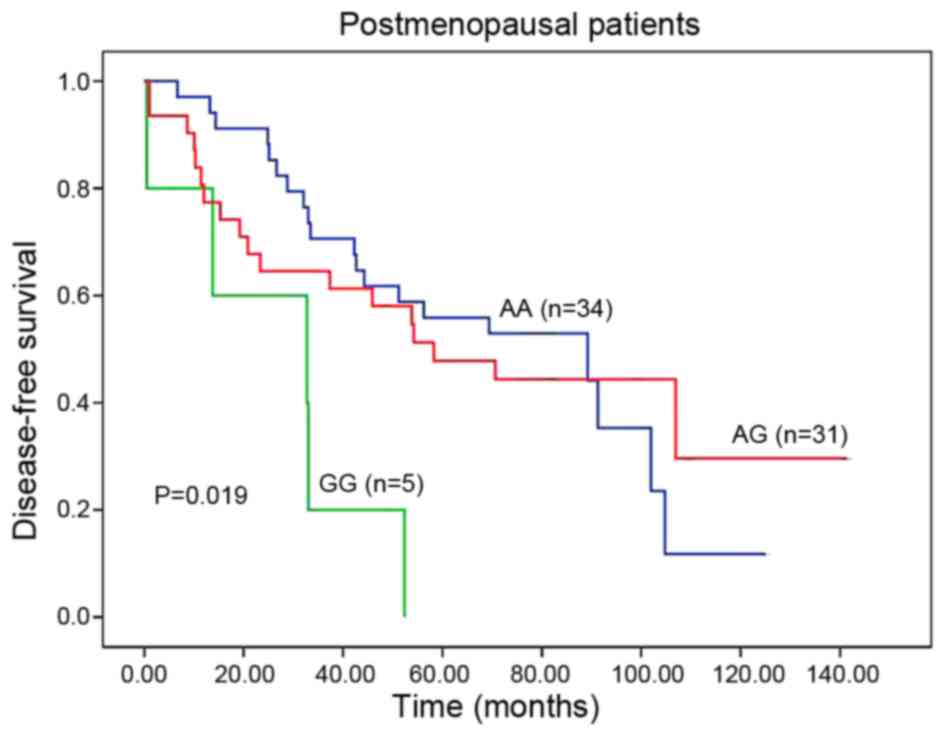
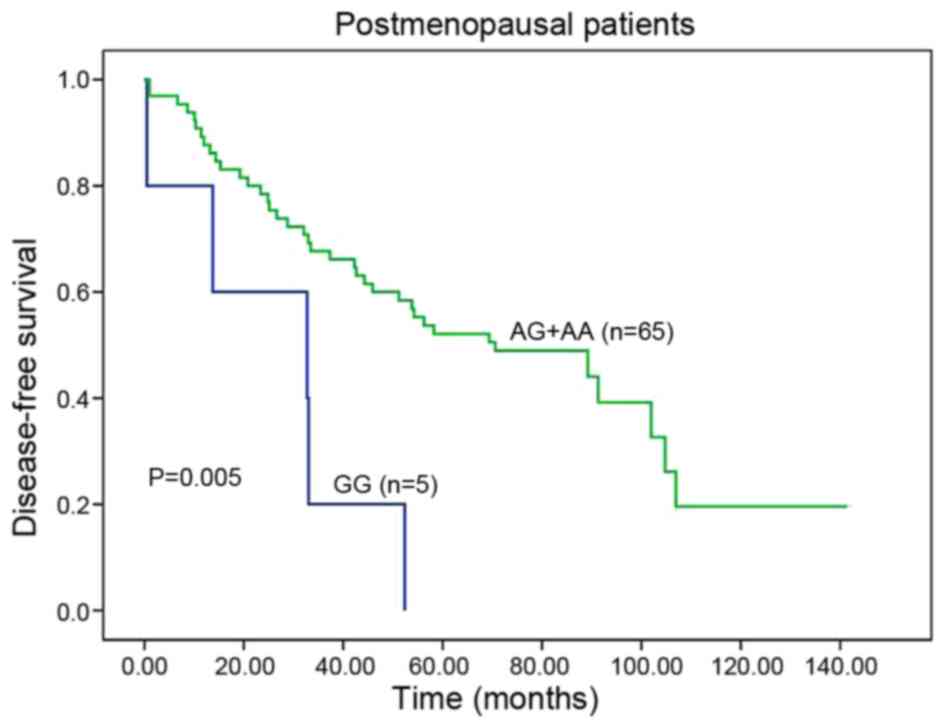
|
1
|
Pedraza AM, Pollan M, Pastor-Barriuso R
and Cabanes A: Disparities in breast cancer mortality trends in a
middle income country. Breast Cancer Res Treat. 134:1199–1207.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hortobagyi GN, de la Garza Salazar J,
Pritchard K, Amadori D, Haidinger R, Hudis CA, Khaled H, Liu MC,
Martin M, Namer M, et al: The global breast cancer burden:
Variations in epidemiology and survival. Clinical Breast Cancer.
6:391–401. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bulun SE, Sebastian S, Takayama K, Suzuki
T, Sasano H and Shozu M: The human CYP19 (aromatase P450) gene:
Update on physiologic roles and genomic organization of promoters.
J Steroid Biochem Mol Biol. 86:219–224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Irahara N, Miyoshi Y, Taguchi T, Tamaki Y
and Noguchi S: Quantitative analysis of aromatase mRNA expression
derived from various promoters (I.4, I.3, PII and I.7) and its
association with expression of TNF-alpha, IL-6 and COX-2 mRNAs in
human breast cancer. Int J Cancer. 118:1915–1921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Neill JS, Elton RA and Miller WR:
Aromatase activity in adipose tissue from breast quadrants: A link
with tumour site. Br Med J (Clin Res Ed). 296:741–743. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agarwal VR, Bulun SE, Leitch M, Rohrich R
and Simpson ER: Use of alternative promoters to express the
aromatase cytochrome P450 (CYP19) gene in breast adipose tissues of
cancer-free and breast cancer patients. J Clin Endocrinol Metab.
81:3843–3849. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bulun SE, Lin Z, Imir G, Amin S, Demura M,
Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, et al:
Regulation of aromatase expression in estrogen-responsive breast
and uterine disease: From bench to treatment. Pharmacol Rev.
57:359–383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Toniolo PG, Levitz M, Zeleniuch-Jacquotte
A, Banerjee S, Koenig KL, Shore RE, Strax P and Pasternack BS: A
prospective study of endogenous estrogens and breast cancer in
postmenopausal women. J Natl Cancer Inst. 87:190–197. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldhirsch A, Gelber RD and Coates AS:
What are the long-term effects of chemotherapy and hormonal therapy
for early breast cancer? Nat Clin Pract Oncol. 2:440–441. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burstein HJ, Prestrud AA, Seidenfeld J,
Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis
CA, Malin J, et al: American society of clinical oncology clinical
practice guideline: Update on adjuvant endocrine therapy for women
with hormone receptor-positive breast cancer. J Clin Oncol.
28:3784–3796. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Y, Mendelson CR and Simpson ER:
Characterization of the sequences of the human CYP19 (aromatase)
gene that mediate regulation by glucocorticoids in adipose stromal
cells and fetal hepatocytes. Mol Endocrinol. 9:340–349. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Santen RJ, Brodie H, Simpson ER, Siiteri
PK and Brodie A: History of aromatase: Saga of an important
biological mediator and therapeutic target. Endocr Rev. 30:343–375.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kristensen VN, Harada N, Yoshimura N,
Haraldsen E, Lonning PE, Erikstein B, Karesen R, Kristensen T and
Borresen-Dale AL: Genetic variants of CYP19 (aromatase) and breast
cancer risk. Oncogene. 19:1329–1333. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haiman CA, Dossus L, Setiawan VW, Stram
DO, Dunning AM, Thomas G, Thun MJ, Albanes D, Altshuler D, Ardanaz
E, et al: Genetic variation at the CYP19A1 locus predicts
circulating estrogen levels but not breast cancer risk in
postmenopausal women. Cancer Res. 67:1893–1897. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dunning AM, Dowsett M, Healey CS, Tee L,
Luben RN, Folkerd E, Novik KL, Kelemen L, Ogata S, Pharoah PD, et
al: Polymorphisms associated with circulating sex hormone levels in
postmenopausal women. J Natl Cancer Inst. 96:936–945. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mitrunen K and Hirvonen A: Molecular
epidemiology of sporadic breast cancer. The role of polymorphic
genes involved in oestrogen biosynthesis and metabolism. Mutat Res.
544:9–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuo SH, Yang SY, Lien HC, Lo C, Lin CH, Lu
YS, Cheng AL, Chang KJ and Huang CS: CYP19 genetic polymorphism
haplotype AASA is associated with a poor prognosis in premenopausal
women with lymph node-negative, hormone receptor-positive breast
cancer. Biomed Res Int. 2013:5621972013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garcia-Casado Z, Guerrero-Zotano A,
Llombart-Cussac A, Calatrava A, Fernandez-Serra A, Ruiz-Simon A,
Gavila J, Climent MA, Almenar S, Cervera-Deval J, et al: A
polymorphism at the 3′-UTR region of the aromatase gene defines a
subgroup of postmenopausal breast cancer patients with poor
response to neoadjuvant letrozole. BMC Cancer. 10:362010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu L, Bai YX, Zhou JH, Sun XW, Sui H,
Zhang WJ, Yuan HH, Xie R, Wei XL, Zhang TT, et al: A polymorphism
at the 3′-UTR region of the aromatase gene is associated with the
efficacy of the aromatase inhibitor, anastrozole, in metastatic
breast carcinoma. Int J Mol Sci. 14:18973–18988. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Colomer R, Monzo M, Tusquets I, Rifa J,
Baena JM, Barnadas A, Calvo L, Carabantes F, Crespo C, Munoz M, et
al: A single-nucleotide polymorphism in the aromatase gene is
associated with the efficacy of the aromatase inhibitor letrozole
in advanced breast carcinoma. Clin Cancer Res. 14:811–816. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simpson ER: Aromatase: Biologic relevance
of tissue-specific expression. Semin Reprod Med. 22:11–23. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Long JR, Kataoka N, Shu XO, Wen W, Gao YT,
Cai Q and Zheng W: Genetic polymorphisms of the CYP19A1 gene and
breast cancer survival. Cancer Epidemiol Biomarkers Prev.
15:2115–2122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai H, Shu XO, Egan KM, Cai Q, Long JR,
Gao YT and Zheng W: Association of genetic polymorphisms in CYP19A1
and blood levels of sex hormones among postmenopausal Chinese
women. Pharmacogenet Genomics. 18:657–664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haiman CA, Stram DO, Pike MC, Kolonel LN,
Burtt NP, Altshuler D, Hirschhorn J and Henderson BE: A
comprehensive haplotype analysis of CYP19 and breast cancer risk:
The multiethnic cohort. Hum Mol Genet. 12:2679–2692. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Theriault RL, Carlson RW, Allred C,
Anderson BO, Burstein HJ, Edge SB, Farrar WB, Forero A, Giordano
SH, Goldstein LJ, et al: Breast cancer, version 3.2013: Featured
updates to the NCCN guidelines. J Natl Compr Canc Netw. 11:753–761.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carbonnelle E, Mesquita C, Bille E, Day N,
Dauphin B, Beretti JL, Ferroni A, Gutmann L and Nassif X: MALDI-TOF
mass spectrometry tools for bacterial identification in clinical
microbiology laboratory. Clin Biochem. 44:104–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rodriguez S, Gaunt TR and Day IN:
Hardy-Weinberg equilibrium testing of biological ascertainment for
Mendelian randomization studies. Am J Epidemiol. 169:505–514. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hudis CA, Barlow WE, Costantino JP, Gray
RJ, Pritchard KI, Chapman JA, Sparano JA, Hunsberger S, Enos RA,
Gelber RD, et al: Proposal for standardized definitions for
efficacy end points in adjuvant breast cancer trials: The STEEP
system. J Clin Oncol. 25:2127–2132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghimenti C, Mello-Grand M, Grosso E,
Scatolini M, Regolo L, Zambelli A and Chiorino G: Regulation of
aromatase expression in breast cancer treated with anastrozole
neoadjuvant therapy. Exp Ther Med. 5:902–906. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bouchard MF, Taniguchi H and Viger RS:
Protein kinase A-dependent synergism between GATA factors and the
nuclear receptor, liver receptor homolog-1, regulates human
aromatase (CYP19) PII promoter activity in breast cancer cells.
Endocrinology. 146:4905–4916. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lønning PE, Helle SI, Johannessen DC, Ekse
D and Adlercreutz H: Influence of plasma estrogen levels on the
length of the disease-free interval in postmenopausal women with
breast cancer. Breast Cancer Res Treat. 39:335–341. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rock CL, Flatt SW, Laughlin GA, Gold EB,
Thomson CA, Natarajan L, Jones LA, Caan BJ, Stefanick ML, Hajek RA,
et al: Reproductive steroid hormones and recurrence-free survival
in women with a history of breast cancer. Cancer Epidemiol
Biomarkers Prev. 17:614–620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Jong PC, Blankenstein MA, van de Ven J,
Nortier JW, Blijham GH and Thijssen JH: Importance of local
aromatase activity in hormone-dependent breast cancer: A review.
Breast. 10:91–99. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang L, Ellsworth KA, Moon I,
Pelleymounter LL, Eckloff BW, Martin YN, Fridley BL, Jenkins GD,
Batzler A, Suman VJ, et al: Functional genetic polymorphisms in the
aromatase gene CYP19 vary the response of breast cancer patients to
neoadjuvant therapy with aromatase inhibitors. Cancer Res.
70:319–328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gennari L, Masi L, Merlotti D, Picariello
L, Falchetti A, Tanini A, Mavilia C, Del Monte F, Gonnelli S,
Lucani B, et al: A polymorphic CYP19 TTTA repeat influences
aromatase activity and estrogen levels in elderly men: Effects on
bone metabolism. J Clin Endocrinol Metab. 89:2803–2810. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma CX, Adjei AA, Salavaggione OE, Coronel
J, Pelleymounter L, Wang L, Eckloff BW, Schaid D, Wieben ED, Adjei
AA, et al: Human aromatase: Gene resequencing and functional
genomics. Cancer Res. 65:11071–11082. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tworoger SS, Chubak J, Aiello EJ, Ulrich
CM, Atkinson C, Potter JD, Yasui Y, Stapleton PL, Lampe JW, Farin
FM, et al: Association of CYP17, CYP19, CYP1B1, and COMT
polymorphisms with serum and urinary sex hormone concentrations in
postmenopausal women. Cancer Epidemiol Biomarkers Prev. 13:94–101.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Haiman CA, Hankinson SE, Spiegelman D, De
Vivo I, Colditz GA, Willett WC, Speizer FE and Hunter DJ: A
tetranucleotide repeat polymorphism in CYP19 and breast cancer
risk. Int J Cancer. 87:204–210. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Haiman CA, Hsu C, de Bakker PI, Frasco M,
Sheng X, Van Den Berg D, Casagrande JT, Kolonel LN, Le Marchand L,
Hankinson SE, et al: Comprehensive association testing of common
genetic variation in DNA repair pathway genes in relationship with
breast cancer risk in multiple populations. Hum Mol Genet.
17:825–834. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Talbott KE, Gammon MD, Kibriya MG, Chen Y,
Teitelbaum SL, Long CM, Gurvich I, Santella RM and Ahsan H: A CYP19
(aromatase) polymorphism is associated with increased premenopausal
breast cancer risk. Breast Cancer Res Treat. 111:481–487. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Erichsen HC and Chanock SJ: SNPs in cancer
research and treatment. Br J Cancer. 90:747–751. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tsigelny IF, Kotlovyi V and Wasserman L:
SNP analysis combined with protein structure prediction defines
structure-functional relationships in cancer related cytochrome
P450 estrogen metabolism. Curr Med Chem. 11:525–538. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bond GL, Hu W, Bond EE, Robins H, Lutzker
SG, Arva NC, Bargonetti J, Bartel F, Taubert H, Wuerl P, et al: A
single nucleotide polymorphism in the MDM2 promoter attenuates the
p53 tumor suppressor pathway and accelerates tumor formation in
humans. Cell. 119:591–602. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Skorupski P, Krol J, Starega J, Adamiak A,
Jankiewicz K and Rechberger T: An alpha-1 chain of type I collagen
Sp1-binding site polymorphism in women suffering from stress
urinary incontinence. Am J Obstet Gynecol. 194:346–350. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen X, Truong TT, Weaver J, Bove BA,
Cattie K, Armstrong BA, Daly MB and Godwin AK: Intronic alterations
in BRCA1 and BRCA2: Effect on mRNA splicing fidelity and
expression. Hum Mutat. 27:427–435. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Duan J, Wainwright MS, Comeron JM, Saitou
N, Sanders AR, Gelernter J and Gejman PV: Synonymous mutations in
the human dopamine receptor D2 (DRD2) affect mRNA stability and
synthesis of the receptor. Hum Mol Genet. 12:205–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jin Y, Dietz HC, Montgomery RA, Bell WR,
McIntosh I, Coller B and Bray PF: Glanzmann thrombasthenia.
Cooperation between sequence variants in cis during splice site
selection. J Clin Invest. 98:1745–1754. 1996. View Article : Google Scholar : PubMed/NCBI
|