Introduction
Cutaneous melanoma is the deadliest form of skin
cancer, which arises from melanocytes and their precursors
(1). Despite major efforts to
identify novel therapeutic tools to treat metastatic melanoma,
durable regression remains a rare event in patients with advanced
disease, and no significant benefit in survival time has been
achieved with targeted inhibitors (2).
A previous study revealed that an acidic tumor
microenvironment is critical to cancer progression and metastasis
(3). The majority of tumors develop
in a pathophysiologic microenvironment that is characterized by low
oxygen tension (pO2), elevated interstitial fluid
pressure, low glucose concentration, high lactate concentration,
low extracellular pH and/or energy deprivation (4,5). This
hostile microenvironment activates a number of transcription
factors, including hypoxia-inducible factor-1, leading to the
upregulated expression of a large number of gene products that
promote malignant progression and metastatic dissemination
(6,7).
In addition, cell surface vacuolar-type H+ (V)-ATPase
activity has been postulated to create an acidic extracellular
microenvironment, which is required for the activation of proteases
critical for tumor cell invasion (8).
V-ATPases are ubiquitously expressed ATP-dependent
proton pumps that may regulate the pH in endomembrane systems
(9). They are overexpressed in a
number of types of metastatic cancer and are positively associated
with invasion and metastasis (10).
It has been demonstrated that pharmacologic or genetic reductions
of V-ATPase activity significantly reduce the migration of invasive
tumor cells in vitro (9). For
example, the V-ATPase inhibitor archazolid abrogated tumor
dissemination in a syngeneic mouse 4T1 breast tumor metastasis
model (9). Consistent with this, the
inhibition of V-ATPase with concanamycin or short hairpin RNA
targeting the V1E subunit reduced matrix metallopeptidase (MMP)-9
activity (11). Bafilomycin and
concanamycin were the first discovered V-ATP inhibitors (12); however, as they also inhibit
mitochondrial ATP enzymes and affect the function of mitochondria
in normal cells, they lead to cell death (13) and so are difficult to apply in
clinical practice. Recently, cleistanthin A (CA), a natural
compound, was demonstrated to inhibit the activity of V-ATPase in
HepG2 cells and to neutralize the pH of lysosomes at nanomolar
concentrations (14); to the best of
our knowledge, no previous studies have assessed how it may affect
tumor cell motility and migration.
The present study aimed to investigate whether CA
may inhibit the V-ATPases of A375 cells and to explore the
potential underlying mechanisms. It was revealed that CA inhibits
the invasion and migration of A375 cells in vitro.
Materials and methods
Chemicals and materials
RPMI-1640 and fetal bovine serum (FBS) were acquired
from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Penicillin and streptomycin were obtained from Thermo Fisher
Scientific, Inc. Matrigel was purchased from Beijing Unique
Biotechnology Co., Ltd. (Beijing, China). A V-ATPase-specific kit
was supplied by Shanghai GenePharma Co., Ltd. (Shanghai, China).
The pH sensitive fluorescent probe
20,70-bis-(2-carboxyethyl)-5-carboxyfluorescein (BCECF), was
acquired from the Beyotime Institute of Biotechnology (Haimen,
China). The pH LysoTracker Red lysosomal fluorescent probe was
purchased from Invitrogen (Thermo Fisher Scientific, Inc.) and the
nuclear dye Hoechst 33258 (5 µg/ml) was purchased from the Beyotime
Institute of Biotechnology. CA was synthesized by the Institute of
Nautical Medicine, Nantong University (Nantong, China). Bafilomycin
A1 was obtained from Shanghai GenePharma Co., Ltd. CA and
bafilomycin A1 were dissolved in dimethylsulfoxide (DMSO) and
stored at −4°C until analysis.
Cell culture
A375 human melanoma cells were kindly provided by
the Institute of Dermatology, Chinese Academy of Medical Sciences
and Peking Union Medical College (Beijing, China). Cells were
cultured in RPMI-1640 supplemented with 10% FBS and antibiotics
(100 U/ml penicillin and 100 µg/ml streptomycin) in humidified air
with 5% CO2 at 37°C throughout the study.
Proliferation assay
Cell proliferation was determined with an MTT
colorimetric assay and trypan blue viable cell counting. Each
experiment was repeated >3 times. For the MTT colorimetric
assay, A375 cells (1×104/well) were plated in 96-well
plates in 200 µl of medium and treated with various concentrations
of CA (0, 0.001, 0.01, 0.03, 0.1, 0.3 and 1 µM) dissolved in 100%
DMSO, with final DMSO concentrations <0.1%. Control cells were
treated with RPMI-1640 supplemented with 0.1% DMSO. Following 24 or
72 h, 100 µl 5 mg/ml MTT was added and the cells were cultured for
another 4 h. This was followed by adding 100 µl 15% SDS per well.
The absorbance at 570 nm was evaluated using a microplate reader
(SN209941; BioTek Instruments, Inc., Winooski, VT, USA), using
wells without cells as blanks and using untreated cells as the
negative control. The viable cell counting using trypan blue was
performed using A375 cells cultured in 6-well plates in RPMI-1640
supplemented with 10% FBS for 24 h. Subsequently, various
concentrations of CA (0, 0.03, 0.1 and 0.3 µM), RPMI-1640
supplemented with 0.1% DMSO and bafilomycin A1 (0.1 µM) were added.
At 24 h, the cells were trypsinized; suspended cells and trypan
blue were mixed 1:1 and the number of viable cells was counted
using a haemocytometer.
Wound-healing assay
The in vitro wound-healing assay was used to
observe the migration of A375 cells following CA treatment. Cells
(1×106 cells/ml) were seeded into a 6-well plate and
incubated for 24 h. The center of the cell monolayer was then
scraped with a sterile micropipette tip to create a straight gap of
constant width. The wells were washed with PBS and the cells were
exposed to various concentrations of CA (0, 0.03, 0.1 and 0.3 µM)
or bafilomycin A1 (0.1 µM) (as the positive control). Wound closure
was imaged at 0 and 24 h using an inverted light microscope
(magnification, ×200) (Leica Microsystems GmbH, Wetzlar,
Germany).
Matrigel invasion assay
For assessment of the motility of A375 melanoma
cells treated with CA and bafilomycin A1, an invasion assay was
performed in a 24-well Transwell plate with 8 µm pore filter
inserts (Corning Incorporated, Corning, NY, USA) coated with 50 µl
Matrigel. The cells were then suspended at a density of
1×105 cells per chamber in 200 µl RPMI-1640 with CA (0,
0.03, 0.1 and 0.3 µM) or bafilomycin A1 (0.1 µM), added to chamber
inserts in wells filled with 0.6 ml of RPMI-1640 supplemented with
20% FBS as the chemoattractant and incubated for 24 h. The cells
and Matrigel on the upper side of inserts were completely removed
by swabbing. The transmigrated cells on the lower side of the
membrane and well bottom were fixed for 30 min with formaldehyde
and stained for 5 min with 0.1% of crystal violet at room
temperature. The number of cells was observed using an inverted
microscope (magnification, ×200).
V-ATPase activity
V-ATPase activity was evaluated using the
V-ATPase-specific kit (Genmed Scientifics, Inc., Arlington, MA,
USA), according to the manufacturer's protocol. Briefly,
8×105 cells untreated or treated with CA or bafilomycin
A1 (0.1 µM) in RPMI-1640 with 10% FBS were incubated for 24 h. The
supernatant was discarded and then cells were washed with reagent A
2–3 times, finally discard cleaning fluid. Each sample had 60 µl
Genmed lysis liquid (reagent B) added to it and the cells were
scraped down into the Eppendorf tube. The tube was incubated on ice
for 30–50 min in the ice to lyse cells and centrifuged at 4°C, 10
min at 13,201 × g. The supernatant contains the cell lysate and its
protein concentration was determined with a bicinchoninic acid
(BCA) assay (Thermo Fisher Scientific, Inc.). V-ATPase activity was
detected using a microplate reader.
Determination of intracellular pH
values
The internal pH (pHi) value was evaluated in the
cell monolayer using the pH sensitive fluorescent probe BCECF. A
standard curve was established using a RF-5301PC fluorescent
spectrophotometer (Shimadzu Corporation, Kyoto, Japan). Cells were
treated with CA (0, 0.03, 0.1 and 0.3 µM) for 24 h prior to the
application of BCECF as previously described (15). The fluorescence intensity at 490 nm
was recorded, and the pHi value was determined according to the pHi
standard curve.
Immunofluorescence staining
analysis
Prior to seeding cells, aseptic glasses were placed
on 6-well plates. A total of 1.2×104 cells in the
logarithmic growth phase were seeded on 6-well plates with
pre-paved glasses and treated with CA (0, 0.03, 0.1 and 0.3 µM) or
bafilomycin A1 (0.1 µM) for 24 h. Then incubated 100 nM lysosomal
fluorescent probe (Invitrogen; Thermo Fisher Scientific, Inc.),
LysoTracker, with CA and bafilomycin A1 (0.1 µM) for 3 h. Cells
were then washed with PBS, fixed with 4% formaldehyde for 15 min at
room temperature and washed again with PBS for 2–3 times, then they
were stained with DAPI (10 µg/ml) for 5 min at room temperature.
Then discarded the dye solution, washed again with PBS for 2–3
times, and pasted the glasses with quenching inhibitor, Finally, a
DMRXA2 Leica laser confocal microscope (magnification, ×63; oil
mirror) was used to observe the cells.
Gelatin zymography
The protein content of supernatants from cells
treated with 0, 0.03, 0.1 and 0.3 µM CA or bafilomycin A1 (0.1 µM)
for 24 h was determined using a BCA assay following centrifugation
at 13,201 × g at 4°C. Equal amounts of protein (25 µg) for each
sample were mixed with 5X loading buffer [50 mmol/l Tris-HCl (pH
6.8), 10% glycerol, 2% SDS and 0.1% bromophenol blue] and loaded
onto a 10% SDS-PAGE containing 0.1% gelatin. Following
electrophoresis, gels were rinsed twice in 0.25% Triton X-100 for
40 min at room temperature and then incubated at 37°C in developing
buffer (50 mmol/l Tris, pH 7.5–7.6, 10 mmol/l CaCl2,
0.02% NaN3 and 150 mmol/l NaCl) for 42 h. The gels were
then stained with 0.05% Coomassie Brilliant Blue R-250 in 30%
methanol and 10% glacial acetic acid for 3 h, and subsequently
destained in 30% methanol and 10% glacial acetic acid for 30 min,
20% methanol and 10% glacial acetic acid for 1 h and 10% methanol
and 5% glacial acetic acid for 2 h. Each staining step was
performed at room temperature. Images were obtained using the Gel
Image Formation system (LI-COR Biosciences, Lincoln, NE, USA) and
analyzed using ImageJ software (National Institutes of Health,
Bethesda, MD, USA). Pro-MMP-9 and activated gelatinase exhibit
gelatinolytic activity in gelatin zymography (16). Thus, the samples were separated by
SDS-PAGE (0.1% gelatin). However, during electrophoresis, SDS binds
with MMPs of samples (the binding is reversible), so that MMPs
cannot perform their role in the decomposition of gelatin. The
activities of MMP-2 and MMP-9 were recovered in the buffer system
with two valence metal ions and the gelatin in the gel was
hydrolyzed at each migration position. Finally, the gels were
stained and decolorized, which appears as white bands on the blue
background. The strength of band was proportional to the activity
of MMP-2 and MMP-9.
Western blot analysis
Cells were seeded into 6 cm dishes and reached
60–80% confluence after 24 h. CA at various concentrations (0,
0.03, 0.1 and 0.3 µM) and bafilomycin A1 (0.1 µM) were added. At 24
h, total cell lysates were collected using lysis buffer (Beyotime
Institute of Biotechnology) containing 0.01% protease inhibitor
cocktail (Thermo Fisher Scientific, Inc.) and sonication with an
ultrasonic processor. The cell lysates were microcentrifuged at
13,201 × g for 10 min at 4°C and the supernatant was collected. The
protein concentrations of total cell lysates were determined with a
BCA assay. The proteins were separated by 10% SDS-PAGE and
electrophoretically transferred onto polyvinylidenefluoride
membranes. The membranes were blocked in 5% skim milk for 1 h at
room temperature. Subsequently the membranes were probed using
primary antibodies against MMP-2 (cat. no. 6E3F8), MMP-9 (cat. no.
ab38898) (1:500; Abcam, Cambridge, UK) and β-actin (cat. no. A1978;
1:5,000; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) overnight
at 4°C and then incubated with the secondary antibody [goat
anti-mouse IRDye 800CW IgG (H+L); cat. no. P/N 925-32210; 1:10,000;
LI-COR Biosciences, Lincoln, NE, USA, and goat anti-rabbit IR Dye
800CW IgG (H+L); cat. no. P/N 925-32211 (just for anti-MMP-9
antibody); 1:10,000; LI-COR Biosciences] for 1.5 h at room
temperature avoiding light. Finally, the protein expression was
detected using an ECL detection kit (Beyotime Institute of
Biotechnology).
Data analysis
All experiments were conducted at least 3 times in
duplicates/triplicates. Results are presented as the mean ±
standard deviation. One-way analysis of variance with a post hoc
Tukey's test was performed using GraphPad Prism 5 software
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
CA treatment inhibits the
proliferation of A375 cells
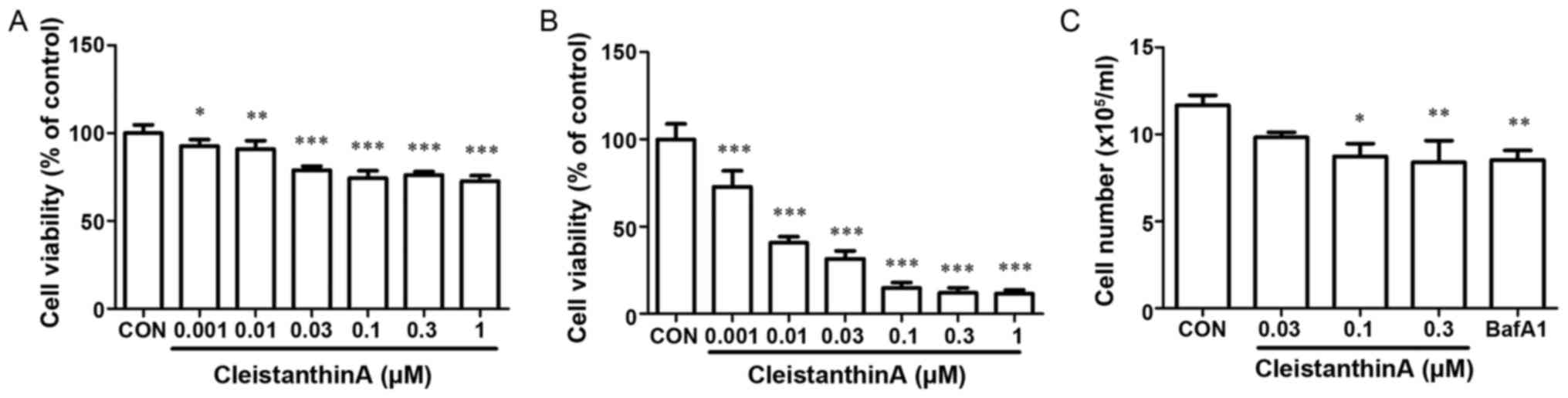
The effect of treatment with CA on cell viability
was determined with an MTT assay. Cells were in various
concentrations (0, 0.001, 0.01, 0.03, 0.1, 0.3 or 1 µM) of CA.
Growth inhibition was induced in a dose- and time-dependent manner
(Fig. 1A and B). As presented in
Fig. 1A, cell viability was not
significantly affected by low concentrations at 24 h compared with
high-concentration treatment. Similar results were obtained from
viable cell counting with trypan blue staining with CA
concentrations of 0.03, 0.1 and 0.3 µM (Fig. 1C). Therefore, a concentration range of
0.03, 0.1 and 0.3 µM CA was selected for the subsequent
experiments.
CA inhibits the activity of V-ATPases,
increases the acidity of the cytoplasm and increases the alkalinity
of the lysosome in A375 cells
To determine whether CA inhibits the expression of
V-ATPase, A375 cells were treated with DMSO, CA (0.03, 0.1 and 0.3
µM) or bafilomycin A1 for 24 h and were analyzed for V-ATPase
activity using a V-ATPase-specific kit.
Following a 24-h CA treatment at various
concentrations, the expression level of V-ATPase was altered when
compared with the control group (Fig.
2A). The expression levels of V-ATPase in the low concentration
group were significantly less compared with in the control group
(P<0.001). A dose-dependent association was observed between the
CA concentration and the expression level of V-ATPase.
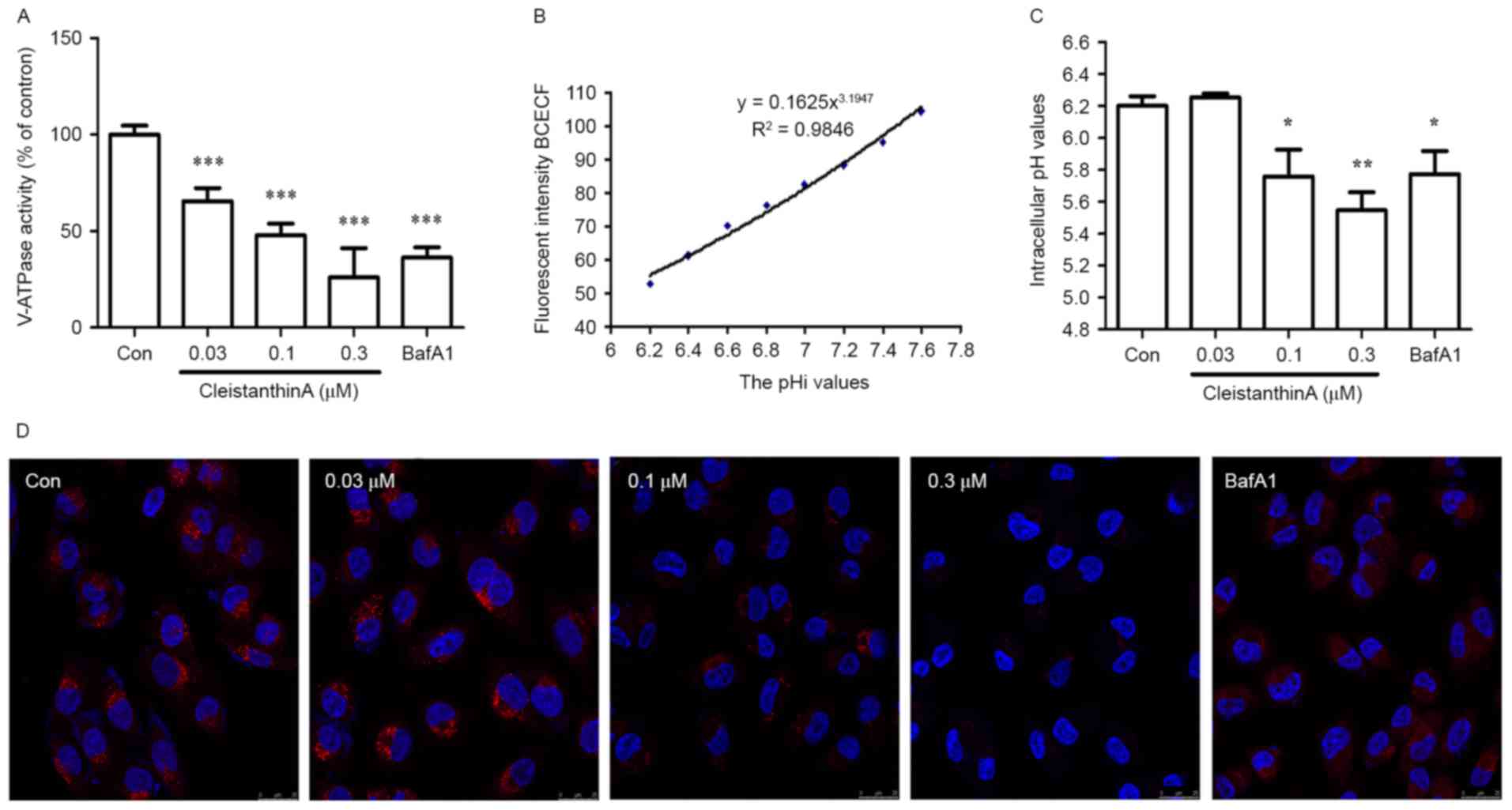 | Figure 2.The effect of CA treatment on the
V-ATPase activity level and pHi values of A375 cells. (A) A375
cells were treated with CA (0, 0.03, 0.1 and 0.3 µM) or bafilomycin
A1 for 24 h. The V-ATPase activity level was determined using a
V-ATPase-specific kit (n=3). (B) Standard curve of fluorescence
intensity of BCECF vs. the pHi value of A375 cells. (C) The pHi
values of A375 cells following CA or bafilomycin A1 treatment for
24 h (n=3). (D) Lysosomal pH. The lysosomal pH was analyzed using
lysosomal fluorescent probe and DAPI staining subsequent to CA or
bafilomycin A1 treatment for 24 h (magnification, ×200).
*P<0.05, **P<0.01 and ***P<0.001 vs. control. CA,
cleistanthin A; V-ATPase, vacuolar-type H+-ATPase; pHi,
intracellular pH; BCECF,
20,70-bis-(2-carboxyethyl)-5-carboxyfluorescein; Con, control. |
Following the inhibition of V-ATPase, hydrogen ions
in the cytoplasm can no longer be transported from the cell.
Therefore, the present study investigated the pHi of A375 cells
treated with CA. The standard curve of fluorescence intensity vs.
the pHi value of A375 cells is presented in Fig. 2B. According to regression analysis,
the mathematic model was established with the equation:
Y=0.1625X3.1947. The pHi values of A375 cells prior
to and following CA treatment with various concentrations for 24 h
were calculated based on this formula. As included in Fig. 2C, it was revealed that the pHi values
in A375 cells treated with CA at concentrations of 0.1 and 0.3 µM
for 24 h were significantly lower compared with that in the control
group (P<0.05 and P<0.01 respectively).
It was determined whether CA can alkalize the
lysosomal pH using the LysoTracker Red lysosomal fluorescent probe.
The lysosome is usually acidic, which induces the production of red
fluorescence; when the lysosomal pH increases, the intensity of red
fluorescence decreases. The present study determined the lysosomal
pH of A375 cells following treatment with CA and bafilomycin A1. It
was revealed that the red fluorescence intensity was decreased in a
dose-dependent manner (Fig. 2D).
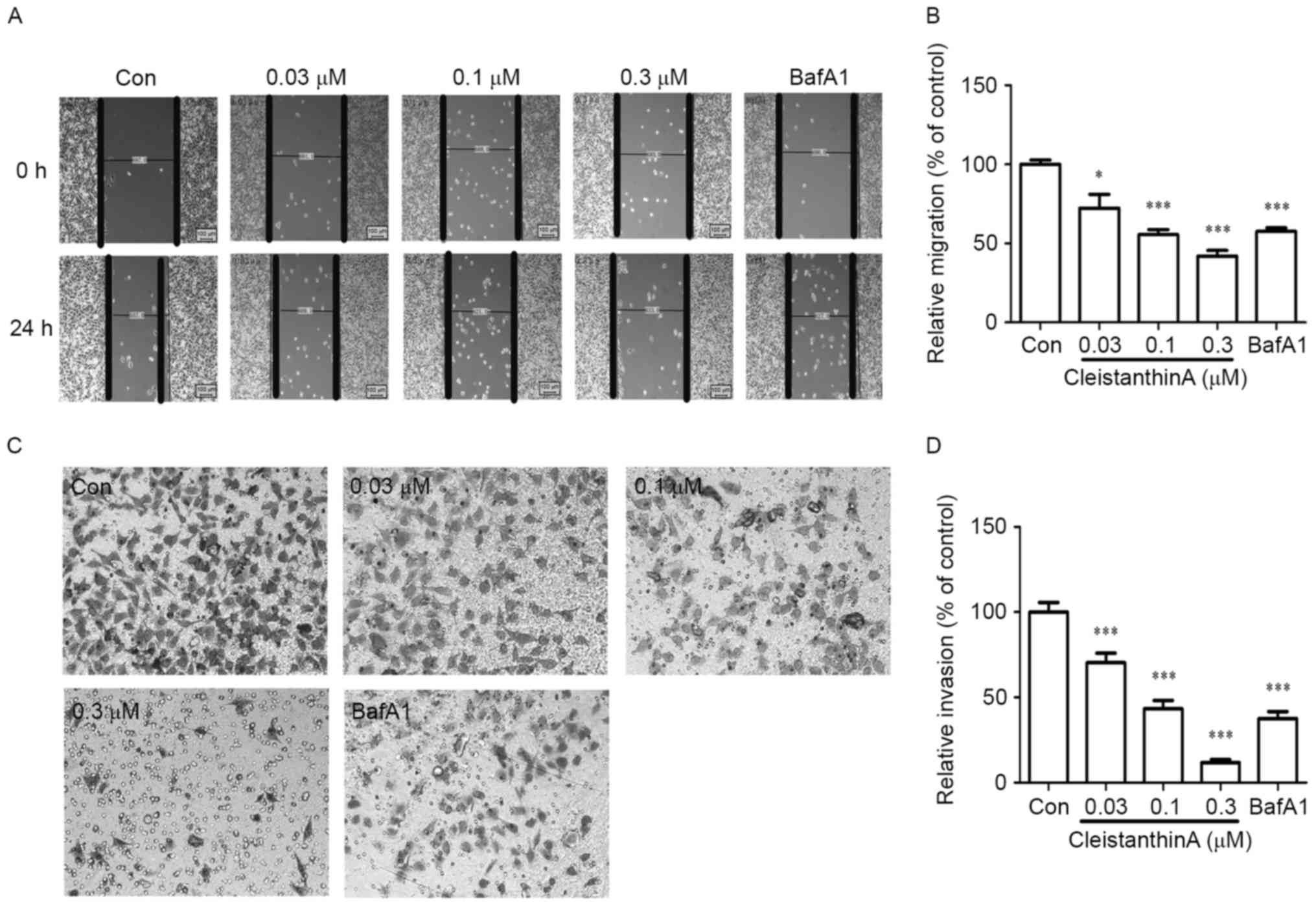
CA inhibits cell migration
The wound-healing assay was used to investigate the
migration of A375 cells cultured with the investigated
concentrations (0, 0.03, 0.1, and 0.3 µM) of CA and the positive
control drug (bafilomycin A1). The results revealed that CA
significantly reduced the migration of A375 cells in a
dose-dependent manner at 24 h (Fig. 3A
and B).
CA inhibits cell invasion
A significant difference was observed in the
motility of A375 cells treated with CA in the Transwell assay.
Following a 24-h treatment with CA, the invasion ability of A375
cells was significantly reduced (P<0.001 vs. no treatment) in
the Matrigel invasion assay (Fig. 3C and
D).
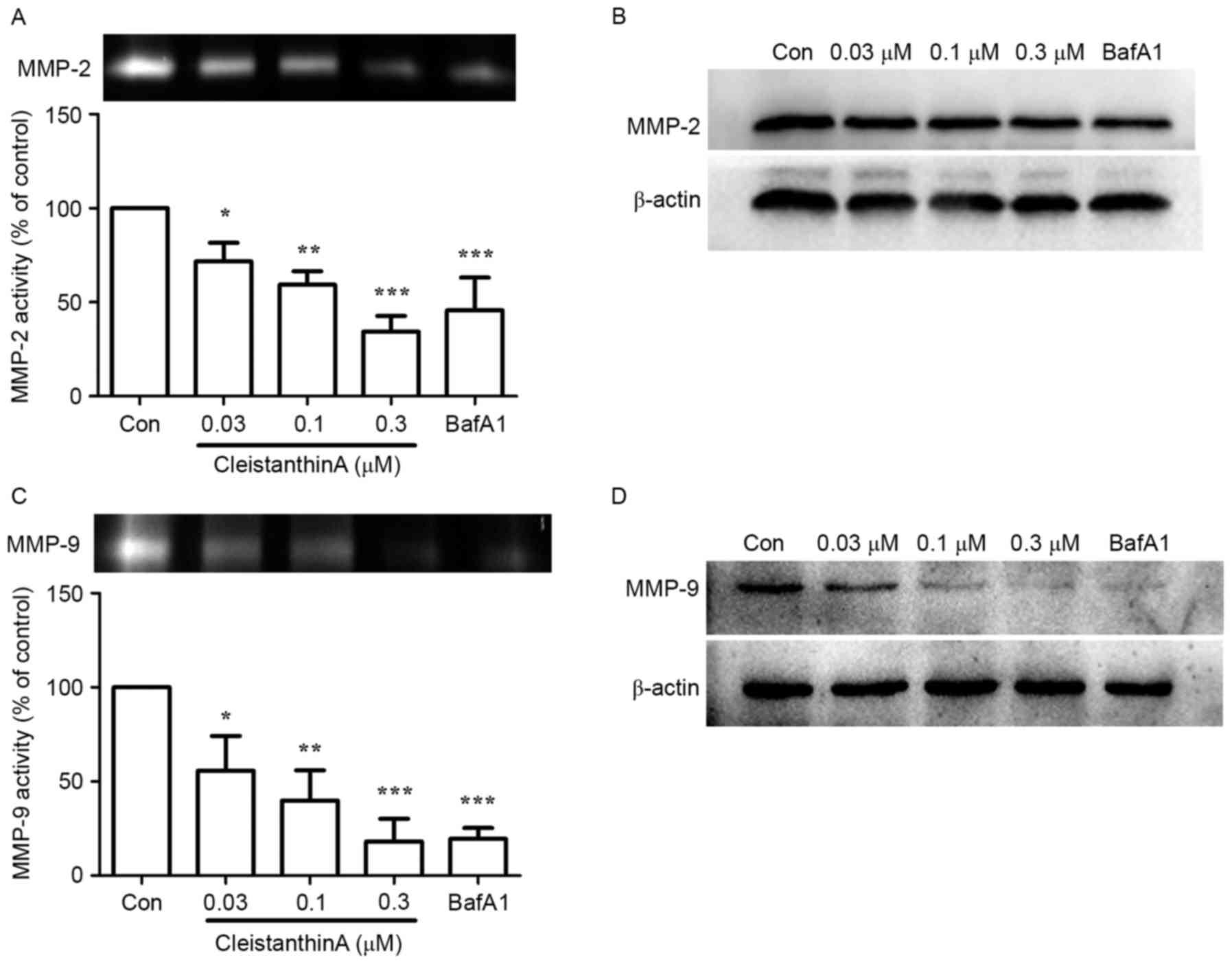
Effect of CA treatment on the
expression and secretion of MMP-2 and MMP-9
It has been reported that V-ATPase inhibition
reduces the activity of MMP-9 (11).
Therefore, the present study investigated whether CA can affect the
activity and expression levels of MMP-9 and −2 with gelatin
zymography and western blot analysis, respectively.
Gelatin zymography was used to analyze the activity
of secreted MMP-2 and −9 in A375 cells treated with or without CA.
Fig. 4A demonstrated that the
activity of MMP-2 in the 0.03, 0.1 and 0.3 µM CA groups was
significantly lower compared with the control group in a
dose-dependent manner (P<0.05, P<0.01, P<0.001,
respectively). The protein expression level of MMP-2 was also
reduced with CA treatment (Fig. 4B).
A similar result was obtained for MMP-9 (Fig. 4C and D).
Discussion
CA, a natural compound, may exhibit
anti-proliferative activity in vitro against MCF-7, HeLa,
HepG2, HCT-116 and U251 cancer cell lines (14). In the present study the toxic effect
of CA on A375 cells was preliminary investigated. It was revealed
that the growth inhibition of A375 cells was induced in a dose- and
time-dependent manner (Fig. 1A and
B); however, the viability of A375 human melanoma cells was not
significantly affected at low concentrations at 24 h, compared with
high-concentration treatment. CA also inhibited cell invasion and
metastasis in a dose-dependent manner at 24 h. Based on these
results, CA may have potential as an anticancer agent.
It was previously reported that blocking the
V-ATPases can inhibit the growth and metastasis of human cancer
cells (10,17). It has also been demonstrated that
diphyllin inhibits V-ATPase, and therefore, lysosomal acidification
in osteoclasts, which leads to the abrogation of bone resorption
(18). The present study suggested
that CA inhibits the expression of V-ATPases on A375 cells at
concentrations of 0.03, 0.1 or 0.3 µM. The administration of CA may
inhibit the concentration of V-ATPases in the cell membrane,
affecting their role in transporting H+ out of the cell,
as V-ATPase is a ubiquitous proton-translocating pump of eukaryotic
cells (19). The pumps are located in
the membranes of vacuoles, lysosomes and other components of the
endomembrane system, as well as in certain specialized plasma
membranes (19). Therefore, it was
inferred that CA may also affect the pH of lysosomes. The present
study evaluated lysosomal pH with immunofluorescence staining. It
was revealed that CA at concentrations of 0.1 and 0.3 µM alkalized
the lysosomal pH.
Tumor invasion and metastasis are multistage and
multi-factorial processes, regulated by complex mechanisms,
including multiple signaling pathways (20). Numerous proteins are associated with
regulating cancer cell adhesion, migration and invasion (21). MMPs are a family of zinc-binding
proteases that contribute to cancer cell invasion by degrading the
extracellular matrix (22,23). MT1-MMP (also known as MMP-14), an
activator of proMMP-2, was the first MMP to be identified and the
most common member of the MTMMP subfamily involved in pericellular
proteolysis associated with cell migration (24,25). The
degradation of the basement membrane, e.g., by MMP-2, may be a
necessary step in cancer invasion (26,27). The
present study demonstrated that the activity and expression levels
of MMP-2 and −9 may be inhibited by CA with gelatin zymography and
western blot analysis.
In conclusion, the present study revealed that CA is
a novel inhibitor of V-ATPases that induced a decrease in A375 cell
migration and invasion via the inhibition of the expression of
MMP-2 and −9. Recently, it was reported that the transcription of
MT1-MMP was regulated by the Wnt signaling pathway (28,29). It is
necessary to determine whether CA inhibits the activation of MMPs
via the Wnt signaling pathway through further study.
References
|
1
|
Liu J, Fukunaga-Kalabis M, Li L and Herlyn
M: Developmental pathways activated in melanocytes and melanoma.
Arch Biochem Biophys. 563:13–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Milito A, Canese R, Marino ML, Borghi
M, Iero M, Villa A, Venturi G, Lozupone F, Iessi E, Logozzi M, et
al: pH-dependent antitumor activity of proton pump inhibitors
against human melanoma is mediated by inhibition of tumor acidity.
Int J Cancer. 127:207–219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chiche J, Brahimi-Horn MC and Pouysségur
J: Tumour hypoxia induces a metabolic shift causing acidosis: A
common feature in cancer. J Cell Mol Med. 14:771–794. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vaupel P, Kallinowski F and Okunieff P:
Blood flow, oxygen and nutrient supply, and metabolic
microenvironment of human tumors: A review. Cancer Res.
49:6449–6465. 1989.PubMed/NCBI
|
|
5
|
Harguindey S, Orive G, Pedraz Luis J,
Paradiso A and Reshkin SJ: The role of pH dynamics and the
Na+/H+ antiporter in the etiopathogenesis and
treatment of cancer. Two faces of the same coin-one single nature.
Biochim Biophys Acta. 1756:1–24. 2005.PubMed/NCBI
|
|
6
|
Rofstad EK: Microenvironment-induced
cancer metastasis. Int J Radiat Biol. 76:589–605. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harris AL: Hypoxia-a key regulatory factor
in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mason SD and Joyce JA: Proteolytic
networks in cancer. Trends Cell Biol. 21:228–237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wiedmann RM, von Schwarzenberg K,
Palamidessi A, Schreiner L, Kubisch R, Liebl J, Schempp C, Trauner
D, Vereb G, Zahler S, et al: The V-ATPase-inhibitor archazolid
abrogates tumor metastasis via inhibition of endocytic activation
of the Rho-GTPase Rac1. Cancer Res. 72:5976–5987. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fais S, De Milito A, You H and Qin W:
Targeting vacuolar H+-ATPases as a new strategy against
cancer. Cancer Res. 67:10627–10630. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chung C, Mader CC, Schmitz JC, Atladottir
J, Fitchev P, Cornwell ML, Koleske AJ, Crawford SE and Gorelick F:
The vacuolar-ATPase modulates matrix metalloproteinase isoforms in
human pancreatic cancer. Lab Invest. 91:732–743. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fortini ME and Bilder D: Endocytic
regulation of Notch signaling. Curr Opin Genet Dev. 19:323–328.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vaccari T, Duchi S, Cortese K, Tacchetti C
and Bilder D: The vacuolar ATPase is required for physiological as
well as pathological activation of the Notch receptor. Development.
137:1825–1832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Ma J, Zhu L and Zhao Y: Synthesis
and identification of cytotoxic diphyllin glycosides as vacuolar
H(+)-ATPase inhibitors. Eur J Med Chem. 82:466–471. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen W, Zou X, Chen M, Liu P, Shen Y,
Huang S, Guo H and Zhang L: Effects of diphyllin as a novel
V-ATPase inhibitor on gastric adenocarcinoma. Eur J Pharmacol.
667:330–338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vandooren J, Geurts N, Martens E, Van den
Steen PE and Opdenakker G: Zymography methods for visualizing
hydrolytic enzymes. Nat Methods. 10:211–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu X, Qin W, Li J, Tan N, Pan D, Zhang H,
Xie L, Yao G, Shu H, Yao M, et al: The growth and metastasis of
human hepatocellular carcinoma xenografts are inhibited by small
interfering RNA targeting to the subunit ATP6L of proton pump.
Cancer Res. 65:6843–6849. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sørensen MG, Henriksen K, Neutzsky-Wulff
AV, Dziegiel MH and Karsdal MA: Diphyllin, a novel and naturally
potent V-ATPase inhibitor, abrogates acidification of the
osteoclastic resorption lacunae and bone resorption. J Bone Miner
Res. 22:1640–1648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu W, Wang L, Wang Y, Xu X, Zou P, Gong M,
Zheng J, You J, Wang H, Mei F and Pei F: A novel tumor metastasis
suppressor gene LASS2/TMSG1 interacts with vacuolar ATPase through
its homeodomain. J Cell Biochem. 114:570–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takahashi M, Tsunoda T, Seiki M, Nakamura
Y and Furukawa Y: Identification of membrane-type matrix
metalloproteinase-1 as a target of the beta-catenin/Tcf4 complex in
human colorectal cancers. Oncogene. 21:5861–5867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma Y, Zou F, Xiong J, Wan W, Yin L, Li X,
Bei Z, Yuan L, Meng S, Wang J and Song G: Effect of Matrine on HPAC
cell migration by down-regulating the expression of MT1-MMP via Wnt
signaling. Cancer Cell Int. 15:592015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Itoh Y and Seiki M: MT1-MMP: A potent
modifier of pericellular microenvironment. J Cell Physiol. 206:1–8.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kajita M, Itoh Y, Chiba T, Mori H, Okada
A, Kinoh H and Seiki M: Membrane-type 1 matrix metalloproteinase
cleaves CD44 and promotes cell migration. J Cell Biol. 153:893–904.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deryugina EI, Ratnikov BI, Postnova TI,
Rozanov DV and Strongin AY: Processing of integrin alpha(v) subunit
by membrane type 1 matrix metalloproteinase stimulates migration of
breast carcinoma cells on vitronectin and enhances tyrosine
phosphorylation of focal adhesion kinase. J Biol Chem.
277:9749–9756. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sato H, Takino T and Miyamori H: Roles of
membrane-type matrix metalloproteinase-1 in tumor invasion and
metastasis. Cancer Sci. 96:212–217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seiki M: Membrane-type 1 matrix
metalloproteinase: A key enzyme for tumor invasion. Cancer Lett.
194:1–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Knopfová L, Beneš P, Pekarčíková L,
Hermanová M, Masařík M, Pernicová Z, Souček K and Smarda J: c-Myb
regulates matrix metalloproteinases 1/9, and cathepsin D:
Implications for matrix-dependent breast cancer cell invasion and
metastasis. Mol Cancer. 11:152012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yousef EM, Tahir MR, St-Pierre Y and
Gaboury LA: MMP-9 expression varies according to molecular subtypes
of breast cancer. BMC Cancer. 14:6092014. View Article : Google Scholar : PubMed/NCBI
|