Introduction
Invasion and migration, which significantly affect
the efficacy of chemotherapeutics (1), have been demonstrated to be the main
characteristics of tumor cells (2).
Invasion and migration are complicated processes that involve a
number of factors (1). For example,
migration is closely associated with the degradation of the
extracellular matrix (ECM). Tumor cells degrade the ECM and
basement membrane (BM) through the function of matrix
metalloproteinases (MMPs) and urokinase plasminogen activator
(uPA), in order to metastasize to other tissues (3). MMP-2 and MMP-9, which are regarded as
the most important factors associated with tumor metastasis, belong
to the MMP family (4). It has been
established that the inhibition of the expression of MMP-2 and
MMP-9 may significantly decrease the degradation of collagen from
the ECM and BM in order to prevent the development, invasion and
metastasis of cancer cells (5).
p38 mitogen-activated protein kinase (MAPK) is an
important signaling transduction factor. Apart from its essential
role of in cell proliferation, differentiation and apoptosis
regulation, this molecule is also involved in the proliferation,
apoptosis and invasion processes of malignant cells (6,7). The
invasion and migration capabilities of cancer cells require the
activation of specific intracellular signaling cascades, including
MMPs. The p38MAPK signaling pathway is considered to serve a key
function in cell invasion and migration; for example, it has been
demonstrated that p38 may induce the expression of MMP1, MMP3 and
MMP13, which regulate matrix remodeling and degradation by
metastatic cancer cells (8). In
addition, p38a may affect hypoxia-inducible factor 1 and vascular
endothelial growth factor, which serve functions in cancer cell
survival, angiogenesis and metastasis (9). Multiple genes and cytokines activate the
p38MAPK signaling pathway to mediate cell invasion and metastasis
in gastric cancer (10,11). It has been established that a specific
p38MAPK signaling pathway inhibitor (SB202190) may reduce the
multidrug resistance gene 1 expression levels by inhibiting the
p38MAPK signaling cascade, thus increasing the sensitivity of
gastric cancer cells to chemotherapeutic drugs (12). A number of in vitro studies
indicated that MMP-2 and MMP-9 are closely associated with the
p38MAPK signaling cascade (13–15).
Silibinin compounds are members of the flavonoids
family, and have received attention for their antitumor activities
(16–18). Evidence indicates that silibinin may
inhibit the proliferation, invasion and migration of tumor cells.
Chang et al (19) demonstrated
that silibinin may inhibit the invasion and migration of cells, and
the growth of tumor cells in nude mice, in addition to enhancing
the chemosensitivity of tumor cells to 5-fluorouracil (5-FU) and
paclitaxel. The present study aimed to investigate the
effectiveness of silibinin and SB203580, a p38MAPK signaling
pathway inhibitor (20–24), on the invasion and migration abilities
of the gastric cancer SGC7901 cell line, in order to examine the
expression of MMP-2 and MMP-9 at the protein and messenger RNA
(mRNA) levels, and to elucidate the role of p38MAPK signaling
cascades in the processes of invasion and migration in SGC7901
cells.
Materials and methods
Materials
SGC7901 cells were purchased from the Chinese
Academy of Medical Sciences' tumor cell library (Beijing, China).
The primary antibodies against p38MAPK (dilution, 1:200; catalog
no. sc-7972), phosphorylated (p-)p38MAPK (dilution, 1:200; catalog
no. sc-17852-R), MMP-2 (dilution, 1:200; catalog no. sc-13594),
MMP-9 (dilution, 1:200; catalog no. sc-21733) and -actin (dilution,
1:3,000; catalog no. 130300) were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The horseradish peroxidase
(HRP)-conjugated goat anti-rabbit immunoglobulin G(IgG) (dilution,
1:2,000; catalog no. ZDR-5306) and the HRP-conjugated goat
anti-mouse IgG (dilution, 1:2,000; catalog no. ZDR-5307) secondary
antibodies were purchased from Beijing Golden Bridge Biotechnology
Co., Ltd. (Beijing, China). The silibinin compound was obtained
from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). The RPMI-1640
culture medium and fetal calf serum were purchased from Gibco;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Matrigel was
purchased from BD Biosciences (Franklin Lakes, NJ, USA). The
Transwell chamber and the TRIzol reagent were obtained from Corning
Incorporated (Corning, NY, USA) and Invitrogen; Thermo Fisher
Scientific, Inc., respectively.
Cell culture
The gastric cancer SGC7901 cells were cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100
µg/ml streptomycin at 37°C in a humidified atmosphere of 5%
CO2.
Wound healing assay
The cells were seeded on 6-well plates at a density
of 2×105 cells/ml per well. The complete culture medium
was replaced by culture medium without FBS subsequent to cell
seeding for 48 h to induce cell synchronization. A 200-µl pipette
tip was used to draw a straight line in the longitudinal center of
each well, and then each well was washed with PBS twice. The cells
were cultured in RPMI-1640 medium containing 50, 100 or 200 µM
silibinin for 24, 48 and 72 h. The image was captured by measuring
the width of a scratch using the IPP 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA). The cell migration distance
formula used was: Cell migration distance (µm)=(initial scratch
width-drug treatment scratch width)/2. In the control
group, complete medium was added only and it considered 0 µM.
Transwell chamber assay
The Transwell assays were performed in Transwell
chambers with 8-µm pore size. The SGC7901 cells were trypsinized
(catalog no. 25200056; Gibco; Thermo Fisher Scientific, Inc.) and
suspended with 1% FBS. Subsequent to counting cells at a density of
2×106 cells/ml, a 100-µl cell suspension was plated into
the upper chamber, and 600 µl medium supplemented with 20% FBS was
placed into the lower chamber. Subsequent to incubation at 37°C for
48 h, the cells on the upper surface of the filters were removed
and the cells adhering to the undersurface of the filter membrane
were fixed with 4% paraformaldehyde for 20 min. Subsequently,
Giemsa stain (2%) was added to stain the transferred cells for 30
min at room temperature, cells were then washed with PBS three
times. The cells on the lower chamber were counted under an
inverted microscope in five random fields. The mean cell numbers
were recorded and analyzed. The experiment was repeated three
times. For the control group, complete medium was added only.
Matrigel-based invasion assay
The Matrigel assays were performed in Transwell
chambers with 8-µm pore size coated with Matrigel at 1:9 dilution
in RPMI-1640 medium for 4 h at 37°C (to allow the Matrigel to
solidify prior to plating cells). The experimental processes were
performed as described above for the Transwell assays.
Western blotting
Equal numbers of cells were lysed in lysis buffer
composed of 0.6 M Tris-HCl (pH 6.8), 10% SDS and a protease
inhibitor cocktail (catalog no. 78430; Thermo Fisher Scientific,
Inc.). Samples were incubated at 4°C for 10 min and then
centrifuged at 10,000 × g for 15 min at 4°C. The supernatants were
transferred, mixed and boiled in sample buffer (10 ml glycerol, 15
ml 20% SDS, 12.5 ml 4X upper tris, 12.5 H2O). The
supernatants were then separated by SDS-PAGE (12% gel) and
transferred to a polyvinylidene fluoride membrane. The membrane was
then incubated at room temperature in a blocking buffer composed of
5% fat-free milk dissolved in 1X 10 mM Tris (pH 7.5), 100 mM NaCl
and 1% Tween 20 (TBST) for 1 h, followed by incubation with the
blocking buffer containing the primary antibodies against p38MAPK,
p-p38MAPK, MMP-2, MMP-9 and β-actin at 4°C overnight. The membrane
was next washed with TBST and incubated with the secondary
antibodies for 1 h at room temperature. The blot was exposed to
Pierce™ ECL Plus Western Blotting Substrate (catalog no.
32132X3; Pierce; Thermo Fisher Scientific, Inc.)
electrochemiluminescence subsequent to TBS washing. The blots were
analyzed using ImageJ software 1.4 (National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
All statistical analyses were carried out using the
SPSS 13.0 statistical software package (SPPS, Inc., Chicago, IL,
USA). Either a two-tailed Student's t-test or a one-way analysis of
variance, followed by the Tukey's test, was performed. P<0.05
was considered to indicate a statistically significant
difference.
Results
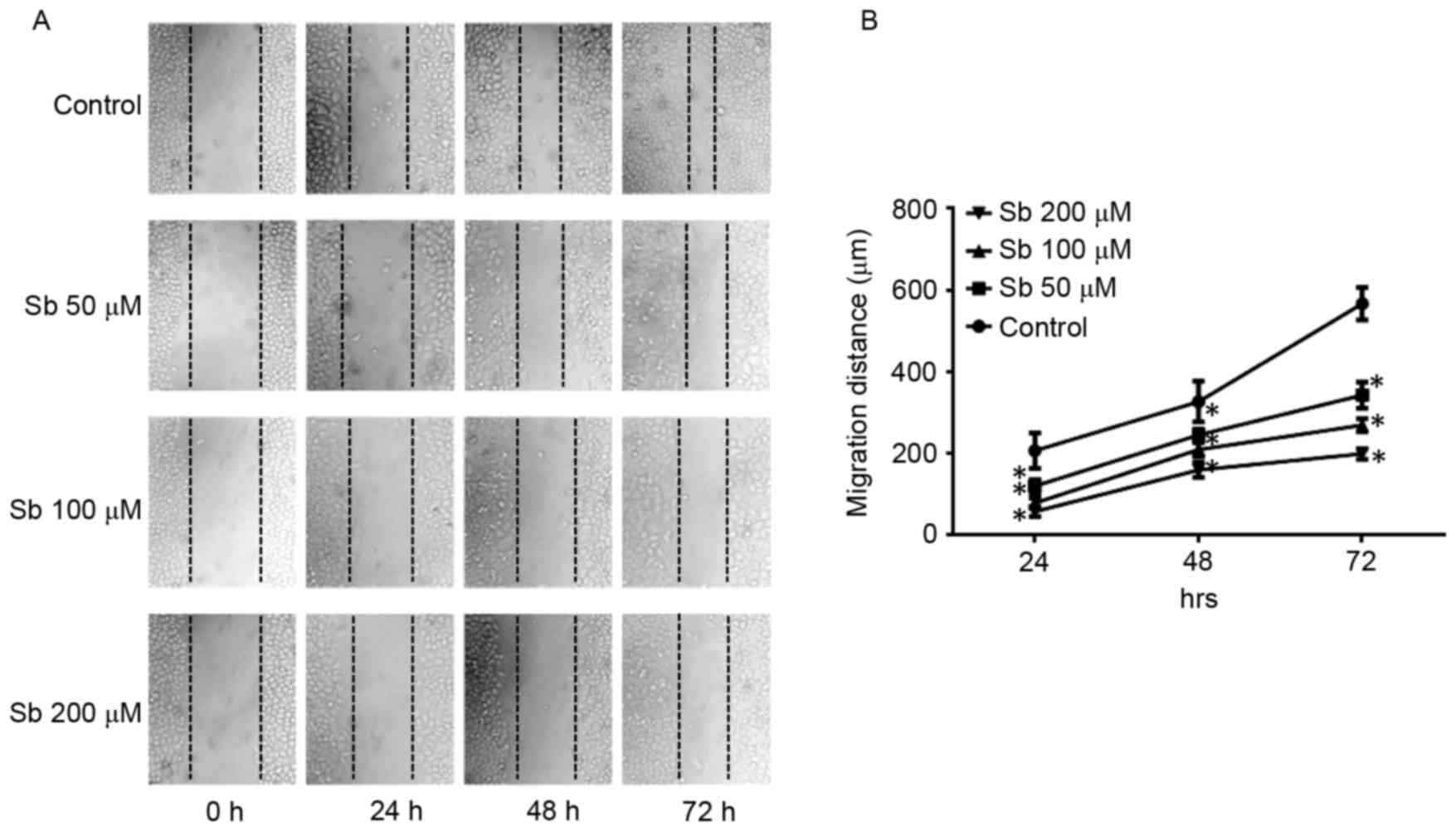
Wound healing assay to detect the
effects of silibinin on SGC7901 cell migration
The wound healing assay indicated that silibinin
significantly reduced the migration distance of SGC7901 cells at
50, 100 and 200 µM silibinin, in a dose- and time-dependent manner
(Fig. 1).
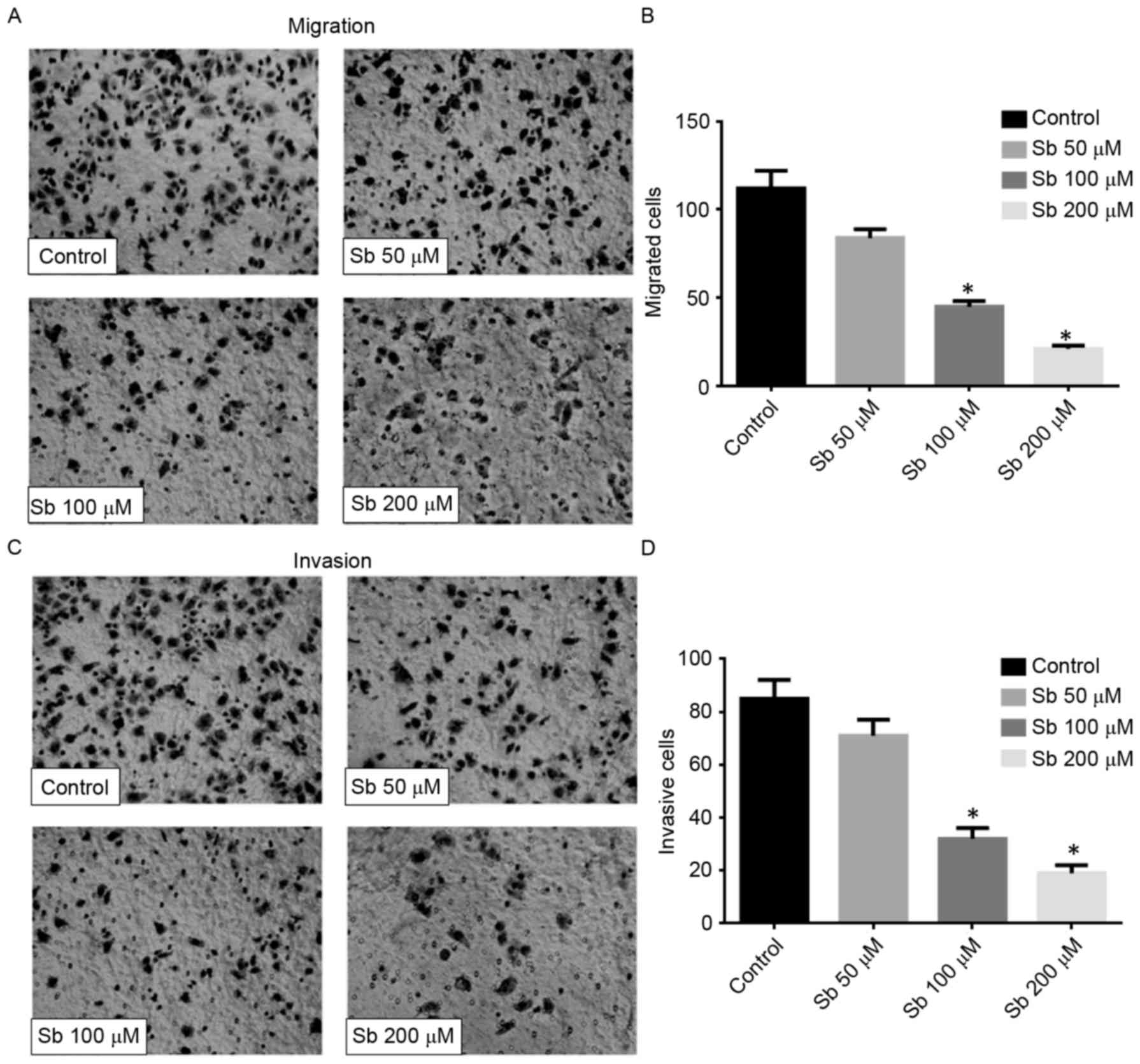
Effect of silibinin on the migration
and invasion of SGC7901 cells
The result of the Transwell migration assay
confirmed that silibinin significantly reduced the motility of
SGC7901 cells, since the number of migrated cells decreased
markedly in a dose-dependent manner (Fig.
2A and B). The Matrigel-based invasion assay also indicated
that silibinin significantly decreased the invasive ability of
SGC7901 cells in a dose-dependent manner (Fig. 2C and D). These data suggested that
silibinin may inhibit cell motility and affect the activity of
MMPs.
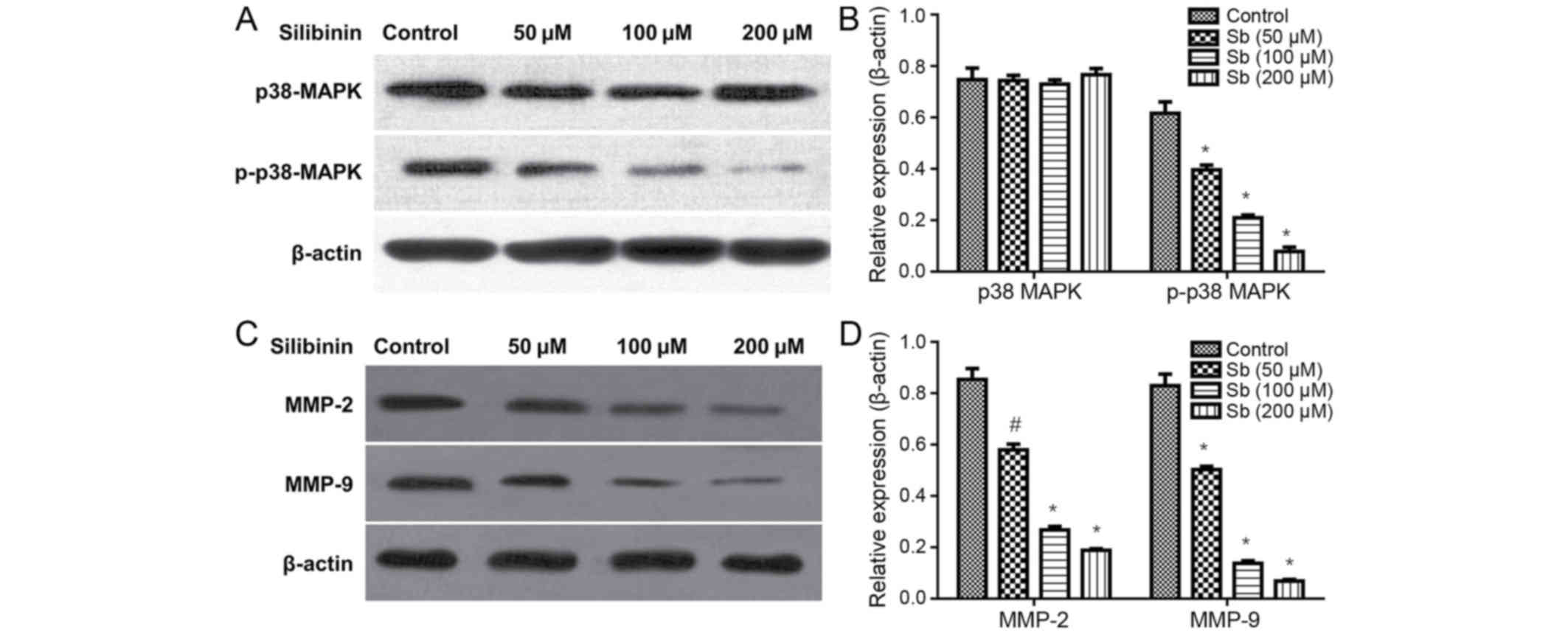
Silibinin effects on cell migration
and invasion inhibition may be associated with p38MAPK signaling
and MMP expression
Subsequent to treatment with 50, 100 and 200 µM
silibinin for 48 h, the total p38MAPK protein levels remained
unchanged, but the expression of p-p38MAPK significantly decreased
in a dose-dependent manner (Fig. 3A and
B). Additionally, it was demonstrated that silibinin decreased
MMP-2 and MMP-9 protein expression levels (Fig. 3C and D), in a dose-dependent
manner.
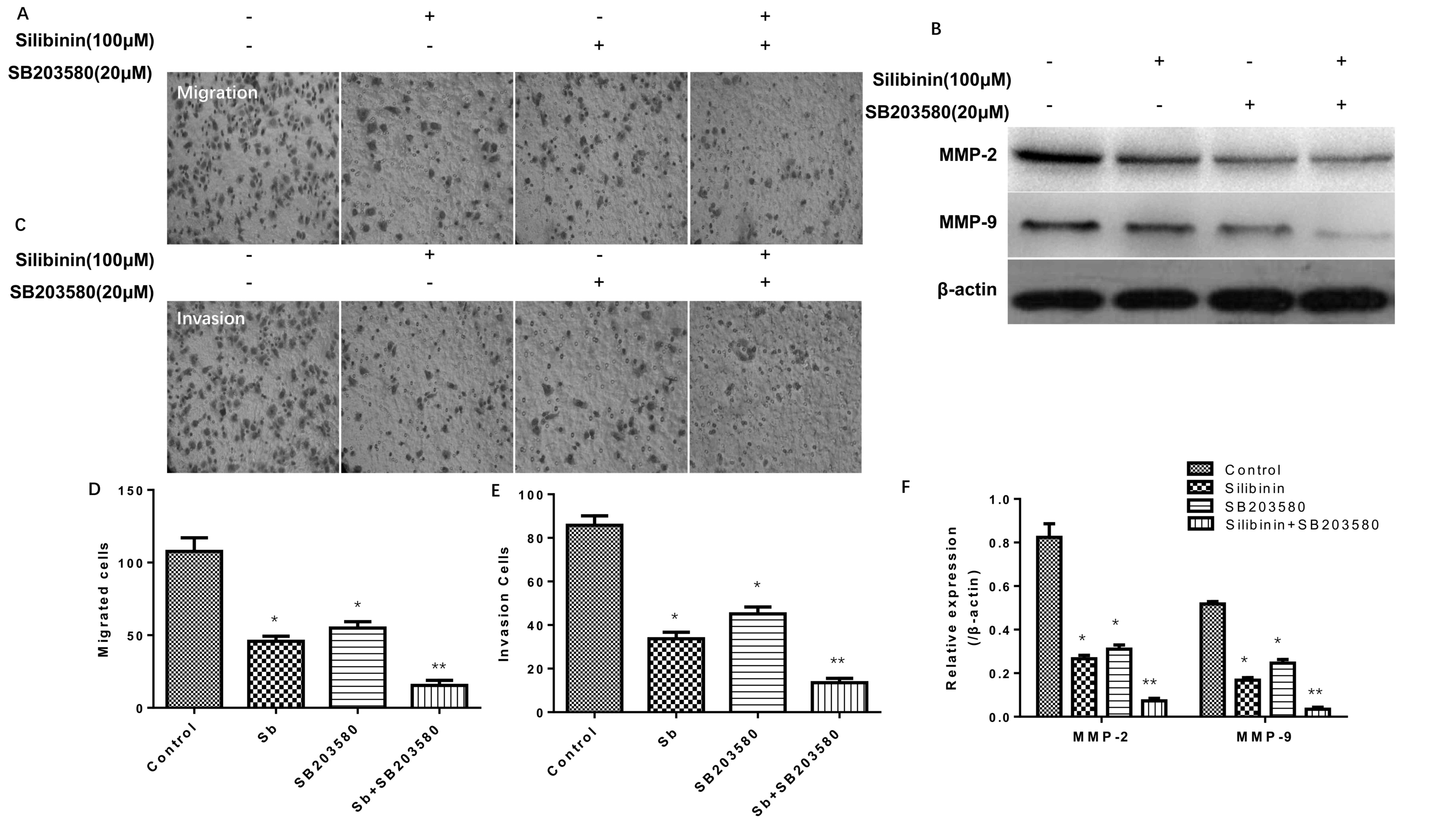
Effectiveness of silibinin combined
with a MAPK inhibitor in Transwell migration and Matrigel-based
invasion assays
The results of the Transwell migration and
Matrigel-based invasion assays (Fig.
4) simultaneously indicated that 100 µM silibinin and 20 µM
SB203580 (a MAPK inhibitor) (25),
independently and in combination, significantly decreased the
migration (Fig. 4A and D) and
invasion (Fig. 4C and E) of SGC7901
cells, particularly when administered in combination. Additionally,
it was observed that silibinin treatment combined with SB203580
decreased the protein expression levels of MMP-2 and MMP-9
concomitantly (Fig. 4B and F).
Discussion
Silibinin has received attention for its
hypothetical anticancer effects. It has been suggested that
silibinin inhibits cell proliferation, migration and invasion in a
number of cancer cell types. Wu et al (26) demonstrated that silibinin
significantly inhibited the invasion and migration of prostate
cancer cells. Similar results were also revealed in an in
vitro breast cancer study (27).
Chang et al (19) demonstrated
that silibinin inhibited the growth of renal cancer 786-0 cells in
a heterograft model, and increased their chemotherapy sensitivity
towards 5-FU and paclitaxel. In the present study, the data
indicate that the migratory and invasive abilities of SGC7901 cells
significantly decreased subsequent to treatment with silibinin in a
dose-dependent manner in wound healing and Transwell assays,
respectively.
The metastasis of tumor cells is a continuous,
multi-step process (3). MMPs are
responsible for remodeling the ECM, and it has been suggested that
MMPs exhibit important effects on cancer progression (28). Amongst the changes that occur in
cancer cells, the ability of tumor cells to modify the surrounding
ECM is key. The ECM is an important regulatory component in
cellular physiology that provides an environment for cell migration
(29). Previous studies have
demonstrated that MMPs act on a diverse group of ECM components,
including collagens, gelatins, fibronectins and laminins, which
serve crucial roles in cancer migration and invasion (30). MMP-2 and MMP-9 are important members
of the MMP family. Data from previous studies have suggested that
MMP-2 and MMP-9 are highly expressed in gastric cancer tissues
compared with their expression in normal tissues (31). Additionally, MMP-2 and MMP-9 were
closely associated with lymph node metastasis, lymphatic invasion
and prognosis of gastric cancer (32). Similar results have also been
demonstrated in vitro, including gastric, ovarian and
laryngeal cancer cells (33–35).
The p38MAPK pathway is an important signal
transduction cascade that is widely expressed in numerous tissues.
This pathway serves a relevant role in a series of cell stress,
cytokines recruitment and gene activation activities, including
cell proliferation and migration (36). Conversely, extracellular
signal-regulated kinase (ERK)1/2, as another transduction factor of
the MAPK signaling cascade, may also promote cell proliferation and
migration under certain circumstances (37). However, it has been identified that
p38MAPK is more active in the process of promoting cell migration,
compared with the ERK1/2 signaling cascade (38).
It has been demonstrated that p38MAPK activation may
increase MMP-2, MMP-9 and uPA expression levels during tumor
metastasis (39). Similarly, the data
in the present study indicated that silibinin significantly
decreases the migratory and invasive abilities of the SGC7901 cell
line in a dose- and time-dependent manner in wound healing and
Transwell assays, respectively. Western blot analysis of the cells
exposed to silibinin, alone or in combination with SB203580,
indicated that the pharmacological mechanism of silibinin may
involve the p38MAPK signaling pathway. In conclusion, the present
study demonstrates that silibinin, as an underlying inhibitor of
p38MAPK, may be regarded as an adjunctive drug for patients with
gastric cancer, particularly those suffering from metastatic renal
cell carcinoma.
References
|
1
|
Mazzocca A and Carloni V: The metastatic
process: Methodological advances and pharmacological challenges.
Curr Med Chem. 16:1704–1717. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bravo-Cordero JJ, Hodgson L and Condeelis
J: Directed cell invasion and migration during metastasis. Curr
Opin Cell Biol. 24:277–283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hulkower KI and Herber RL: Cell migration
and invasion assays as tools for drug discovery. Pharmaceutics.
3:107–124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loukopoulos P, Mungall BA, Straw RC,
Thornton JR and Robinson WF: Matrix metalloproteinase-2 and −9
involvement in canine tumors. Vet Pathol. 40:382–394. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koul HK, Pal M and Koul S: Role of p38 MAP
kinase signal transduction in solid tumors. Genes Cancer.
4:342–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dolado I and Nebreda AR: Regulation of
tumorigenesis by p38 MAP kinase. Topics Curr Genet. 20:99–128.
2008. View Article : Google Scholar
|
|
9
|
Emerling BM, Platanias LC, Black E,
Nebreda AR, Davis RJ and Chandel NS: Mitochondrial reactive oxygen
species activation of p38 mitogen-activated protein kinase is
required for hypoxia signaling. Mol Cell Biol. 25:4853–4862. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee KH, Kim SW and Kim JR: Reactive oxygen
species regulate urokinase plasminogen activator expression and
cell invasion via mitogen-activated protein kinase pathways after
treatment with hepatocyte growth factor in stomach cancer cells. J
Exp Clin Cancer Res. 28:732009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boukerche H, Aissaoui H, Prévost C, Hirbec
H, Das SK, Su ZZ, Sarkar D and Fisher PB: Src kinase activation is
mandatory for MDA-9/syntenin-mediated activation of nuclear
factor-kappaB. Oncogene. 29:3054–3066. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo X, Ma N, Wang J, Song J, Bu X, Cheng
Y, Sun K, Xiong H, Jiang G, Zhang B, et al: Increased p38-MAPK is
responsible for chemotherapy resistance in human gastric cancer
cells. BMC Cancer. 8:3752008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chou RH, Hsieh SC, Yu YL, Huang MH, Huang
YC and Hsieh YH: Fisetin inhibits migration and invasion of human
cervical cancer cells by down-regulating urokinase plasminogen
activator expression through suppressing the p38 MAPK-dependent
NF-kB signaling pathway. PLoS One. 8:e719832013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng CY, Yang HW, Chu YH, Chang YC, Hsieh
MJ, Chou MY, Yeh KT, Lin YM, Yang SF and Lin CW: Caffeic Acid
phenethyl ester inhibits oral cancer cell metastasis by regulating
matrix metalloproteinase-2 and the mitogen-activated protein kinase
pathway. Evid Based Complement Alternat Med. 2012:7325782012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang XF, Zhou QM, Du J, Zhang H, Lu YY and
Su SB: Baicalin suppresses migration, invasion and metastasis of
breast cancer via p38MAPK signaling pathway. Anticancer Agents Med
Chem. 13:923–931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
DE Oliveira DT, Savio AL, Marcondes JP,
Barros TM, Barbosa LC, Salvadori DM and DA Silva GN: Cytotoxic and
toxicogenomic effects of silibinin in bladder cancer cells with
different TP53 status. J Biosci. 42:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Polachi N, Bai G, Li T, Chu Y, Wang X, Li
S, Gu N, Wu J, Li W, Zhang Y, et al: Modulatory effects of
silibinin in various cell signaling pathways against liver
disorders and cancer-A comprehensive review. Eur J Med Chem.
123:577–595. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jahanafrooz Z, Motameh N and Bakhshandeh
B: Comparative evaluation of Silibinin effects on cell cycling and
apoptosis in human breast cancer MCF-7 and T47D cell lines. Asian
Pac J Cancer Prev. 17:2661–2665. 2016.PubMed/NCBI
|
|
19
|
Chang HR, Chen PN, Yang SF, Sun YS, Wu SW,
Hung TW, Lian JD, Chu SC and Hsieh YS: Silibinin inhibits the
invasion and migration of renal carcinoma 786-O cells in vitro,
inhibits the growth of xenografts in vivo and enhances
chemosensitivity to 5-fluorouracil and paclitaxel. Mol Carcinog.
50:811–823. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lali FV, Hunt AE, Turner SJ and Foxwell
BM: The pyridinyl imidazole inhibitor SB203580 blocks
phosphoinositide-dependent protein kinase activity, protein kinase
B phosphorylation, and retinoblastoma hyperphosphorylation in
interleukin-2-stimulated T cells independently of p38
mitogen-activated protein kinase. J Biol Chem. 275:7395–7402. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Birkenkamp KU, Tuyt LM, Lummen C, Wierenga
AT, Kruijer W and Vellenga E: The p38 MAP kinase inhibitor SB203580
enhances nuclear factor-kappa B transcriptional activity by a
non-specific effect upon the ERK pathway. Br J Pharmacol.
131:99–107. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barancik M, Htun P, Strohm C, Kilian S and
Schaper W: Inhibition of the cardiac p38-MAPK pathway by SB203580
delays ischemic cell death. J Cardiovasc Pharmacol. 35:474–483.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin N, Wang Q, Zhang X, Jiang D, Cheng H
and Zhu K: The selective p38 mitogen-activated protein kinase
inhibitor, SB203580, improves renal disease in MRL/lpr mouse model
of systemic lupus. Int Immunopharmacol. 11:1319–1326. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang H, Wu S and Xing D: YAP accelerates
A(25–35)-induced apoptosis through upregulation of Bax expression
by interaction with p73. Apoptosis. 16:808–821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lali FV, Hunt AE, Turner SJ and Foxwell
BM: The pyridinyl imidazole inhibitor SB203580 blocks
phosphoinositide-dependent protein kinase activity, protein kinase
B phosphorylation, and retinoblastoma hyperphosphorylation in
interleukin-2-stimulated T cells independently of p38
mitogen-activated protein kinase. J Biol Chem. 275:7395–7402. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu KJ, Zeng J, Zhu GD, Zhang LL, Zhang D,
Li L, Fan JH, Wang XY and He DL: Silibinin inhibits prostate cancer
invasion, motility and migration by suppressing vimentin and MMP-2
expression. Acta Pharmacol Sin. 30:1162–1168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dastpeyman M, Motamed N, Azadmanesh K,
Mostafavi E, Kia V, Jahanian-Najafabadi A and Shokrgozar MA:
Inhibition of silibinin on migration and adhesion capacity of human
highly metastatic breast cancer cell line, MDA-MB-231, by
evaluation of 1-integrin and downstream molecules, Cdc42, Raf-1 and
D4GDI. Med Oncol. 29:2512–2518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metall oproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kleiner DE and Stetler-Stevenson WG:
Matrix metalloproteinases and metastasis. Cancer Chemother
Pharmacol. 43 Suppl:S42–S51. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hidalgo M and Eckhardt SG: Development of
matrix metalloproteinase inhibitors in cancer therapy. J Natl
Cancer Inst. 93:178–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gerstein ES, Sini L, Ryabov AB, Dvorova
EK, Yurchenko AA, Stilidi IS, Kushlinskii NE and Davydov MI:
Comparative enzyme immunoassay of matrix metalloproteinases-2,-7,-9
and their tissue inhibitor-2 in tumors and plasma of patients with
gastric cancer. Bull Exp Biol Med. 148:899–902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sampieri CL, de la Peña S, Ochoa-Lara M,
Zenteno-Cuevas R and León-Córdoba K: Expression of matrix
metalloproteinases 2 and 9 in human gastric cancer and superficial
gastritis. World J Gastroenterol. 16:1500–1505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weng Y, Cai M, Zhu J, Geng J, Zhu K, Jin X
and Ding W: Matrix metalloproteinase activity in early-stage lung
cancer. Onkologie. 36:256–259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dragutinović VV, Radonjić NV, Petronijević
ND, Tatić SB, Dimitrijević IB, Radovanović NS and Krivokapić ZV:
Matrix metalloproteinase-2 (MMP-2) and −9 (MMP-9) in preoperative
serum as independent prognostic markers in patients with colorectal
cancer. Mol Cell Biochem. 355:173–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Ma J, Guo Q, Duan F, Tang F, Zheng
P, Zhao Z and Lu G: Overexpression of MMP-2 and MMP-9 in esophageal
squamous cell carcinoma. Dis Esophagus. 22:664–667. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bradham C and McClay DR: p38 MAPK in
development and cancer. Cell Cycle. 5:824–828. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang C, Jacobson K and Schaller MD: MAP
kinases and migration. J Cell Sci. 117:4619–4628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang ZS, Luo P, Dai SH, Liu ZB, Zheng XR
and Chen T: Salvianolic acid B induces apoptosis in human glioma
U87 cells through p38-mediated ROS generation. Cell Mol Neurobiol.
33:921–928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pan F, Ma S, Cao W, Liu H, Chen F, Chen X
and Shi R: SDF-1 upregulation of MMP-2 is mediated by p38 MAPK
signaling in pancreatic cancer cell lines. Mol Biol Rep.
40:4139–4146. 2013. View Article : Google Scholar : PubMed/NCBI
|