Introduction
Cervical cancer is the third most common type of
cancer among females, worldwide (1).
The annual global incidence of cervical cancer for 2008 was
529,800; the annual mortality rate was 275,100. Cervical cancer
rates remain high within Hispanic/Latina, African descent and Asian
female populations; additionally, cervical cancer is the second
highest cause of cancer-associated mortality for females living in
developing countries (2). Cervical
cancer is characterized by local invasion, pelvic lymph nodes and
distant organ metastasis (3).
Following treatment, the five-year survival rate of early-stage
cervical cancer may be as high as 90%. However, patients with local
advanced or distant metastatic cervical cancer still have a poor
prognosis; in particular, stage IV has a survival rate of ~20%
(4). Therefore, understanding the
molecular mechanisms underlying cervical cancer invasiveness would
be of clinical value for the identification of effective
therapeutic strategies and novel therapeutic targets.
Stomatin was first isolated from human erythrocytes
and is an integral membrane protein, which is widely expressed in
numerous types of cells (5). Stomatin
is the founding member of a family of proteins that includes
stomatin-like protein (SLP)-1, 2 and 3 in mammals (6,7). Unlike
SLP-1 and SLP-3, SLP-2 does not share an N-terminal transmembrane
domain with stomatin, which is a distinguishing feature (8). Therefore, SLP-2 may link stomatin or
other integral membrane proteins to the peripheral cytoskeleton
(8). The function of SLP-2 remains
largely unknown. Previous studies have suggested that SLP-2 may
serve a role in stabilizing the mitochondrial inner membrane,
regulating ion channel conductance and the organization of
sphingolipid and cholesterol-rich lipid rafts (9). Previous studies revealed that SLP-2 is
overexpressed in numerous types of cancer tissues and is involved
in the progression and development of cancer (10–12). Zhang
et al (10) revealed that
SLP-2 was upregulated ≥6 times in esophageal squamous cell
carcinoma tissues, and that antisense transfection of the SLP-2
gene led to S-phase arrest and decreased expression of SLP-2 in the
TE12 cell line (10). SLP-2 has been
reported as overexpressed in laryngeal squamous cell carcinoma when
compared with the adjacent normal laryngeal epithelium, and SLP-2
expression correlates with clinical stage (11). Zhang et al (12) demonstrated decreased cell growth,
proliferation, tumorigenicity and cell adhesion in the antisense
SLP-2 transfectants.
In the present study, the expression levels of SLP-2
in cervical cancer were evaluated by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR),
western blotting and immunohistochemistry; to the best of our
knowledge, this is the first study to do so. Thus, SLP-2 expression
levels are correlated with pelvic lymph node metastasis, in
addition to the prognosis of patients with early-stage cervical
cancer. To investigate the possible biological function and
underlying mechanisms of SLP-2, SLP-2 small interfering (si)RNA was
transfected into HeLa-HCC94 cells, in the current study. Antisense
transfection of SLP-2 in cervical cancer cells was identified to
reduce the rate of apoptosis. Taken together, the results suggest
that SLP-2 serves an important role in cervical cancer progression
and pathogenesis.
Materials and methods
Cell lines
Cervical cancer cell lines, HeLa, CaSki, HCC94, SiHa
and C33A, were obtained from the Cell Bank of Type Culture
Collection, Chinese Academy of Sciences, Shanghai, China. HeLa,
SiHa and C33A cells were maintained in Eagle's minimal essential
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
CaSki and Hcc94 cells were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.), all supplemented with 10% fetal
bovine serum (HyClone, Logan, UT, USA) in a humid incubator at 37°C
with 5% CO2.
Patients and tissue specimens
Between January 1999 and December 2005, 300
cancerous and corresponding adjacent normal tissues from patients
(20–71 years old) with early-stage (FIGO stage Ib-IIa) cervical
cancer as well as 10 normal cervical tissues from patients with
hysteromyoma were collected by the Department of Gynecologic
Oncology (Cancer Center, Sun Yat-sen University, Guangzhou, China).
For RT-qPCR, western blotting and immunohistochemistry, 19
surgically resected cervical cancer and paired adjacent tissues
were obtained by the Department of Gynecologic Oncology (Cancer
Center, Sun Yat-sen University, Guangzhou, China) and stored in
−80°C for further use. The present study was approved by the
Medical Ethics Committee of the Cancer Center (Sun Yat-Sen
University). Written informed consent was obtained from all
patients prior to enrollment in the present study.
Clinicopathological information for the tissue samples is presented
in Table I.
 | Table I.Clinical/pathological characteristics
of the study cohort (n=300). |
Table I.
Clinical/pathological characteristics
of the study cohort (n=300).
| Clinical/pathological
characteristics | No. patients (%) |
|---|
| Age (years) |
|
|
<40 | 142 (47) |
| ≥40 | 158 (53) |
| SCCA, ng/ml |
|
|
≤1.5 | 189 (67) |
|
>1.5 | 92 (33) |
| FIGO stage |
|
|
Ib1 | 170 (56) |
|
Ib2 | 71 (24) |
|
IIa1 | 27 (9) |
|
IIa2 | 32 (11) |
| Histological
type |
|
|
Squamous cell carcinoma | 264 (88) |
|
Adenocarcinoma | 20 (7) |
|
Adenosquamous carcinoma | 16 (5) |
| Differentiation
grade |
|
| G1 | 22 (8) |
| G2 | 117 (39) |
| G3 | 159 (53) |
| Tumor size
(cm) |
|
|
<4 | 192 (66) |
| ≥4 | 99 (34) |
| Deep stromal
invasion |
|
|
Negative | 134 (47) |
|
Positive | 152 (53) |
| LVSI |
|
|
Negative | 286 (95) |
|
Positive | 14 (5) |
| Positive
parametrium |
|
|
Negative | 293 (98) |
|
Positive | 7 (2) |
| Pelvic lymph node
metastasis (+) |
|
|
Negative | 253 (84) |
|
Positive | 47 (16) |
| Recurrence |
|
|
Negative | 259 (86) |
|
Positive | 41 (14) |
| Vital status at
follow-up |
|
|
Alive | 267 (89) |
|
Cervical cancer associated
mortality | 32 (11) |
RNA extraction, reverse transcription
and RT-qPCR
Total RNA was isolated using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. cDNA was generated from 2 µg
pretreated RNA with an iScript cDNA Synthesis kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). RT-qPCR was used to
determine SLP-2 mRNA expression levels in tissues and cell lines,
using a Bio-Rad CFX96 sequence detection system with
SsoFast® EvaGreen™ Supermix (Bio-Rad Laboratories,
Inc.). The transcript amount for SLP-2 was normalized to the
housekeeping gene GAPDH to control the variability in expression
levels and analyzed using the 2−ΔΔCq method described by
the previous study (13). Sequences
of RT-qPCR primers were designed using the Primer Express Software
version 2.0 (Applera Corporation, Norwalk, CT, USA). Primers were
as follows: SLP-2 forward, 5′-GTGACTCTCGACAATGTAAC-3′ and reverse,
5′-TGATCTCATAACGGAGGCAG-3′, with annealing conditions of 57°C for
30 sec; GAPDH forward, 5′-AATCCCATCACCATCTTCCA-3′ and reverse,
5′-CCTGCTTCACCACCTTCTTG-3′, with annealing conditions of 55°C for
30 sec.
Western blotting
Cells and ground frozen tissues were harvested and
lysed in sampling buffer [62.5 mmol/l Tris-HCl (pH 6.8), 2% SDS,
10% glycerol and 5% 2-h-mercaptoethanol]. Protein concentration was
determined using a Bradford assay (Bio-Rad Laboratories, Inc.). A
total of 20 µg of proteins were separated by 10% SDS-PAGE, prior to
being transferred onto a polyvinylidene fluoride membrane (GE
Healthcare Life Sciences, Chalfont, UK). Subsequent to being
blocked in 5% non-fat dry milk, the membrane was incubated for 12 h
at 4°C with an anti-SLP-2 rabbit polyclonal antibody (dilution,
1:3,000; cat. no. AP20280c; Abgent, Inc., San Diego, CA, USA), then
the membranes were washed with PBST and exposed to a horseradish
peroxidase-conjugated anti-rabbit secondary antibody (dilution,
1:2,000; cat. no. NA934; GE Healthcare Life Sciences) for 1 h at
room temperature. Protein bands were visualized using an enhanced
chemiluminescence kit (GE Healthcare Life Sciences). An
anti-a-tubulin polyclonal antibody (dilution, 1:1,000; cat. no.
SAB4500087; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) was
used as the loading control.
Immunohistochemistry
Immunohistochemical staining was performed using
Histostain-Plus kits (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Briefly, 4 µm
paraffin-embedded tissue sections were deparaffinized in xylene,
rehydrated in ethanol and rinsed in distilled water. Endogenous
peroxidase activity was blocked with 3% H2O2
and antigen retrieval was performed with 1 mM EDTA buffer (cat.
no.7011 V; pH 8.0; Cell Signaling Technology, Inc., Danvers, MA,
USA) for 10 min at 100°C. Following incubation with an anti-SLP-2
rabbit polyclonal primary antibody (dilution 1:100; cat. no.
10348-1-AP; ProteinTech Group, Inc., Chicago, IL, USA) at 4°C
overnight, and a biotinylated anti-rabbit secondary antibody
(dilution, 1:200; cat. no. TA130017; OriGene Technologies, Inc.,
Rockville, MD, USA) at 37°C for 15 min, the tissue sections were
immersed in streptavidin horseradish peroxidase (OriGene
Technologies, Inc.) at 37°C for 15 min and developed with
diaminobenzidine (OriGene Technologies, Inc.). Slides were
evaluated by two pathologists blind to the clinical
characteristics. Immunoreactivity score was determined by adding
the score of the percentage of positive cells (0, 0%; 1, 1-10%; 2,
11–50%; 3 51–70%; 4 71–100%) and the intensity of staining (0, no
staining; 1, weak; 2, moderate; 3, strong). Tissue samples with an
SLP-2 immunohistochemistry (IHC) final score >3 were defined as
having high expression.
Transfection
Synthesis and purification of three siRNAs targeting
SLP-2 was determined by Guangzhou RiboBio Co., Ltd. (Guangzhou,
China). The siRNA sequences were as follows: siRNA#1,
5′-CCGTTATGAGATCAAGGATATdTdT-3′; siRNA#2,
5′-GATGCAAGTCTTGATGAGGAAdTdT-3′; siRNA#3,
5′-GCAAATCGATGGAGTCCTTTAdTdT-3′. The negative control (NC)-siRNA
sequence, 5′-UUCUCCGAACGUGUCACGUTT-3′, was used as the control.
Transfections in cells at ~70% confluency were performed with
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Cell survival
Cells 1×103 cells/well were seeded in
6-well plates and incubated in a humid incubator at 37°C with 5%
CO2 for 24 h, followed by 25 µg/ml cisplatin (cat. no.
479306; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) treatment at
37°C with 5% CO2 for 48 h. Cell apoptosis was assessed
by the TUNEL system (Promega Corporation, Madison, WI, USA) and an
Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit
(EMD Millipore, Billerica, MA, USA), and then analyzed using flow
cytometry (EPICS XL flow cytometer; Breckman Coulter, Inc., Brea,
CA, USA) and a fluorescence microscope equipment with a digital
camera. Three independent experiments were done, in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS
software standard version 18.0 (SPSS, Inc., Chicago, IL, USA). The
association between the expression levels of SLP-2 and patient
clinical features was analyzed by the χ2 test. Factors
predictive of pelvic lymph node metastasis were analyzed by Binary
Logistic Regression. Survival analysis was carried out by the
Kaplan-Meier method. The Cox proportional hazards model was used to
explore possible prognostic factors. Cell apoptosis was analyzed by
an independent-sample t-test (two-tailed). Data are presented as
the mean ± standard error of the mean. P<0.05 was considered to
indicate a statistically significant difference.
Results
Upregulation of SLP-2 in cervical
cancer cell lines and cervical cancer tissues
To determine SLP-2 protein expression, western
blotting, RT-qPCR and IHC assay were carried out in various
cervical cancer cell lines (HeLa, SiHa, C33A, CaSki and Hcc94),
normal cervical tissues and fresh cervical cancer tissues (T), with
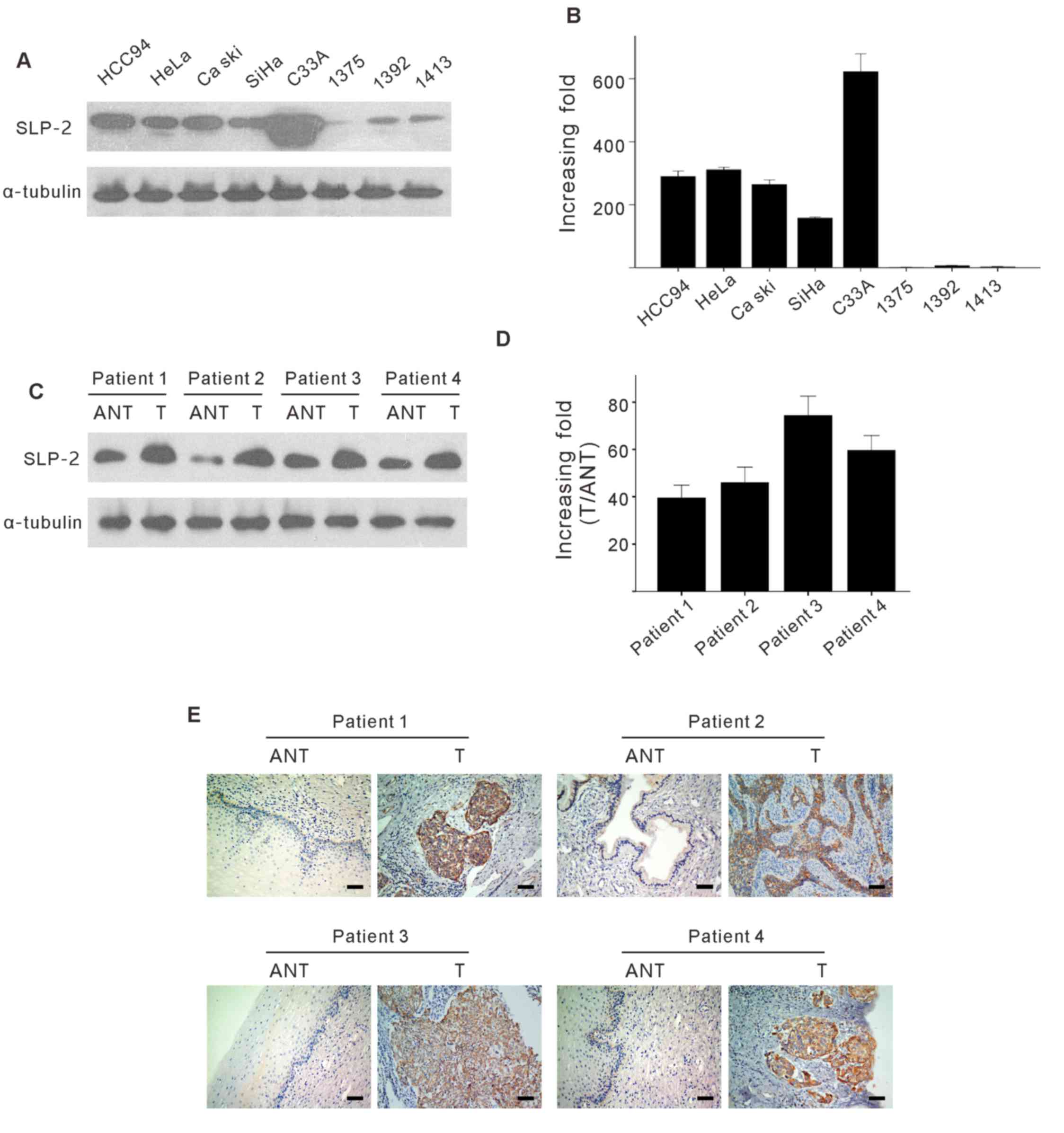
paired adjacent noncancerous tissues (ANT). As presented in
Fig. 1A and B, SLP-2 proteins and
mRNA expression was upregulated in the examined cervical cancer
cell lines, by comparison with normal cervical tissues.
Furthermore, comparative analysis revealed that SLP-2 was highly
expressed in all cancer tissues from patients with cervical cancer,
as compared with the paired adjacent noncancerous tissue expression
levels (Fig. 1C and D). This was also
confirmed by IHC in the four aforementioned paired tissues
(Fig. 1E). Taken together, these
results suggest that SLP-2 is upregulated at the protein and mRNA
level in cervical cancer. Subsequently, IHC analysis was carried
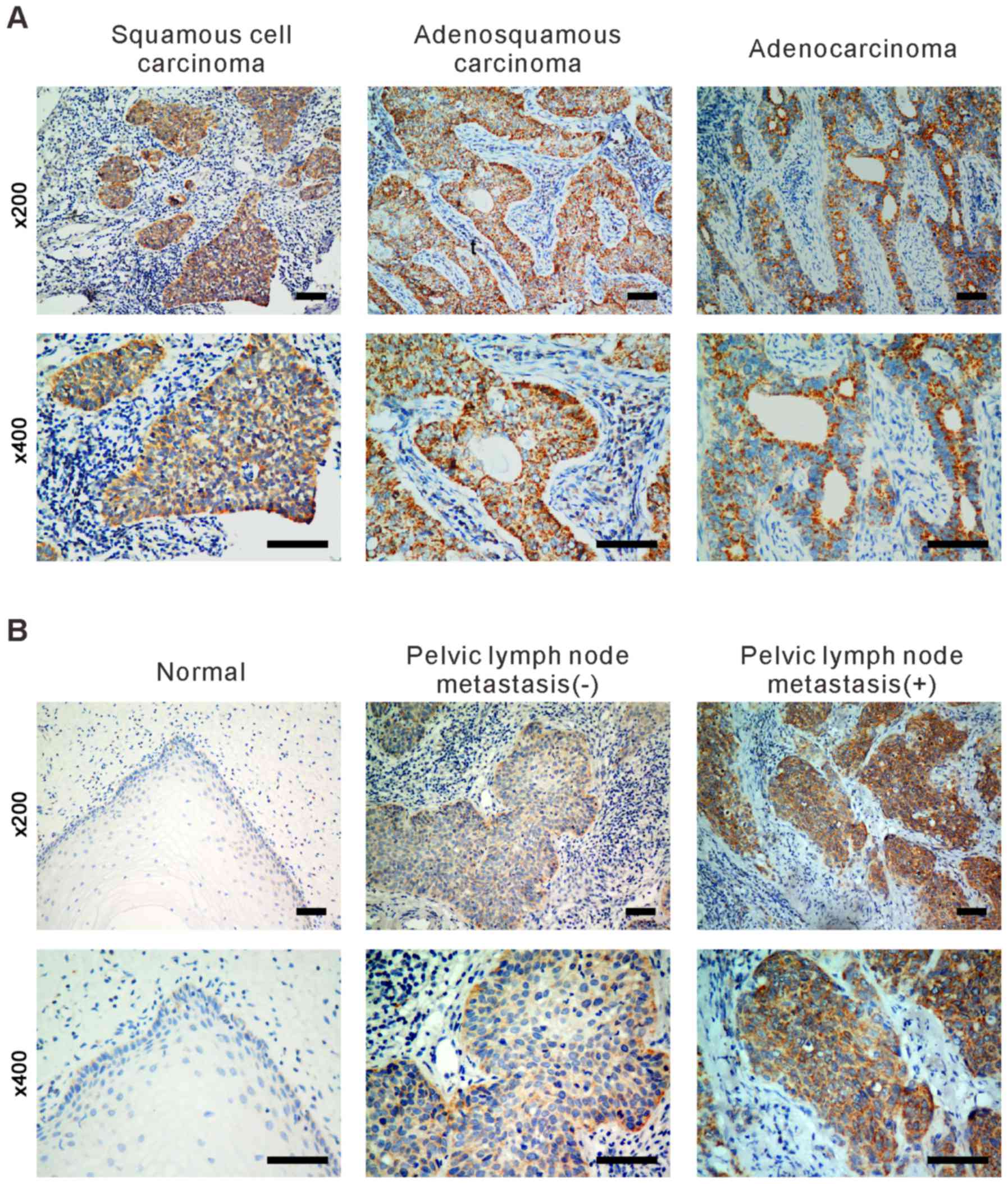
out to examine SLP-2 protein expression in 10 paraffin-embedded
normal cervical tissue samples and in 300 cases of FIGO stage
Ib-IIa sectioned cervical cancer tissues, including three
histological types of cervical cancer (squamous cell carcinoma,
adenocarcinoma and adenosquamous carcinoma). The detected
expression levels of SLP-2 in paraffin-embedded cervical cancer
tissues were as follows: SLP-2 strongly positive 24% (72/300),
positive 48% (144/300), weakly positive 20.3% (61/300) and negative
7.7% (23/300). By contrast, SLP-2 was marginally or not detected in
the normal cervical tissues or in areas surrounding the cancerous
tissues in all tumor samples. As presented in Fig. 2, expression of SLP-2 was higher in
patients with lymph node metastasis when compared with those with
no lymph node metastasis. Immunostaining revealed that SLP-2 was
localized to the cytoplasm.
SLP-2 is positively correlated with
squamous cell carcinoma antigen (SCCA), deep stromal invasion,
lymphovascular space involvement and, in particular, pelvic lymph
node metastasis
As presented in Table
II, statistical analysis of the IHC results demonstrated a
significant correlation between SLP-2 protein expression and
clinical characteristics, including the SCCA (χ2=9.014;
P=0.003), deep stromal invasion (χ2=5.321; P=0.021),
lymphovascular space involvement (χ2=4.050; P=0.044) and
pelvic lymph node metastasis (χ2=38.668; P<0.001) of
patients with cervical cancer, whereas it was not associated with
age, gender, stage of cancer, differentiation grade, positive
parametrium or histological type. Furthermore, in logistic
regression analysis, including the variables of tumor size, deep
stromal invasion, positive parametrium, lymph vascular space
involvement, SCC and SLP-2 expression, revealed that SLP-2 protein
expression (P<0.001; OR=6.810) and SCCA ≥1.5 ng/ml (P<0.001;
OR=5.361) in cervical cancer was significantly associated with the
lymph node metastasis (Table
III).
 | Table II.Association between SLP-2 expression
and clinical/pathological characteristics (n=300). |
Table II.
Association between SLP-2 expression
and clinical/pathological characteristics (n=300).
|
| No. patients
(%) |
|
|
|---|
|
|
|
|
|
|---|
|
Clinical/pathological characteristics | Low/no SLP-2
expression | High
SLP-2expression | χ2 | P-value |
|---|
| Total no. of
patients | 228 (76) | 72 (24) |
|
| Age (years) |
|
|
|
|
|
<40 | 112 (49) | 30 (42) | 1.220 | 0.269 |
|
≥40 | 116 (51) | 42 (58) |
|
|
| SCCA (ng/ml) |
|
|
|
|
|
<1.5 | 154 (72) | 35 (52) | 9.014 | 0.003a |
|
≥1.5 | 60 (28) | 32 (48) |
|
|
| FIGO stage |
|
|
|
|
|
Ib1 | 129 (57) | 41 (57) | 0.066 | 0.996 |
|
Ib2 | 54 (24) | 17 (24) |
|
|
|
IIa1 | 21 (9) | 6 (8) |
|
|
|
IIa2 | 24 (10) | 8 (11) |
|
|
| Differentiation
grade |
|
|
|
|
| G1 | 19 (8) | 3 (4) | 2.898 | 0.235 |
| G2 | 92 (41) | 25 (35) |
|
|
| G3 | 115 (51) | 44 (61) |
|
|
| Tumor size
(cm) |
|
|
|
|
|
<4 | 145 (66) | 47 (66) | 0.002 | 0.964 |
| ≥4 | 75 (34) | 24 (34) |
|
|
| Deep stromal
invasion |
|
|
|
|
|
Negative | 110 (51) | 24 (35) | 5.321 | 0.021a |
|
Positive | 107 (49) | 45 (65) |
|
|
| LVSI |
|
|
|
|
|
Negative | 221 (97) | 65 (90) | 4.050 | 0.044a |
|
Positive | 7 (3) | 7 (10) |
|
|
| Positive
parametrium |
|
|
|
|
|
Negative | 225 (99) | 68 (94) | 2.656 | 0.103 |
|
Positive | 3 (1) | 4 (6) |
|
|
| Positive surgical
margin |
|
|
|
|
|
Negative | 215 (94) | 63 (87) | 3.721 | 0.054 |
|
Positive | 13 (6) | 9 (13) |
|
|
| Pelvic lymph node
metastasis |
|
|
|
|
Negative | 209 (92) | 44 (61) | 38.668 |
<0.001a |
|
Positive | 19 (8) | 28 (39) |
|
|
| Recurrence |
|
|
|
|
|
Negative | 201 (88) | 58 (81) | 2.680 | 0.102 |
|
Positive | 27 (12) | 14 (19) |
|
|
 | Table III.Multivariate analysis of risk factors
of lymph node metastasis. |
Table III.
Multivariate analysis of risk factors
of lymph node metastasis.
| Parameters | B | Wald | P-value | OR | 95% confidence
interval |
|---|
| SCCA | 1.679 | 12.217 |
<0.001a | 5.361 |
2.091–13.745 |
| Tumor size | 0.464 | 0.792 | 0.374 | 1.591 | 0.572–4.424 |
| LVSI | 1.084 | 2.004 | 0.157 | 2.956 |
0.659–13.253 |
| Deep stromal
invasion | 0.372 | 0.665 | 0.415 | 1.451 | 0.593–3.553 |
| Positive
parametrium | 2.788 | 3.252 | 0.071 | 16.255 |
0.785–336.589 |
| SLP-2 expression
level | 1.918 | 19.797 |
<0.001a | 6.810 |
2.925–15.855 |
| Age | −0.147 | 0.115 | 0.734 | 0.863 | 0.369–2.017 |
| FIGO stage | −0.196 | 0.548 | 0.459 | 0.822 | 0.489–1.382 |
| Histological
type | 0.856 | 3.425 | 0.064 | 2.355 | 0.951–5.832 |
| Differentiation
grade | −0.064 | 0.033 | 0.856 | 0.938 | 0.469–1.877 |
| Surgical
margin | −1.110 | 0.925 | 0.336 | 0.329 | 0.034–3.166 |
SLP-2 expression is associated with
the prognosis of patients with cervical cancer
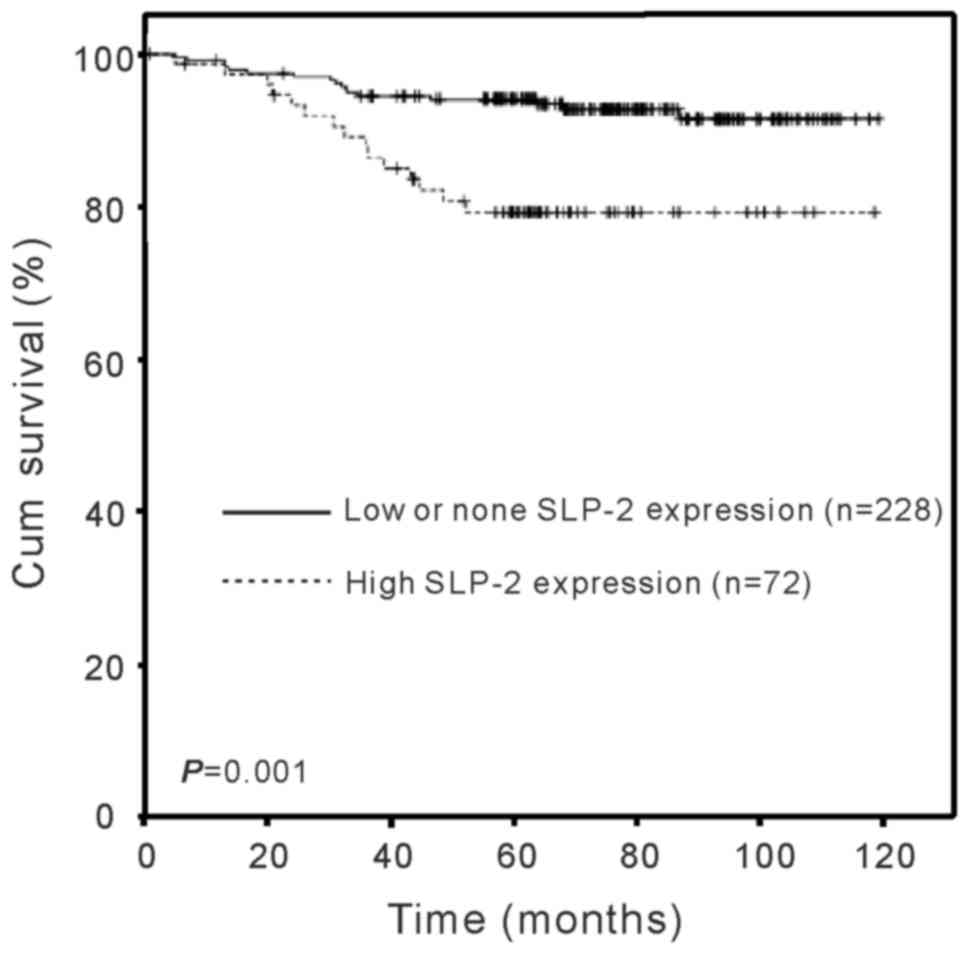
Analysis of patient survival was conducted in order
to determine whether SLP-2 expression was associated with the
survival time. As presented in Fig.
3, the duration of survival was significantly different between
the patients with low/none and high SLP-2 expression levels
(log-rank test, χ2=11.615; P=0.001), with the high SLP-2
expression group exhibiting a shorter overall survival time,
indicating that the expression of SLP-2 was inversely correlated
with survival time. The cumulative five year survival rate was 92%
in the low/none SLP-2 expression group, whereas it was 78% in the
high SLP-2 expression group. Furthermore, multivariate COX analysis
was performed to determine whether the SLP-2 expression level is an
independent prognostic factor of patient outcome. As presented in
Table IV, SLP-2 expression levels
were identified as independent prognostic factors for patients with
cervical cancer (P=0.001; relative risk=3.881). Taken together, the
data suggests that SLP-2 may be a novel and potentially useful
independent biomarker for the prognosis of patients with cervical
cancer.
 | Table IV.Multivariate survival analysis of
patients with cervical cancer. |
Table IV.
Multivariate survival analysis of
patients with cervical cancer.
| Parameters | B | Wald | P-value | RR | 95% confidence
interval |
|---|
| Age | 0.102 | 0.059 | 0.807 | 1.108 | 0.486–2.523 |
| FIGO stage | −0.220 | 0.585 | 0.444 | 0.802 | 0.457–1.410 |
| Tumor size | 0.145 | 0.072 | 0.789 | 1.156 | 0.400–3.343 |
| SCCA | 0.206 | 0.188 | 0.665 | 1.229 | 0.483–3.127 |
| SLP-2 level | 1.356 | 10.276 | 0.001a | 3.881 | 1.694–8.894 |
| Histological
type | 0.526 | 1.874 | 0.171 | 1.693 | 0.797–3.597 |
| Differentiation
grade | −0.254 | 0.603 | 0.438 | 0.776 | 0.409–1.473 |
| Deep stromal
invasion | 0.337 | 0.578 | 0.447 | 1.401 | 0.587–3.343 |
| Positive
parametrium | 11.726 | 0.005 | 0.941 | 123,740.450 |
0.000–1.163E140 |
| Surgical
margin | −10.578 | 0.004 | 0.947 | 0.000 |
0.000–2.385E130 |
| LVSI | 0.457 | 0.392 | 0.532 | 1.579 | 0.378–6.603 |
Downregulation of SLP-2 enhanced
cellular apoptosis
SLP-2 expression levels were revealed in the present
study to correlate with SCCA, deep stromal invasion, LVSI and
pelvic lymph node metastasis. The function of SLP-2 is closely
associated with the mitochondrial membrane (9,14);
therefore, it has been suggested by the present study that SLP-2
serves a role in the resistance of cervical cancer cells to
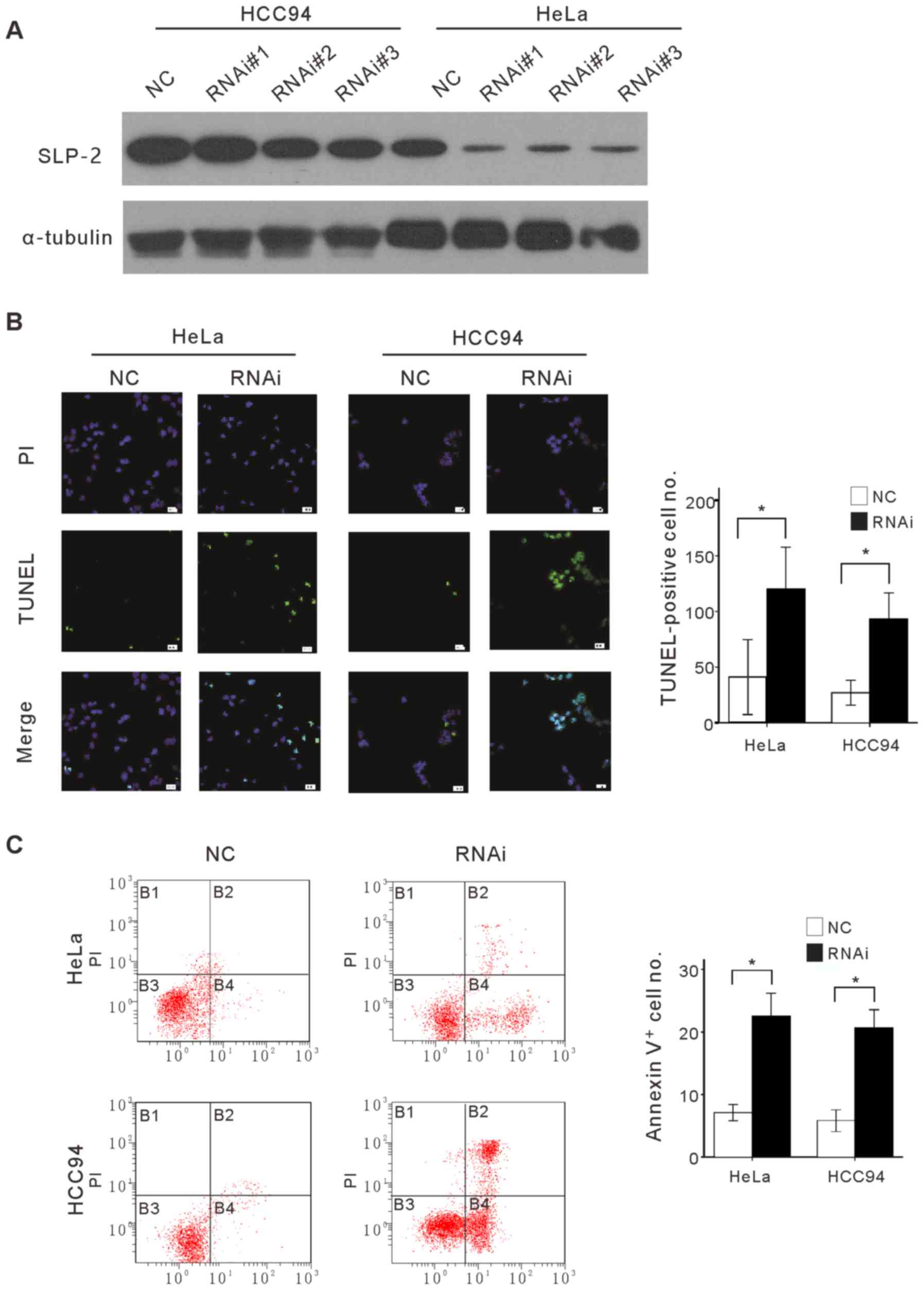
apoptosis. Endogenous SLP-2 in HeLa and Hcc94 cells was first
knocked down by using specific siRNAs and the sensitivity of the
modified cells to apoptosis was evaluated, in the current study. As
presented in Fig. 4A, three siRNAs
knocked down endogenous SLP-2 protein in cervical cancer cell
lines. For subsequent evaluation, siRNA#3 was selected due to its
higher efficiency. The apoptotic nature of induced cell death was
confirmed by TUNEL and Annexin V binding assays on SLP-2 knocked
down cells and control cells treated with 25 µg/ml cisplatin, a
widely used chemotherapeutic drug for cervical cancer treatment
(Fig. 4B and C). TUNEL and Annexin V
binding assays revealed that the number of apoptotic cells, when
treated with cisplatin in SLP-2 knocked-down cells was
significantly higher than that in control cells. Taken together,
the results indicated that cellular depletion of SLP-2 impaired the
ability of cervical cancer cells to resist cisplatin-induced cell
death.
Discussion
In the present study, the expression of SLP-2 was
revealed to be upregulated in cervical cancer at the mRNA and
protein level, in comparison with normal cervical tissue expression
levels. Meanwhile, western blotting and RT-qPCR analysis
demonstrated the overexpression of SLP-2 in cervical cancer cell
lines, when compared with their normal counterparts. A previous
study revealed that SLP-2 is overexpressed in numerous types of
human cancer tissues (10–12). In addition, the present study revealed
that SLP-2 downregulation enhances the sensitivity to apoptosis
inducers in cancer tissues. Therefore, it was concluded that SLP-2
may be fundamentally important in human tumorigenesis.
Based on the National Comprehensive Cancer Network
(NCCN) guidelines, for patients with early stage disease who
possess negative lymph nodes following surgery and pathologic risk
factors including large primary tumor size, deep stromal invasion
and LVSI, pelvic radiation is recommended (15–17). In
the present study, it was demonstrated that the survival time was
significantly different between patients with low/none and high
SLP-2 expression. Additionally, multivariate COX analysis further
confirmed that SLP-2 expression level is an independent prognostic
factor of patient outcome. Furthermore, the present study revealed
that SLP-2 protein expression correlated significantly with the
previously mentioned pathologic risk factors including deep stromal
invasion and LVSI. As a result, it was suggested that high SLP-2
expression is an important pathologic risk factor for determining
the necessity of pelvic radiation following surgery.
Patients with stage IB or IIA tumors usually undergo
surgery, however radiation therapy or concurrent chemoradiation may
be the chosen method of treatment (18,19). A
number of specialists suggest that if the lymph nodes are positive,
surgery should be abandoned and the patients should receive
chemoradiation (20). Therefore, if
pathological factors predict the pelvic lymph node metastasis,
gynecologic oncologists may select the method of treatment to avoid
unnecessary surgical intervention. Currently, there are no
efficient techniques for diagnosing lymph node metastasis,
particularly for those are smaller than 0.5 cm. (21–23) The
present study demonstrated that high expression of SLP-2 was
closely associated with lymph node metastasis. In addition,
logistic regression analysis revealed that SLP-2 protein expression
in cervical cancer was an independent risk factor for lymph node
metastasis. Therefore, if SLP-2 expression levels are high,
chemoradiation may be the better option for patients with suspected
lymph node metastasis diagnosed by computed tomography or magnetic
resonance imaging scans. Taken together, the results suggest that
SLP-2 may be a novel and potential biomarker that may assist in
guiding treatment.
SLP-2 is expressed in a number of normal types of
cell in addition to cancer cells; it is associated with the inner
mitochondrial membrane and faces the intermembrane space (9,24–27). The function of SLP-2 association with
the mitochondrial membrane marks a suitable starting point for
investigating the potential role of SLP-2 in tumorigenesis. It is
well known that mitochondria serve an important role in the
regulation of apoptosis, which indicates that mitochondria could
determine cell outcomes (28,29). Four major events are involved in this
process, including the regulation of calcium concentration within
the cytoplasm, modification of mitochondrial membrane permeability,
dissipation of mitochondrial transmembrane potentials and the
alteration of mitochondrial functions by Bcl-2 family members
(29). Previous studies revealed that
SLP-2 contributes to mitochondrial membrane stability and regulates
the functions of its ion channels (24,25). SLP-2
interacts with the mitochondrial fusion mediators mitofusin 1,
mitofusin 2 and optic atrophy 1, which may participate in
mitochondrial fusion (9,26). Hajek et al (9) demonstrated that knockdown of SLP-2 by
the siRNA approach reduced the mitochondrial membrane potential. Da
Cruz et al (24) demonstrated
that SLP-2 serves a role in the regulation and stability of
mitochondrial proteins, including prohibitins and subunits of the
respiratory chain complexes. Da Cruz et al (25) also revealed that SLP-2 negatively
modulates the mitochondrial sodium-calcium exchange. As
aforementioned, elevation of the calcium concentration participates
directly in signal transduction and performance of early apoptosis
via the Ras-mitogen-activated protein kinase (MAPK) signaling
pathway (30,31). Mitochondria serve an important
functional role at the intracellular calcium level but the
underlying mechanisms require further investigation. SLP-2, located
in the mitochondrial membrane, is able to stabilize the
mitochondrial membrane and regulate mitochondrial ion channels
(24–26). The present study revealed that
silencing of SLP-2 induces apoptosis in cervical cancer cell lines,
indicating that SLP-2 may inhibit apoptosis by affecting
mitochondrial membrane permeability and regulating the internal
flow of calcium ions. Further study into the regulation of calcium
and the Ras-MAPK signaling pathway by SLP-2 is required.
In conclusion, SLP-2 serves an important role in
apoptosis and is closely associated with the occurrence and
development of cervical cancer; therefore, it may be a potent
diagnostic marker and therapeutic target.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Guangdong Province, China (grant no.
S2013010016194).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barnholtz-Sloan J, Patel N, Rollison D,
Kortepeter K, MacKinnon J and Giuliano A: Incidence trends of
invasive cervical cancer in the United States by combined race and
ethnicity. Cancer Causes Control. 20:1129–1138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Benedet JL, Bender H, Jones H III, Ngan HY
and Pecorelli S: FIGO staging classifications and clinical practice
guidelines in the management of gynecologic cancers. FIGO Committee
on Gynecologic Oncology. Int J Gynaecol Obstet. 70:209–262. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sherman ME, Wang SS, Carreon J and Devesa
SS: Mortality trends for cervical squamous and adenocarcinoma in
the United States. Relation to incidence and survival. Cancer.
103:1258–1264. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stewart GW: Stomatin. Int J Biochem Cell
Biol. 29:271–274. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seidel G and Prohaska R: Molecular cloning
of hSLP-1, a novel human brain-specific member of the band 7/MEC-2
family similar to Caenorhabditis elegans UNC-24. Gene. 225:23–29.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldstein BJ, Kulaga HM and Reed RR:
Cloning and characterization of SLP3: A novel member of the
stomatin family expressed by olfactory receptor neurons. J Assoc
Res Otolaryngol. 4:74–82. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y and Morrow JS: Identification and
characterization of human SLP-2, a novel homologue of stomatin
(band 7.2b) present in erythrocytes and other tissues. J Biol Chem.
275:8062–8071. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hajek P, Chomyn A and Attardi G:
Identification of a novel mitochondrial complex containing
mitofusin 2 and stomatin-like protein 2. J Biol Chem.
282:5670–5681. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang LY, Ding F, Liu ZM, Li WD, Liu ZH
and Li YD: Effect of stomatin-like protein 2 (SLP-2) gene on growth
and proliferation of esophageal squamous carcinoma cell line TE12.
Ai Zheng. 24:155–159. 2005.(In Chinese). PubMed/NCBI
|
|
11
|
Cao WF, Zhang LY, Liu MB, Tang PZ, Liu ZH
and Sun BC: Prognostic significance of stomatin-like protein 2
overexpression in laryngeal squamous cell carcinoma: Clinical,
histologic and immunohistochemistry analyses with tissue
microarray. Hum Pathol. 38:747–752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Ding F, Cao W and Liu Z, Liu W,
Yu Z, Wu Y, Li W, Li Y and Liu Z: Stomatin-like protein 2 is
overexpressed in cancer and involved in regulating cell growth and
cell adhesion in human esophageal squamous cell carcinoma. Clin
Cancer Res. 12:1639–1646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chevallet M, Lescuyer P, Diemer H, van
Dorsselaer A, Leize-Wagner E and Rabilloud T: Alterations of the
mitochondrial proteome caused by the absence of mitochondrial DNA:
A proteomic view. Electrophoresis. 27:1574–1583. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marchiole P, Buénerd A, Benchaib M, Nezhat
K, Dargent D and Mathevet P: Clinical significance of lympho
vascular space involvement and lymph node micrometastases in
early-stage cervical cancer: A retrospective case-control
surgico-pathological study. Gynecol Oncol. 97:727–732. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chernofsky MR, Felix JC, Muderspach LI,
Morrow CP, Ye W, Groshen SG and Roman LD: Influence of quantity of
lymph vascular space invasion on time to recurrence in women with
early-stage squamous cancer of the cervix. Gynecol Oncol.
100:288–293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Monk BJ, Wang J, Im S, Stock RJ, Peters WA
III, Liu PY, Barrett RJ II, Berek JS, Souhami L, Grigsby PW, et al:
Rethinking the use of radiation and chemotherapy after radical
hysterectomy: A clinical-pathologic analysis of a gynecologic
oncology group/southwest oncology group/radiation therapy oncology
group trial. Gynecol Oncol. 96:721–728. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Keys HM, Bundy BN, Stehman FB, Muderspach
LI, Chafe WE, Suggs CL III, Walker JL and Gersell D: Cisplatin,
radiation, and adjuvant hysterectomy compared with radiation and
adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl
J Med. 340:1154–1161. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morris M, Eifel PJ, Lu J, Grigsby PW,
Levenback C, Stevens RE, Rotman M, Gershenson DM and Mutch DG:
Pelvic radiation with concurrent chemotherapy compared with pelvic
and para-aortic radiation for high-risk cervical cancer. N Engl J
Med. 340:1137–1143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Selman TJ, Mann C, Zamora J, Appleyard TL
and Khan K: Diagnostic accuracy of tests for lymph node status in
primary cervical cancer: A systematic review and meta-analysis.
CMAJ. 178:855–862. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anzai Y, Piccoli CW, Outwater EK, Stanford
W, Bluemke DA, Nurenberg P, Saini S, Maravilla KR, Feldman DE,
Schmiedl UP, et al: Evaluation of neck and body metastases to nodes
with ferumoxtran 10-enhanced MR imaging: Phase III safety and
efficacy study. Radiology. 228:777–788. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim JH, Beets GL, Kim MJ, Kessels AG and
Beets-Tan RG: High-resolution MR imaging for nodal staging in
rectal cancer: Are there any criteria in addition to the size? Eur
J Radiol. 52:78–83. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Perigaud C, Bridji B, Roussel JC, Sagan C,
Mugniot A, Duveau D, Baron O and Despins P: Prospective
preoperative mediastinal lymph node staging by integrated positron
emission tomography-computerised tomography in patients with
non-small-cell lung cancer. Eur J Cardiothorac Surg. 36:731–736.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Da Cruz S, Parone PA, Gonzalo P, Bienvenut
WV, Tondera D, Jourdain A, Quadroni M and Martinou JC: SLP-2
interacts with prohibitins in the mitochondrial inner membrane and
contributes to their stability. Biochim Biophys Acta. 1783:904–911.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Da Cruz S, De Marchi U, Frieden M, Parone
PA, Martinou JC and Demaurex N: SLP-2 negatively modulates
mitochondrial sodium-calcium exchange. Cell Calcium. 47:11–18.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tondera D, Grandemange S, Jourdain A,
Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke
I, Merkwirth C, et al: SLP-2 is required for stress-induced
mitochondrial hyperfusion. EMBO J. 28:1589–1600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Cao W, Yu Z and Liu Z:
Downregulation of a mitochondria associated protein SLP-2 inhibits
tumor cell motility, proliferation and enhances cell sensitivity to
chemotherapeutic reagents. Cancer Biol Ther. 8:1651–1658. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adrain C and Martin SJ: The mitochondrial
apoptosome: A killer unleashed by the cytochrome seas. Trends
Biochem Sci. 26:390–397. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Velde C Vande, Cizeau J, Dubik D, Alimonti
J, Brown T, Israels S, Hakem R and Greenberg AH: BNIP3 and genetic
control of necrosis-like cell death through the mitochondrial
permeability transition pore. Mol Cell Biol. 20:5454–5468. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Berridge MJ, Lipp P and Bootman MD: The
versatility and universality of calcium signalling. Nat Rev Mol
Cell Biol. 1:11–21. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
McConkey DJ and Nutt LK: Calcium flux
measurements in apoptosis. Methods Cell Biol. 66:229–246. 2001.
View Article : Google Scholar : PubMed/NCBI
|