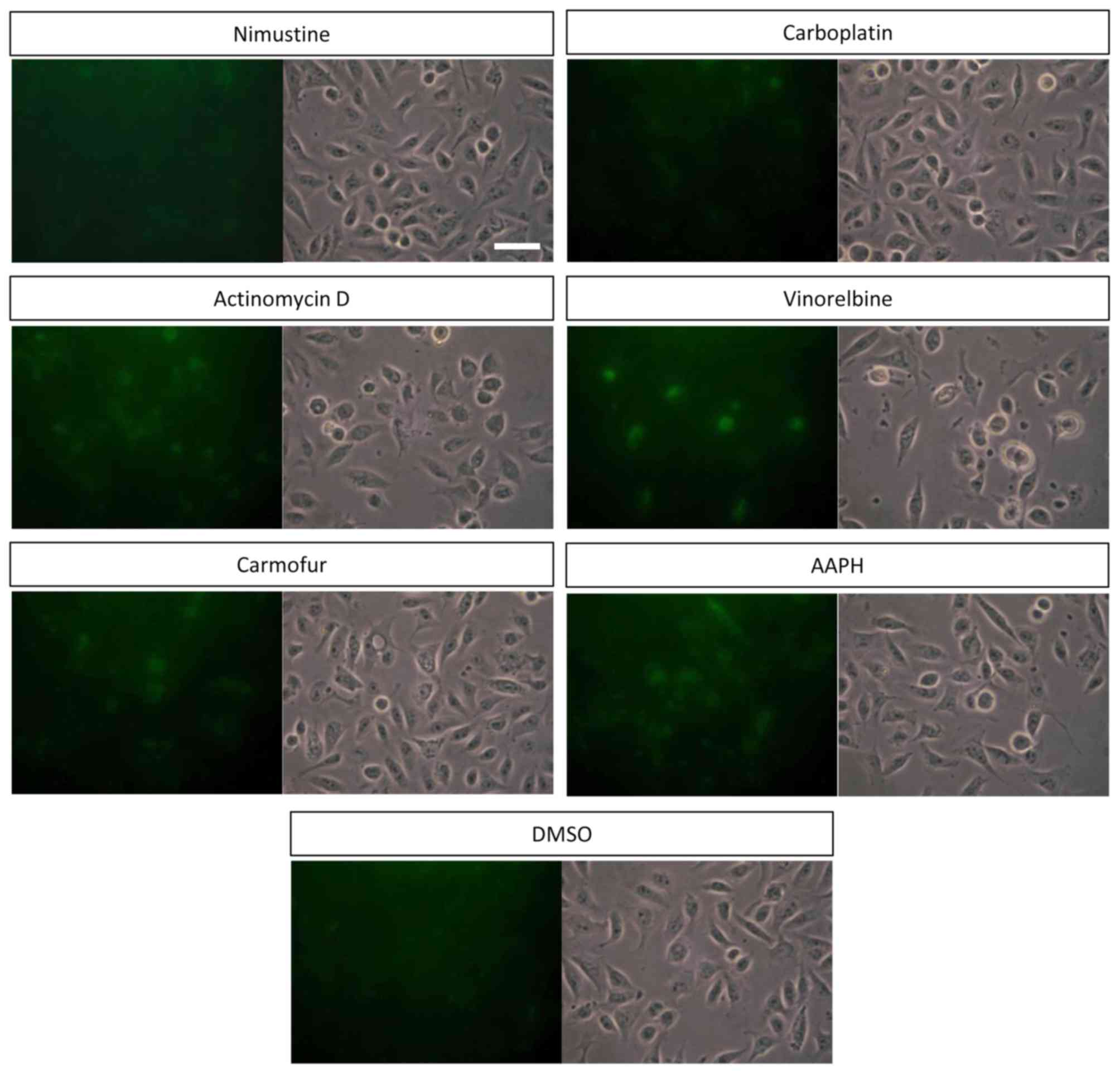 | Figure 1.Cell staining of DLD-1 cells treated
with anticancer drugs using CellROX® Green reagent.
DLD-1 cells were treated with 10 µM nimustine, actinomycin D,
carmofur, carboplatin and vinorelbine, or 10 µM AAPH as a positive
control for 24 h. Following incubation, cells were stained with
CellROX® Green reagent. Left, CellROX® green
fluorescence; right, bright field. Scale bar, 50 µm. AAPH, 2
2′-azobis (2-amidinopropane) dihydrochloride; DMSO, dimethyl
sulfoxide. |
 | Table I.CellROX® signaling
intensity in DLD-1 cells and oxidant activity using the ORAC (Cu2+)
assay in the absence of cells. |
Table I.
CellROX® signaling
intensity in DLD-1 cells and oxidant activity using the ORAC (Cu2+)
assay in the absence of cells.
| Anticancer drug | CellROX®
signal | Reduction of
fluorescence intensity in the ORAC assay, % | Reduction of
fluorescence intensity (%) in the ORAC (Cu2+) assay |
|---|
| Nimustine | − | 58.2 | 42.7 |
| Dacarbazine | − | 98.9 | 99.8 |
| Cyclophosphamide | − | 99.5 | 103.0 |
| Temozolomide | − | 102.4 | 106.0 |
| Mitoxantrone | − | 51.8 | 32.2 |
| Doxorubicin | ++ | 83.5 | 89.2 |
| Actinomycin D | ++ | 88.2 | 92.3 |
| Mitomycin C | + | 102.6 | 98.0 |
| Gemcitabine | − | 93.2 | 44.5 |
| Mercaptopurine | + | 99.4 | 78.4 |
| Carmofur | + | 85.8 | 90.5 |
| Fluorouracil | − | 104.3 | 98.9 |
| Cytarabine | − | 106.1 | 110.4 |
| Carboplatin | − | 104.2 | 94.2 |
| Oxaliplatin | − | 103.3 | 101.6 |
| Vinorelbine | +++ |
33.0 |
19.9 |
| Vinblastine | +++ |
79.3 |
49.1 |
| Camptothecin | ++ |
98.9 |
99.5 |
| Paclitaxel | ++ | 110.3 | 101.1 |
| Etoposide | − |
96.9 | 109.8 |
| AAPH | + |
60.0 |
53.5 |
| DMSO | − | 100.0 | 100.0 |
Quantification of oxidant activity
using the ORAC (Cu2+) assay
The ORAC and ORAC (Cu2+) assays are
methods for measuring the antioxidant ability of food. In the
present study, the ORAC (Cu2+) assay was used to
determine the oxidant activities of anticancer drugs in the absence
of cells by measuring the reduction of fluorescence intensity
derived from fluorescein. As demonstrated in Table I, the abilities of nimustine,
mitoxantrone, gemcitabine and vinblastine to reduce the
fluorescence intensity were comparable to that of AAPH, whereas
vinorelbine markedly reduced the fluorescence intensity. These
results suggested that nimustine, mitoxantrone, gemcitabine,
vinblastine and vinorelbine may directly generate intracellular
ROS. In addition, the ORAC assay indicated that nimustine and
mitoxantrone reduced the intensity of fluorescence to the same
extent as AAPH, whereas vinorelbine reduced the intensity of
fluorescence (Table I). However, the
reduction in fluorescence intensity of anticancer drugs in the ORAC
assay was less extensive, compared with in the ORAC
(Cu2+) assay.
As the reduction of fluorescence intensity by
anticancer drugs in the ORAC (Cu2+) assay was attributed
to the greater stability of a radical structure of anticancer
drugs, the calculation of the radical structural energy of
anticancer drugs, including doxorubicin, mitoxantrone, cytarabine,
gemcitabine, camptothecin, etoposide, paclitaxel, vinblastine,
vinorelbine and AAPH was performed. As the radical structural
energy of the most stable structure of each anticancer drug,
Δ(E+ZPE) of doxorubicin was 80.6 kcal/mol, that of mitoxantrone was
87.9 kcal/mol, that of cytarabine was 83.6 kcal/mol, that of
gemcitabine was 89.1 kcal/mol, that of camptothecin was 73.1
kcal/mol, that of etoposide was 76.6 kcal/mol and that of AAPH is
5.7 kcal/mol. Similarly, ΔE of paclitaxel was 88.7 kcal/mol, that
of vinblastine was 77.3 kcal/mol and that of vinorelbine was 77.2
kcal/mol. These results indicated that the anticancer drugs were
identified as exhibiting a higher radical structural energy
compared with AAPH. Therefore, it was concluded that the results of
an ORAC (Cu2+) assay was not associated with the radical
structural energy of an anticancer drug.
Discussion
Anticancer drugs often cause a variety of adverse
events and induction of ROS has been reported as one of the side
effects of anticancer therapies (11–14,27,28).
However, the ability of numerous anticancer drugs to induce
oxidative stress has not been examined. In the present study, the
oxidative stress levels induced by 20 anticancer drugs were
investigated using cell staining with CellROX® in
vitro and in the absence of cells using the ORAC
(Cu2+) assay. The data of the current study suggests
that certain anticancer drugs have the ability to induce oxidative
stress. DLD-1 cells treated with actinomycin D, doxorubicin,
camptothecin, paclitaxel, vinblastine or vinorelbine exhibited a
strong CellROX® signal; however, it cannot be concluded
that the ROS signal was directly derived from treatment with the
various anticancer drugs. As numerous cells treated with these
anticancer drugs died, the drug-treated DLD-1 cells were stained
with Annexin V-Cy3 as a marker of apoptosis for 24 h. Cells that
were treated with actinomycin D, doxorubicin, mitomycin C,
vinblastine or vinorelbine exhibited a signal with Annexin V-Cy3
(data not shown), suggesting that the ROS signal detected in cells
treated with these anticancer drugs is partially dependent on cell
death. Taken together, these data suggest that anticancer drugs
that are CellROX®-positive/ORAC
(Cu2+)-negative, such as actinomycin D, doxorubicin,
mitomycin C, carmofur, mercaptopurine, camptothecin and paclitaxel
disrupt the oxidant-antioxidant balance.
The ORAC and ORAC (Cu2+) assays were
applied in order to analyze the oxidant activities of anticancer
drugs in the absence of cells. In the ORAC assay, the abilities of
nimustine and mitoxantrone to reduce the fluorescence intensity
were comparable to that of AAPH, whereas vinorelbine markedly
reduced the fluorescence intensity. The ORAC (Cu2+)
assay demonstrated that the reduction in fluorescence intensity of
anticancer drugs was enhanced by the addition of Cu2+,
such that gemcitabine and vinblastine reduced the fluorescence
intensities to an equal extent as nimustine, and mitoxantrone.
Although these anticancer drugs were predicted to be able to reduce
the fluorescence intensity of fluorescein similar to the radical
initiator AAPH, the underlying mechanism remains unknown.
Therefore, the radical structural energies of anticancer drugs were
estimated using computational chemistry. The radical structural
energies of the anticancer drugs were identified to be less stable
compared with those of AAPH. This suggests that these anticancer
drugs reduced the fluorescence intensity through a different
mechanism compared with AAPH. Furthermore, nimustine, mitoxantrone
and gemcitabine were identified as
CellROX®-negative/ORAC (Cu2+)-positive. This
may be due to an active antioxidant system against nimustine,
mitoxantrone and gemcitabine being present in the cells. However,
further analyses are required to elucidate a mechanism of action
for each anticancer drug.
The signals of CellROX® indicated the
presence of intracellular ROS in DLD-1 cells treated with each
anticancer drug. Hence, the CellROX®-positive anticancer
drugs, actinomycin D, doxorubicin, mitomycin C, carmofur,
mercaptopurine, camptothecin, paclitaxel, vinblastine, and
vinorelbine, ultimately induce oxidative stress in the cells and
may cause side effects in cancer patients. Furthermore, the results
of the ORAC (Cu2+) assay implied that ORAC
(Cu2+)-positive anticancer drugs, nimustine,
mitoxantrone, gemcitabine, vinblastine, and vinorelbine, may
themselves be oxidants. In addition, the present results suggest
that CellROX®-positive/ORAC (Cu2+) -positive
anticancer drugs such as vinblastine and vinorelbine have a strong
capacity to induce oxidative stress.
In conclusion, the results of the present study
demonstrate that certain anticancer drugs have the ability to
induce oxidative stress in the presence or absence of cells. The
profiling of anticancer drugs in this study may provide an insight
into the ROS-induced mechanisms of anticancer drugs. A detailed
examination of the induction of oxidative stress by anticancer
drugs may aid in developing novel approaches to reduce the side
effects of cancer therapies.
Acknowledgements
The present study was funded by a Grant-in-Aid for
Scientific Research of Seikei University (grant no. 2014).
References
|
1
|
Yoshpe-Purer Y and Eylan E: Disinfection
of water by hydrogen peroxide. Health Lab Sci. 5:233–238.
1968.PubMed/NCBI
|
|
2
|
Tauber AI and Babior BM: Evidence for
hydroxyl radical production by human neutrophils. J Clin Invest.
60:374–379. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosen H and Klebanoff SJ: Bactericidal
activity of a superoxide anion-generating system. A model for the
polymorphonuclear leukocyte. J Exp Med. 149:27–39. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sawyer DT and Tsang PK: The interactions
of superoxide ion (O2-.) with metallo-porphyrins [(C1(8)TPP)M,
M=Fe, Mn, Co Zn]; models for biological systems and superoxide
dismutases. Free Radic Res Commun. 12–13:75–86. 1991. View Article : Google Scholar
|
|
5
|
Dreher D and Junod AF: Differential
effects of superoxide, hydrogen peroxide and hydroxyl radical on
intracellular calcium in human endothelial cells. J Cell Physiol.
162:147–153. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Landriscina M, Maddalena F, Laudiero G and
Esposito F: Adaptation to oxidative stress, chemoresistance, and
cell survival. Antioxid Redox Signal. 11:2701–2716. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cross CE, Halliwell B, Borish ET, Pryor
WA, Ames BN, Saul RL, McCord JM and Harman D: Oxygen radicals and
human disease. Ann Intern Med. 107:526–545. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Klaunig JE, Wang Z, Pu X and Zhou S:
Oxidative stress and oxidative damage in chemical carcinogenesis.
Toxicol Appl Pharmacol. 254:86–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weinberg F and Chandel NS: Reactive oxygen
species-dependent signaling regulates cancer. Cell Mol Life Sci.
66:3663–3673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mercuro G, Cadeddu C, Piras A, Dessì M,
Madeddu C, Deidda M, Serpe R, Massa E and Mantovani G: Early
epirubicin-induced myocardial dysfunction revealed by serial tissue
Doppler echocardiography: Correlation with inflammatory and
oxidative stress markers. Oncologist. 12:1124–1133. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cadeddu C, Piras A, Mantovani G, Deidda M,
Dessì M, Madeddu C, Massa E and Mercuro G: Protective effects of
the angiotensin II receptor blocker telmisartan on
epirubicin-induced inflammation, oxidative stress, and early
ventricular impairment. Am Heart J. 160:487.e1–e7. 2010. View Article : Google Scholar
|
|
13
|
Minotti G, Menna P, Salvatorelli E, Cairo
G and Gianni L: Anthracyclines: Molecular advances and
pharmacologic developments in antitumor activity and
cardiotoxicity. Pharmacol Rev. 56:185–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang J, Nakamura H and Iyer AK:
Tumor-targeted induction of oxystress for cancer therapy. J Drug
Target. 15:475–486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamada T, Egashira N, Imuta M, Yano T,
Yamauchi Y, Watanabe H and Oishi R: Role of oxidative stress in
vinorelbine-induced vascular endothelial cell injury. Free Radic
Biol Med. 48:120–127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
DeLoughery Z, Luczak MW and Zhitkovich A:
Monitoring Cr intermediates and reactive oxygen species with
fluorescent probes during chromate reduction. Chem Res Toxicol.
27:843–851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okamura DM, Bahrami NM, Ren S, Pasichnyk
K, Williams JM, Gangoiti JA, Lopez-Guisa JM, Yamaguchi I, Barshop
BA, Duffield JS and Eddy AA: Cysteamine modulates oxidative stress
and blocks myofibroblast activity in CKD. J Am Soc Nephrol.
25:43–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ting CH, Huang HN, Huang TC, Wu CJ and
Chen JY: The mechanisms by which pardaxin, a natural cationic
antimicrobial peptide, targets the endoplasmic reticulum and
induces c-FOS. Biomaterials. 35:3627–3640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao G, Alessio HM and Cutler RG:
Oxygen-radical absorbance capacity assay for antioxidants. Free
Radic Biol Med. 14:303–311. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao G, Verdon CP, Wu AH, Wang H and Prior
RL: Automated assay of oxygen radical absorbance capacity with the
COBAS FARA II. Clin Chem. 41:1738–1744. 1995.PubMed/NCBI
|
|
21
|
Ou B, Hampsch-Woodill M and Prior RL:
Development and validation of an improved oxygen radical absorbance
capacity assay using fluorescein as the fluorescent probe. J Agric
Food Chem. 49:4619–4626. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dávalos A, Gómez-Cordovés C and Bartolomé
B: Extending applicability of the oxygen radical absorbance
capacity (ORAC-fluorescein) assay. J Agric Food Chem. 52:48–54.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang D, Ou B and Prior RL: The chemistry
behind antioxidant capacity assays. J Agric Food Chem.
53:1841–1856. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jomova K and Valko M: Advances in
metal-induced oxidative stress and human disease. Toxicology.
283:65–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gupte A and Mumper RJ: Elevated copper and
oxidative stress in cancer cells as a target for cancer treatment.
Cancer Treat Rev. 35:32–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawashima T, Manda S, Uto Y, Ohkubo K,
Hori H, Matsumoto K, Fukuhara K, Ikota N, Fukuzumi S, Ozawa T, et
al: Kinetics and mechanism for the scavenging reaction of the
2,2-diphenyl-1-picrylhydrazyl radical by synthetic artepillin C
analogues. Bull Chem Soc Jpn. 85:877–883. 2012. View Article : Google Scholar
|
|
27
|
Tewey KM, Rowe TC, Yang L, Halligan BD and
Liu LF: Adriamycin-induced DNA damage mediated by mammalian DNA
topoisomerase II. Science. 226:466–468. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vrdoljak AL, Berend S, Zeljezić D,
Piljac-Zegarac J, Plestina S, Kuca K, Radić B, Mladinić M and
Kopjar N: Irinotecan side effects relieved by the use of HI-6
oxime: In vivo experimental approach. Basic Clin Pharmacol Toxicol.
105:401–409. 2009. View Article : Google Scholar : PubMed/NCBI
|















