Introduction
Prostate cancer (PCa) is the second most frequent
malignant neoplastic disease among men, with 241,740 cases in
America, and 28,170 deaths from PCa in 2012; in the Mexican
population, the incidence of PCa is underrated, but there is a high
occurrence of high-grade lesions (1,2). Due to
its impact, and to promptly treat this illness, several programs
for prevention and early diagnosis are currently active. The
primary diagnosis tools for PCa are the level measurement of total
prostate specific antigen (t-PSA) in serum along with clinical and
digital rectal examination; nevertheless, none of these method is
specific enough to differentiate cases of adenocarcinoma (Ad) from
benign prostatic hyperplasia (BPH) (3,4). To
distinguish between prostatic pathologies and to determine
progressiveness of PCa, is useful to perform a histological
inspection of biopsies with Gleason score (GS) grading since it
allows the physicians to distinguish benign and malignant
neoplasias. However, the specificity of histology interpretation
could decrease depending on the number of analyzed biopsies, the
captured area and the expertise of the pathologist. There is
sufficient evidence suggesting that angiogenesis plays an important
role in PCa. PCa cells secrete proteic factors such as the vascular
endothelial growth factor (VEGF), which is extensively studied and
known as the major angiogenic marker. VEGF acts as a direct
mediator in endothelial cell proliferation, vascular permeation,
tumor growth promotion, and metastasis. Several authors report that
there are higher levels of VEGF in biopsies and serum of PCa
patients as compared to healthy individuals (5–8). Although
there is a correlation between levels of VEGF in serum and the
stages of the disease, its validity as a prognosis marker is still
controversial because VEGF is also augmented in BPH and its plasma
concentration does not concur with the clinical classification as
benign or malignant forms (9–13).
Other protein related to angiogenesis is the pigment
epithelium-derived factor (PEDF), an antiangiogenic factor with
antitumoral properties (14). In PCa
and other solid tumors, low levels of PEDF are associated with
higher vascular density and to a metastatic phenotype, indicating a
decrease of its expression along with tumor progression (15,16).
Likewise, tumor growth in PCa diminishes when treated with
recombinant PEDF or with diverse epitopes of this protein (14,17,18). Also,
the levels of PEDF are lower in serum and biopsies of PCa,
suggesting that it as a prediction marker of the disease (19,20).
However, there are not studies about the levels of PEDF in PCa and
BPH as a diagnosis marker.
Angiogenesis depends on the critical equilibrium
between pro- and anti-angiogenic factors (VEGF/PEDF). Several
studies in vivo and in vitro show an association
between an increase in the VEGF/PEDF ratio and a bad prognosis in
nasopharyngeal carcinoma and ophthalmic neovascular illnesses,
suggesting that the VEGF/PEDF ratio in serum could be useful as a
prognostic value for other diseases (21–25).
Though, there are no reports of the differences in the measurements
of VEGF/PEDF ratio between PCa and BPH. In here, we aim to describe
the serum levels of VEGF, PEDF and the VEGF/PEDF ratio among
patients recently diagnosed with PCa or BPH and whether these
measurements are related to the detection of both proteins in
prostate biopsies. The combination of these data might allow the
discrimination of the grade of angiogenesis associated with the
disease, and it could become a valuable theranostic tool.
Materials and methods
The present study was performed under the approval
of the ethics and research local committees. All participants gave
their informed consent through a written format, under the 1975
Helsinki's Declaration and the nationally approved guidelines
(26). Patients with PCa (n=40) and
BPH (n=57) were recently diagnosed by digital rectal exam, serum
t-PSA measurement (t-PSA>4.0 ng/ml) and by detection of diffuse
growth at the prostatic transition zone, near the bladder base,
with nodular and heterogeneous echo. Acinar Ad and BPH diagnosis
were confirmed by histological examination, using a biopsy
extracted with a guided transrectal ultrasound (TRUS). None of the
PCa and BPH patients had history of other malignancies, previous
surgery or any other PCa treatments (deprivation therapy,
chemotherapy or androgen radiotherapy), neither presented active
infections at the time of their blood test.
Healthy adult volunteers (n=35) were recruited from
the blood bank under the criteria established by the Mexican
Official Standard NOM-253-SSA1-2012 (27), showing no complaints or signs of
malignancies or inflammatory diseases and with t-PSA<4 ng/ml.
Diabetes mellitus, cardiovascular disease, and other systemic
diseases were excluded both in ill and healthy individuals.
Serum sample collection and
measurement of VEGF and PEDF
Venous blood samples were collected after an
overnight fast, serum was separated and stored at −80°C. VEGF serum
levels were quantified using the Quantikine assay kit (R&D
Systems) according to the manufacturer's instructions. PEDF was
measured using an enzyme-linked immunosorbent assay (ELISA) kit
(ChemiKine™; Chemicon International; Millipore Inc., Billerica, MA,
USA). To prevent PEDF from associating with other circulating
proteins that may interfere with its total serum quantification,
samples were pre-treated with urea (8 M final concentration) for 60
min on ice and diluted in dilution buffer before being applied in
duplicate to ELISA plates, as recommended by the manufacturer. All
plates VEGF and PEDF were read at 450 nm using a microplate reader
(Eon™Microplate Spectrophotometer; bioTek, Winooski, VT, USA).
Immunohistochemical staining
A range of 9–12 cores was taken at the initial
prostate biopsy, which were divided into three biopsies per
paraffin block. Tissue specimens were processed using conventional
procedures for paraffin embedding. Three-micron sections were
serially cut. The pathologist analyzed hematoxylin/eosin-stained
slides for classification. Subsequently, the highest score Gleason
representative paraffin block (containing at least 2 cores
positive) was sectioned, dewaxed and rehydrated up to wash buffer
(Dako wash; North America, Inc.) and loaded onto Shandon sequenza
chamber (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Labeled
polymer-based immunodetection system (Mouse/Rabbit PolyVue™ HRP/DAB
Detection System; Diagnostic BioSystems, Pleasanton, CA, USA) was
used as recommended by the manufacturer's protocol. Monoclonal
mouse anti-VEGF antibody (1:50; SC-7269; Santa Cruz Biotech, Inc.,
Santa Cruz, CA, USA) or polyclonal goat PEDF antibody (1:200;
AF1177; Millipore, R&D Systems, Minneapolis, MN, USA) were
applied. Then enhancer Polyvue Plus and HRP were added, and
incubated with DAB plus/chromogen substrate. Histological
observation and image capture were performed using an Axio
Imager.A2 (ZEISS, Oberkochen, Germany). To prevent artifactual
formation, VEGF or PEDF staining were processed the same day. The
criterion of analysis was applied to the regions where VEGF
staining showed a higher intensity.
The intensity of VEGF-A and PEDF expression in the
biopsies selected above were evaluated in the entire tissue,
subsequently three to five fields by cylinder were captured
(magnification, ×40) and then processed by Image-Pro Plus 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA), which
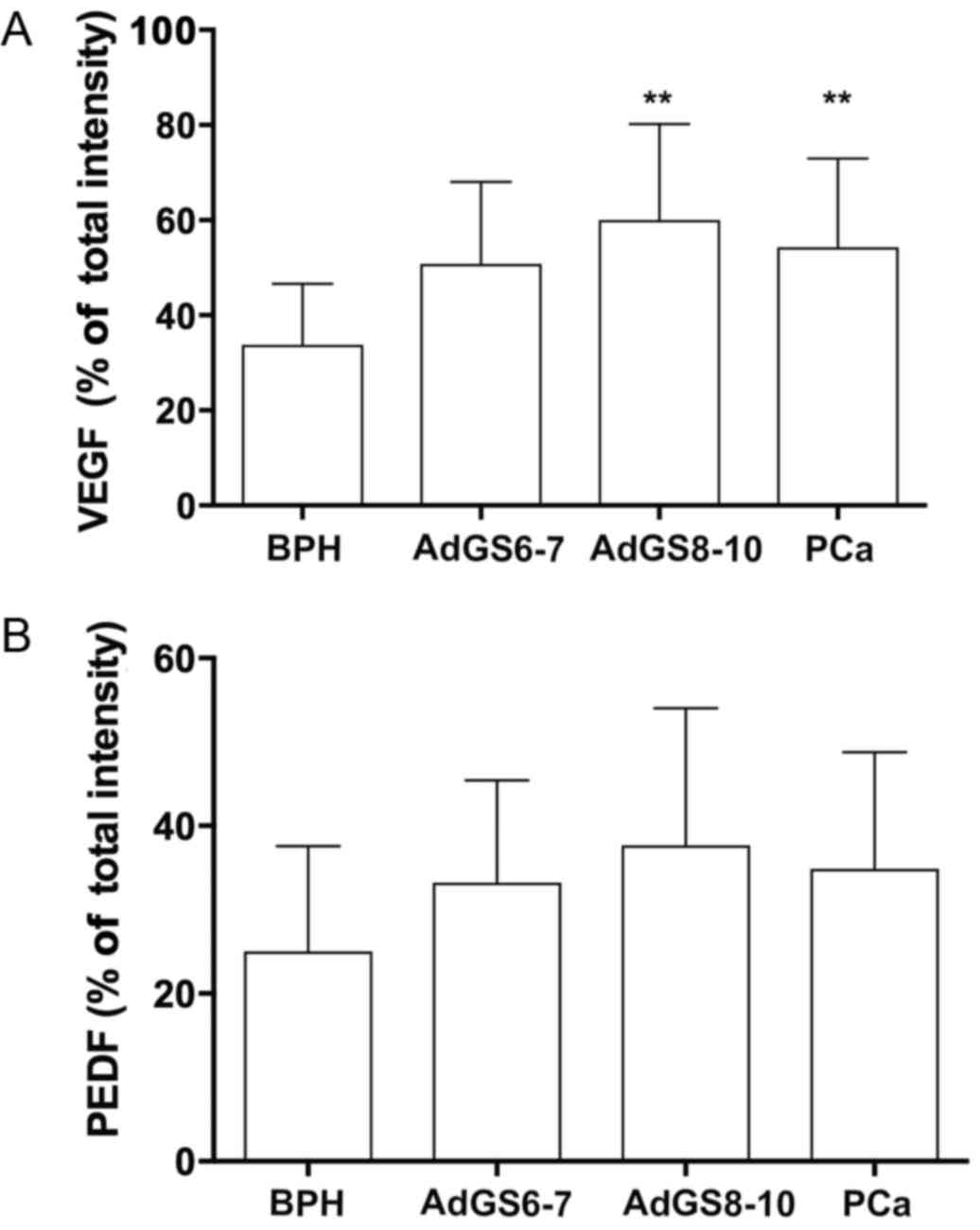
detected the brown spots of the image. According to the data of
detected area, samples were classified as four grades: none (0–50),
mild (51–166), moderate (167–283), and strong (284–400), and the
percentage was plotted as total intensity. Two blinded observers
independently performed analysis and immunostaining interpretation.
A third observer was required in the discording cases.
Statistical analysis
All data were analyzed by SPSS v.20 (IBM SPSS Inc.,
Armonk, NY, USA) using descriptive statistics. Data were presented
as the mean ± SD. One-way analysis of variance (ANOVA) with Tukey
post hoc test was used to compare groups of normally distributed
data of the variables studied; Pearson's correlation for serum
levels or Spearman's correlation coefficients for serum vs.
immunostained intensity percentage in biopsies were used to test
associations between variables. Student's t-test was used to
compare median for t-PSA with the AdGS. P<0.05 was considered to
indicate a statistically significant difference.
Results
Group description
Table I describes the
clinical parameters of the enrolled patients and healthy
participants. The means of age between patients and healthy
participants were similar (P=0.109). BMI means were similar in BPH
and PCa (P=0.170) but higher as compared to healthy individuals
(P=0.001). The t-PSA values in the serum of PCa and BPH patients
were higher than 4 ng/ml, and there was not a significant
difference between them, but both were different from the healthy
group (P=0.001). According to the clinical and histological
evaluation, from the 40 cases diagnosed as PCa (PCa total), nine
were classified as Ad with GS 6 (AdGS6); sixteen were AdGS7,
thirteen were AdGS8, and two were AdGS10. PCa cases were grouped
into two subsets, AdGS6-7 (well and moderately differentiated), and
AdGS8-10 (poorly differentiated or undifferentiated) to further
analysis. There was a difference statistically significant between
the t-PSA mean values of AdGS6-7 and AdGS8-10 (P=0.045).
 | Table I.Data comparison of Age, BMI and t-PSA
of patients with PCa, AdGS6-7, AdGS8-10, BPH and healthy
participants. |
Table I.
Data comparison of Age, BMI and t-PSA
of patients with PCa, AdGS6-7, AdGS8-10, BPH and healthy
participants.
| Characteristic | PCa(n=40) | BPH(n=57) | Healthy(n=35) |
|---|
| Age |
65.32±4,28 |
64.35±5,56 |
62.80±5,41 |
| BMI |
26.05±2,88a |
27.20±3,59a |
23.18±1,59 |
| t-PSA |
12.81±1.76a |
14.88±2.83a |
1.08±0.14 |
|
|
9.41±3.73b,c | – | – |
|
|
17.91±9.80d | – | – |
VEGF and PEDF measurements in
serum
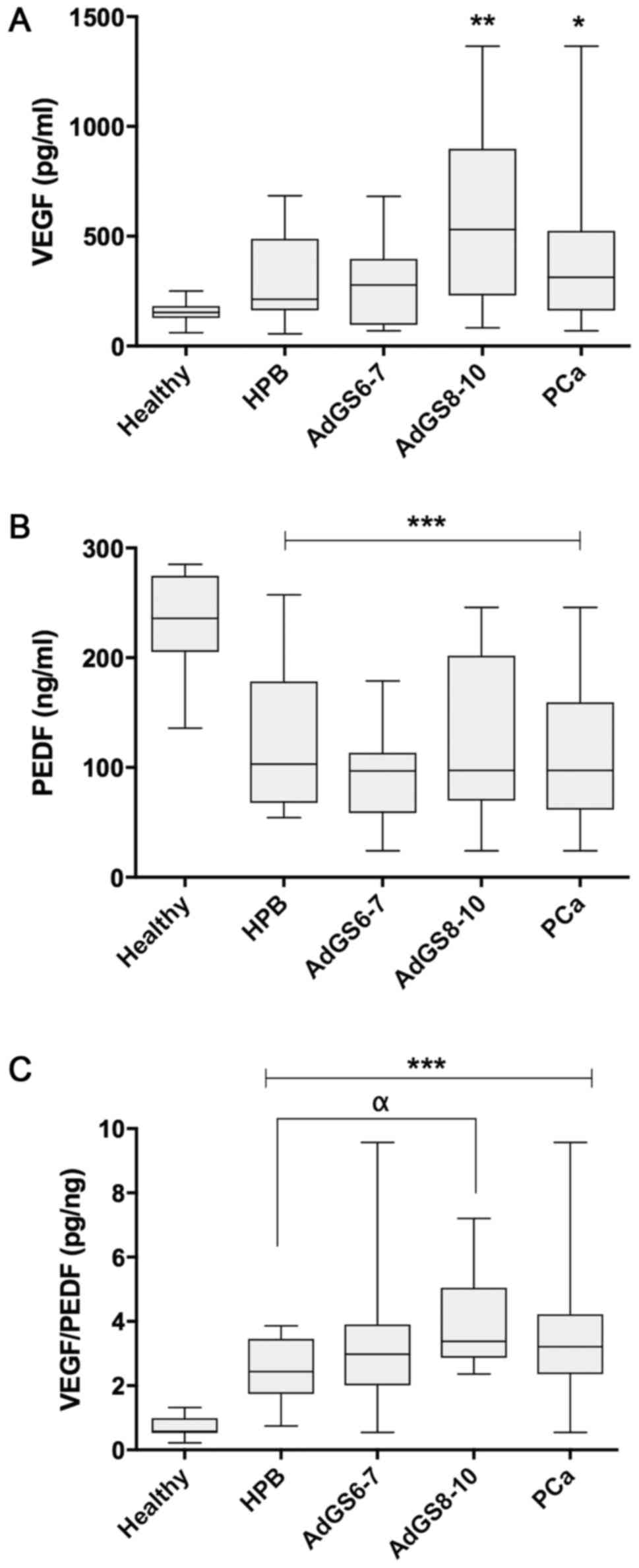
The VEGF levels (pg/ml) were increased in PCa
compared to healthy individuals (360.55±292.10 vs. 157.1±49.73;
P=0.039), but there was not a difference with BPH cases
(298.17±178.6; P=0.274). Nonetheless, the stratification of PCa in
GS showed that only AdGS8-10 (475.7±405.7) had a significant
increase compared to the control group (P=0.009) (Fig. 1A).
Fig. 1B indicates that
the means of PEDF values in ng/ml were significantly inferior in
BPH (122.15±58.84), AdGS6-7 (98.93±38.02), AdGS8-10 (121.58±84.13)
and PCa (107.99±59.79) when weighed against the healthy group
(233.6±9.25; P=0.001), although there was no significant difference
between the prostatic diseases (P>0.05).
When analyzing the VEGF/PEDF ratio (pg/ng) among the
samples, its mean was 2.47±0.94 in BPH, 3.18±1.11 in PCa and
0.71±0.3 in the healthy group. There are statistically significant
differences between all groups compared to the healthy group
(P=0.001); meanwhile, there was no difference between PCa or AdG6-7
vs. BPH. Nevertheless, AdGS8-10 was significant compared to BPH
(3.71±1.25; P=0.015) (Fig. 1C).
Table II illustrates
the correlation coefficient analyses between serum levels of t-PSA,
VEGF, PEDF and VEGF/PEDF ratio from healthy, PCa and BPH groups. In
PCa, VEGF presented a positive correlation with t-PSA (P=0.042),
PEDF (P=0.001), and the ratio (P=0.004); in AdGS6-7 and AdGS8-10
groups a positive correlation was also found between VEGF and PEDF
(P=0.003 and P=0.001, respectively). For BPH there was a positive
correlation between VEGF and PEDF (P=0.001), and it was negative
when the t-PSA vs. ratio analysis was performed (P=0.008). As it is
expected, the correlation of VEGF with VEGF/PEDF ratio was positive
and significant between PCa and BPH (P=0.004 and P=0.003). In
healthy individuals, a strong negative correlation was observed
between VEGF and PEDF with the ratio (P=0.001 and P=0.001).
 | Table II.Pearson's Correlation Coefficient of
PSA, VEGF, PEDF and Ratio VEGF/PEDF serum in PCa, BPH and healthy
groups. |
Table II.
Pearson's Correlation Coefficient of
PSA, VEGF, PEDF and Ratio VEGF/PEDF serum in PCa, BPH and healthy
groups.
|
| Serum |
|---|
|
|
|
|---|
| Group |
| VEGF | PEDF | Ratio |
|---|
| PCa | t-PSA | 0.458a | 0.389 | 0.316 |
|
| VEGF | – | 0.900c | 0.613b |
|
| PEDF | – | – | 0.259 |
| AdGS6-7 | t-PSA | −0.129 | 0.037 | −0.251 |
|
| VEGF | – | 0.775b | 0.695 |
|
| PEDF | – | – | 0.112 |
| AdGS8-10 | t-PSA | 0.460 | 0.426 | 0.314 |
|
| VEGF | – | 0.927b | 0.556 |
|
| PEDF | – | – | 0.262 |
| BPH | t-PSA | −0.300 | 0.077 | −0.578b |
|
| VEGF | – | 0.690b | 0.620b |
|
| PEDF | – | – | −0.079 |
| Healthy | t-PSA | −0.046 | −0.051 | −0.008 |
|
| VEGF | – | −.347 | −0.883c |
|
| PEDF | – | – | −0.730c |
Immunohistochemical analysis
Immunohistochemical analysis was performed to
demonstrate if the serum levels of these proteins were related to
its expression intensity in prostatic tissues. VEGF staining
presented a diffuse pattern; meanwhile, PEDF showed a granular
staining. Representative photomicrographs and the analysis of the
staining intensity in prostatic tissues are illustrated in Figs. 2–4. In
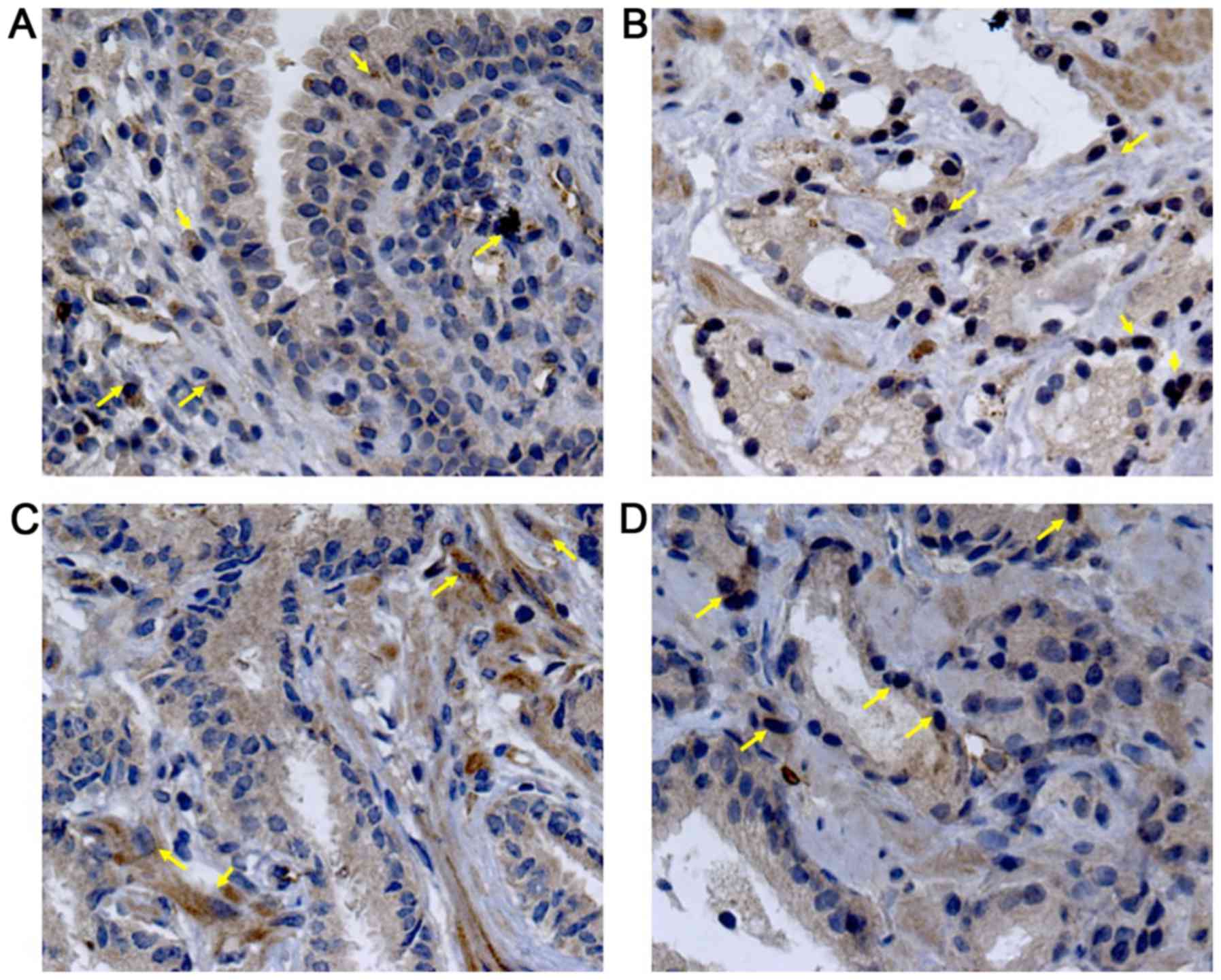
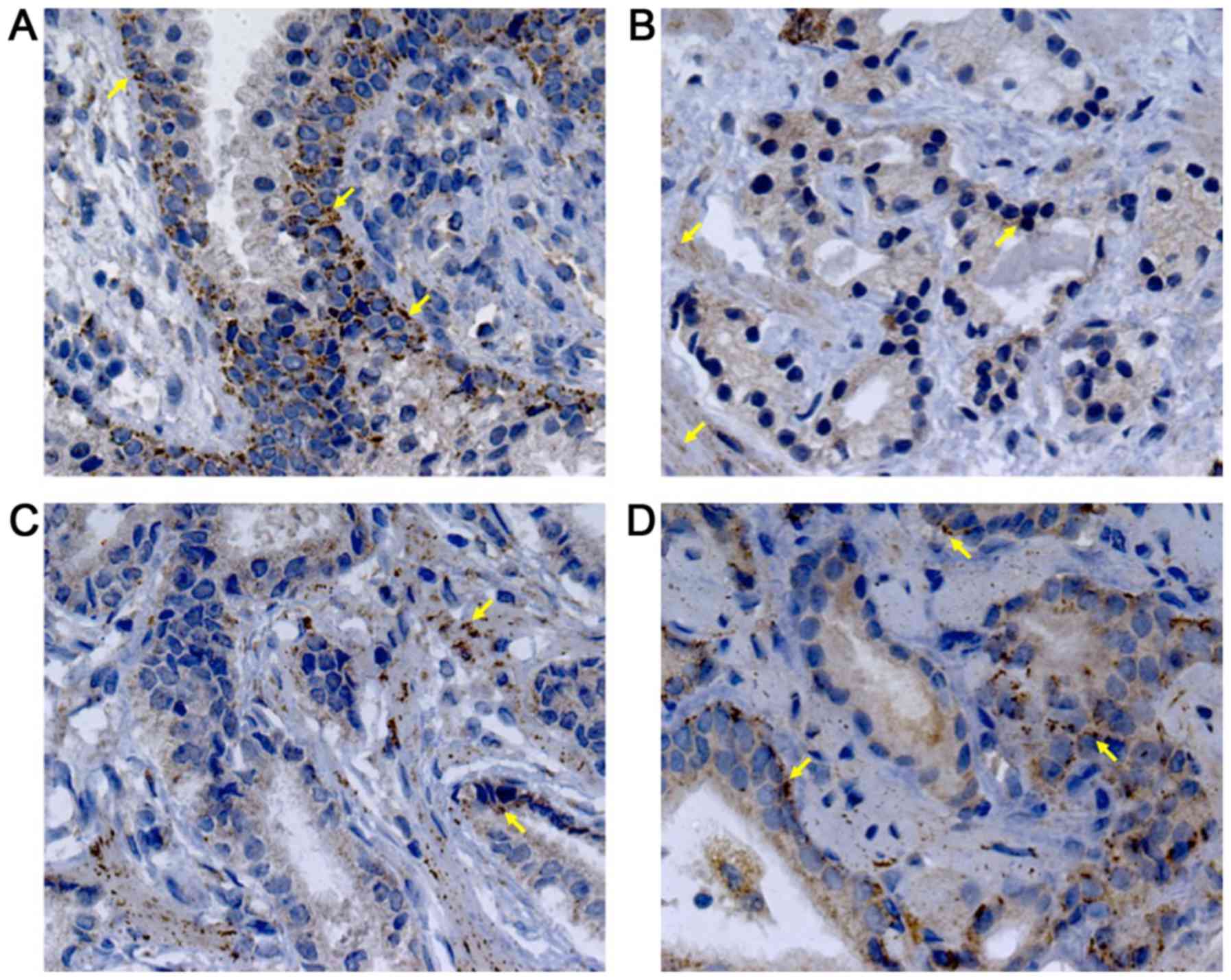
BPH tissues, there was a moderate VEGF staining (33.75±12.86, 4A),
confined mainly into the cytoplasm of glandular epithelial cells,
endothelial cells and stromal fibroblasts (Fig. 2A). In contrast, PEDF staining showed a
mild intensity (24.73±12.85, 4B) and it was limited to the
perinuclear region of basal cells (Fig.
3A). The percentage of VEGF staining in PCa (49.90±18.31) was
different to BPH (P=0.003). Particularly, AdGS6-7 mean staining was
45±13.31 with a mild to moderate intensity (Fig. 2B and C) but was no different from BPH.
However, we found intensity from moderate to high in AdGS8-10
(Fig. 2D) with mean staining values
of 57.25±23 (Fig. 4A), which was
statistically significant compared to BPH (P=0.002). On the other
hand, PEDF staining intensity for PCa was 31±13.72; with a mild
staining for AdGS6-7 (Fig. 3B and C)
with a mean intensity 29.58±9.4 (Fig.
4B) and mild to moderate in the AdGS8-10 (Fig. 3D) with 33.13±19.07 mean intensity
(Fig. 4B). However, on the
microscopic examination, most tissue samples of BPH and PCa showed
superior staining areas for VEGF over PEDF (P<0.05).
A correlation analysis of serum VEGF and PEDF levels
with staining intensity in tissues was additionally performed
(Table III). In BPH we found no
association in both measurements. In PCa a positive correlation was
shown for VEGF and PEDF (P=0.002 and 0.001, respectively),
nonetheless, in AdGS6-7 a correlation was seen only with VEGF
values (P=0.048). AdGS8-10 displayed a correlation with PEDF values
(P=0.004) and a positive tendency with VEGF; showing that the
heterogeneity found in serum corresponds to the observations in
biopsies.
 | Table III.Spearman's Correlation Coefficient of
serum values VEGF and PEDF with immunostaining intensity percentage
in prostatic diseases. |
Table III.
Spearman's Correlation Coefficient of
serum values VEGF and PEDF with immunostaining intensity percentage
in prostatic diseases.
|
| Serum |
|---|
|
|
|
|---|
| Biopsy (%) | VEGF | PEDF |
|---|
| BPH |
|
VEGF | 0.166 | – |
|
PEDF | – | −0.198 |
| PCa |
|
VEGF | 0.661b | – |
|
PEDF | – | 0.661b |
| AdGS6-7 |
|
VEGF | 0.580a | – |
|
PEDF | – | 0.344 |
| AdGS8-10 |
|
VEGF | 0.611 | – |
|
PEDF | – | 0.881b |
Discussion
Increased levels of t-PSA determine possible
anomalies in the prostate, so it has been proposed as a prognostic
biomarker in PCa. However, it remains contradictory since its
positive predictive value is ~30% (4). Other biomarkers have been proposed to
improve this value such as a factors related to angiogenesis (VEGF
and MMP9), and to cell processes like PCA3, ANXA3 and TERT
(28).
We describe for the first time the behavior
simultaneously of VEGF and PEDF in benign and malignant prostate
environments, both serum and tissue in individuals without
comorbidities related to chronic inflammation (9,29).
VEGF is narrowly related to the malignancy grade and
metastasis of PCa, suggesting that it has a diagnostic and
prognostic value of this illness. Our results and other studies
reveal that serum expression of VEGF is not correlated, neither can
discriminate a benign form (30–33). We
have shown that levels of VEGF in the serum of PCa and BPH are not
significantly different. Probably the inflammatory response in BPH
causes an increase in the VEGF expression leading to stromal
hypervascularization, endothelial vessel permeability (34–36), or it
might occur through a decrease in the androgen receptors and
inhibition of apoptosis in epithelial cells (10).
On the other hand, PEDF is a glycoprotein with
antitumor properties, because it diminishes the tumor volume and
metastases, by acting directly on migration and differentiation
into type I tumor-associated macrophages (TAM-1) (37–40),
suggesting that it could be used as a predictor of the disease and
with therapeutic utility (19,20).
Nonetheless, little is known about the levels of PEDF in serum. Ide
H et al reported that there are lower levels in BPH in
comparison with PCa patients (41).
However, we found that PEDF levels were not different in both
pathologies but were lower compared to healthy individuals. There
is a high expression of PEDF in our PCa group, particularly on
AdGS8-10, probably due to the aforementioned (15).
Some studies have linked an increase in the
angiogenic balance VEGF/PEDF as a prognostic marker in neovascular
diseases (21–25). On prostatic diseases, the measurement
of VEGF/PEDF ratio in serum has been unexplored; this is the first
study that shows data of their expression in PCa. We observed that
the VEGF/PEDF ratio in AdGS8-10 patients was higher as compared to
BPH and, even more, to healthy individuals (Fig. 1C). We suggest that the VEGF/PEDF ratio
is a kind of normalization of the individually measured data,
denoting that the simultaneous measurement of VEGF and PEDF, not
the isolated observation of their levels, could help to determine
the disease status in an individualized manner. We interpret this
idea through the correlation between VEGF, PEDF, and t-PSA
(Table III). VEGF was associated to
t-PSA only in AdGS8-10 meanwhile this association was negative in
BPH. Conversely, PEDF did not present association with t-PSA in any
pathology. These results show that levels of t-PSA are not related
to VEGF and PEDF in benign hyperplasia and lower grades of PCa
(AdGS6-7).
On the other hand, the relationship of VEGF with
PEDF shows a positive significance in both GSs and BPH; indicating
that both are increased independently of the pathology. Suggesting
that the individual analysis of VEGF or PEDF does not differentiate
between benign and malignant forms; except for healthy individuals
where a negative tendency was shown. Regarding the VEGF/PEDF ratio,
there is a significant relation with the decrease of t-PSA in BPH.
While in healthy individuals it is maintained in balance.
Additionally, PEDF and VEGF were detected by
immunostaining in biopsies. It was noticeable that the intensity of
PEDF was lower compared to VEGF in most samples. Doll et al
reported a downregulation of PEDF expression in PCa and high levels
in BPH (42). Perhaps our divergence
is due to the origin of the samples (patients vs. animal model,
respectively) (43). Furthermore, we
found marked differences in the localization of PEDF among
glandular regions. For instance, in BPH, PEDF is located in the
cytoplasmic region of basal epithelium, meanwhile, in malignant
glands, it was found in the acinar cytoplasm. The intensity of VEGF
in the PCa glands was higher compared to hyperplastic glands. We
observed that AdGS8-10 significantly contributed to the higher
staining intensity. As it has been previously found, VEGF is
increased according to the severity of PCa; nonetheless, our data
were unable to discriminate between early stages (AdG6-7) and the
benign hyperplastic disease. These particularities could allow the
histological discrimination between malignant and benign regions
constituting relevant information for the pathological analysis.
Nonetheless, these results should be verified using ELISA to
quantitatively assess VEGF and PEDF expression in tissues,
specially with those from prostatectomies where the volume of
biological material is abundant.
To study if the serum values of VEGF and PEDF in PCa
and BPH were similar to its staining intensity in biopsies, we
perform a correlation analysis. Interestingly, we found that in PCa
had a significant difference and a positive trend with its levels
in biopsies, this is to say, the phenomenon in the tumor is
reflected by the circulating levels of both proteins. Similarly,
PEDF had a higher correlation between the levels in serum and
biopsy, contrary to the common pre-conception, we found a
simultaneous increment of both pro-angiogenic (VEGF), and
anti-angiogenic (PEDF) factors.
Our results seem to reveal a fine-tuning performed
by the balance of VEGF and PEDF levels. Several anti-angiogenic
mechanisms, where PEDF acts upon VEGF in a direct or indirect
manner, have been proposed in physiological conditions. First, an
interference of the VEGFR1 signaling through transmembranal
excision activated by the PEDF-induced gamma-secretase (44). Second, antagonist activity of PEDF
upon VEGFR1 and VEGFR2 to promote its internalization and
degradation inside endothelial cells (45). Finally, stimulation by PEDFR/PPARγ
signaling that leads to apoptosis of endothelial cells via FAS-L
(17,39,46). On
the contrary, the growth of malignant cells is caused by
alterations in the balance between VEGF and PEDF, releasing in
consequence matrix metalloproteases (MMPs) that influence migration
and proliferation of endothelial cells and extracellular PEDF
degradation (14).
Prospective and simultaneous measurements of serum
levels of VEGF, PEDF or the use of their ratio, along with other
diagnostic methods, including t-PSA could be clinically relevant
for determining the progression of the disease in a personalized
manner, and allow the physicians to make better decisions in
doubtful cases. However, these are only preliminary descriptive
data and further research is required to determine the role VEGF
and PEDF in PCa.
Acknowledgements
The authors would like to thank Mr. Alejandro
Herrera-Mundo for his support and valuable consultation on the
histological procedures and immunohistochemistry techniques.
References
|
1
|
Brawley OW: Trends in prostate cancer in
the United States. J Natl Cancer Inst Monogr. 2012:152–156. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gomez-Guerra LS, Martinez-Fierro ML,
Alcantara-Aragon V, Ortiz-Lopez R, Martinez-Villarreal RT,
Morales-Rodriguez IB, Garza-Guajardo R, Ponce-Camacho MA and
Rojas-Martinez A: Population based prostate cancer screening in
north Mexico reveals a high prevalence of aggressive tumors in
detected cases. BMC Cancer. 9:912009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thompson IM, Pauler DK, Goodman PJ, Tangen
CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford
ED, et al: Prevalence of prostate cancer among men with a
prostate-specific antigen level < or =4.0 ng per milliliter. N
Engl J Med. 350:2239–2246. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Catalona WJ, Richie JP, Ahmann FR, Hudson
MA, Scardino PT, Flanigan RC, DeKernion JB, Ratliff TL, Kavoussi
LR, Dalkin BL, et al: Comparison of digital rectal examination and
serum prostate specific antigen in the early detection of prostate
cancer: Results of a multicenter clinical trial of 6,630 men. J
Urol. 197:S200–S207. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu ZQ, Fang JM, Xiao YY, Zhao Y, Cui R,
Hu F and Xu Q: Prognostic role of vascular endothelial growth
factor in prostate cancer: A systematic review and meta-analysis.
Int J Clin Exp Med. 8:2289–2298. 2015.PubMed/NCBI
|
|
6
|
Gyftopoulos K, Vourda K, Sakellaropoulos
G, Perimenis P, Athanasopoulos A and Papadaki E: The angiogenic
switch for vascular endothelial growth factor-A and
cyclooxygenase-2 in prostate carcinoma: Correlation with
microvessel density, androgen receptor content and Gleason grade.
Urol Int. 87:464–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nordby Y, Andersen S, Richardsen E, Ness
N, Al-Saad S, Melbø-Jørgensen C, Patel HR, Dønnem T, Busund LT and
Bremnes RM: Stromal expression of VEGF-A and VEGFR-2 in prostate
tissue is associated with biochemical and clinical recurrence after
radical prostatectomy. Prostate. 75:1682–1693. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duque JL, Loughlin KR, Adam RM, Kantoff P,
Mazzucchi E and Freeman MR: Measurement of plasma levels of
vascular endothelial growth factor in prostate cancer patients:
Relationship with clinical stage, Gleason score, prostate volume,
and serum prostate-specific antigen. Clinics (Sao Paulo).
61:401–408. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Silva SA, Gobbo MG, Pinto-Fochi ME,
Rafacho A, Taboga SR, Almeida EA, Góes RM and Ribeiro DL: Prostate
hyperplasia caused by long-term obesity is characterized by high
deposition of extracellular matrix and increased content of MMP-9
and VEGF. Int J Exp Pathol. 96:21–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stefanou D, Batistatou A, Kamina S,
Arkoumani E, Papachristou DJ and Agnantis NJ: Expression of
vascular endothelial growth factor (VEGF) and association with
microvessel density in benign prostatic hyperplasia and prostate
cancer. In vivo. 18:155–160. 2004.PubMed/NCBI
|
|
11
|
Walsh K, Sriprasad S, Hopster D, Codd J
and Mulvin D: Distribution of vascular endothelial growth factor
(VEGF) in prostate disease. Prostate Cancer Prostatic Dis.
5:119–122. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Botelho F, Pina F and Lunet N: VEGF and
prostatic cancer: A systematic review. Eur J Cancer Prev.
19:385–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peyromaure M, Goulvestre C, Fulla Y,
Grabar S, Debré B and Dinh-Xuan AT: Serum levels of vascular
endothelial growth factor in patients undergoing prostate biopsy
for suspicion of prostate cancer. Urology. 66:687–691. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Becerra SP and Notario V: The effects of
PEDF on cancer biology: Mechanisms of action and therapeutic
potential. Nat Rev Cancer. 13:258–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Halin S, Wikström P, Rudolfsson SH,
Stattin P, Doll JA, Crawford SE and Bergh A: Decreased pigment
epithelium-derived factor is associated with metastatic phenotype
in human and rat prostate tumors. Cancer Res. 64:5664–5671. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu QJ, Gong CY, Luo ST, Zhang DM, Zhang S,
Shi HS, Lu L, Yan HX, He SS, Li DD, et al: AAV-mediated human PEDF
inhibits tumor growth and metastasis in murine colorectal
peritoneal carcinomatosis model. BMC Cancer. 12:1292012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gong Q, Qiu S, Li S, Ma Y, Chen M, Yao Y,
Che D, Feng J, Cai W, Ma J, et al: Proapoptotic PEDF functional
peptides inhibit prostate tumor growth-a mechanistic study. Biochem
Pharmacol. 92:425–437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mirochnik Y, Aurora A, Schulze-Hoepfner
FT, Deabes A, Shifrin V, Beckmann R, Polsky C and Volpert OV: Short
pigment epithelial-derived factor-derived peptide inhibits
angiogenesis and tumor growth. Clin Cancer Res. 15:1655–1663. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qingyi Z, Lin Y, Junhong W, Jian S,
Weizhou H, Long M, Zeyu S and Xiaojian G: Unfavorable prognostic
value of human PEDF decreased in high-grade prostatic
intraepithelial neoplasia: A differential proteomics approach.
Cancer Invest. 27:794–801. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Byrne JC, Downes MR, O'Donoghue N, O'Keane
C, O'Neill A, Fan Y, Fitzpatrick JM, Dunn M and Watson RW: 2D-DIGE
as a strategy to identify serum markers for the progression of
prostate cancer. J Proteome Res. 8:942–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grossniklaus HE, Zhang Q, You S, McCarthy
C, Heegaard S and Coupland SE: Metastatic ocular melanoma to the
liver exhibits infiltrative and nodular growth patterns. Hum
Pathol. 57:165–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi D, Xie F, Zhang Y, Tian Y, Chen W, Fu
L, Wang J, Guo W, Kang T, Huang W and Deng W: TFAP2A regulates
nasopharyngeal carcinoma growth and survival by targeting HIF-1α
signaling pathway. Cancer Prev Res (Phila). 7:266–277. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jeng KS, Sheen IS, Jeng WJ and Su JC: PEDF
effectively decreases VEGF to PEDF messenger RNA ratio of the inner
edge of rat hepatocellular carcinoma induced by diethyl
nitrosamine-an ‘in vivo’ study. Hepatogastroenterology.
59:1484–1490. 2012.PubMed/NCBI
|
|
24
|
Yang H, Xu Z, Iuvone PM and Grossniklaus
HE: Angiostatin decreases cell migration and vascular endothelium
growth factor (VEGF) to pigment epithelium derived factor (PEDF)
RNA ratio in vitro and in a murine ocular melanoma model. Mol Vis.
12:511–517. 2006.PubMed/NCBI
|
|
25
|
Bai YJ, Huang LZ, Zhou AY, Zhao M, Yu WZ
and Li XX: Antiangiogenesis effects of endostatin in retinal
neovascularization. J Ocul Pharmacol Ther. 29:619–626. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Regulation on Research for Health of the
General Health Law. http://www.salud.gob.mx/unidades/cdi/nom/compi/rlgsmis.html
|
|
27
|
Official Mexican Norm number
NOM-253-SSA1-2012, for the disposal of human blood and its
components for therapeutic purposes. http://www.cnts.salud.gob.mx/descargas/PROY_A_NOM_2-1.pdf
|
|
28
|
Jamaspishvili T, Kral M, Khomeriki I,
Student V, Kolar Z and Bouchal J: Urine markers in monitoring for
prostate cancer. Prostate Cancer Prostatic Dis. 13:12–19. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim NH, Oh JH, Seo JA, Lee KW, Kim SG,
Choi KM, Baik SH, Choi DS, Kang YS, Han SY, et al: Vascular
endothelial growth factor (VEGF) and soluble VEGF receptor FLT-1 in
diabetic nephropathy. Kidney Int. 67:167–177. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shariat SF, Anwuri VA, Lamb DJ, Shah NV,
Wheeler TM and Slawin KM: Association of preoperative plasma levels
of vascular endothelial growth factor and soluble vascular cell
adhesion molecule-1 with lymph node status and biochemical
progression after radical prostatectomy. J Clin Oncol.
22:1655–1663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li H, Kantoff PW, Ma J, Stampfer MJ and
George DJ: Prediagnostic plasma vascular endothelial growth factor
levels and risk of prostate cancer. Cancer Epidemiol Biomarkers
Prev. 14:1557–1561. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mao K, Badoual C, Camparo P, Delongchamps
NB, Vieillefond A, Dinh-Xuan AT and Peyromaure M: The prognostic
value of vascular endothelial growth factor (VEGF)-A and its
receptor in clinically localized prostate cancer: A prospective
evaluation in 100 patients undergoing radical prostatectomy. Can J
Urol. 15:4257–4262. 2008.PubMed/NCBI
|
|
33
|
Soulitzis N, Karyotis I, Delakas D and
Spandidos DA: Expression analysis of peptide growth factors VEGF
FGF2, TGFB1, EGF and IGF1 in prostate cancer and benign prostatic
hyperplasia. Int J Oncol. 29:305–314. 2006.PubMed/NCBI
|
|
34
|
Lekas A, Lazaris AC, Deliveliotis C,
Chrisofos M, Zoubouli C, Lapas D, Papathomas T, Fokitis I and
Nakopoulou L: The expression of hypoxia-inducible factor-1alpha
(HIF-1alpha) and angiogenesis markers in hyperplastic and malignant
prostate tissue. Anticancer Res. 26:2989–2993. 2006.PubMed/NCBI
|
|
35
|
Shih SJ, Dall'Era MA, Westphal JR, Yang J,
Sweep CG, Gandour-Edwards R and Evans CP: Elements regulating
angiogenesis and correlative microvessel density in benign
hyperplastic and malignant prostate tissue. Prostate Cancer
Prostatic Dis. 6:131–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Voss M, Trojan L, Steidler A, Weiss C,
Grobholz R, Alken P and Michel MS: Serum vascular endothelial
growth factor C level in patients with prostate cancer and benign
prostatic hyperplasia. Anal Quant Cytol Histol. 30:199–202.
2008.PubMed/NCBI
|
|
37
|
Matsui T, Ojima A, Higashimoto Y, Taira J,
Fukami K and Yamagishi SI: Pigment epithelium-derived factor
inhibits caveolin-induced interleukin-8 gene expression and
proliferation of human prostate cancer cells. Oncol Lett.
10:2644–2648. 2015.PubMed/NCBI
|
|
38
|
Nelius T, Martinez-Marin D, Hirsch J,
Miller B, Rinard K, Lopez J, de Riese W and Filleur S: Pigment
epithelium-derived factor expression prolongs survival and enhances
the cytotoxicity of low-dose chemotherapy in castration-refractory
prostate cancer. Cell Death Dis. 5:e12102014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hirsch J, Johnson CL, Nelius T, Kennedy R,
Riese WD and Filleur S: PEDF inhibits IL8 production in prostate
cancer cells through PEDF receptor/phospholipase A2 and regulation
of NFκB and PPARγ. Cytokine. 55:202–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nelius T, Samathanam C, Martinez-Marin D,
Gaines N, Stevens J, Hickson J, de Riese W and Filleur S: Positive
correlation between PEDF expression levels and macrophage density
in the human prostate. Prostate. 73:549–561. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ide H, Yamagishi S, Lu Y, Sakamaki K,
Nakajima A, Horiuchi A, Kitamura K, Hisasue S, Muto S, Yamaguchi R
and Horie S: Circulating pigment epithelium-derived factor (PEDF)
is associated with pathological grade of prostate cancer.
Anticancer Res. 35:1703–1708. 2015.PubMed/NCBI
|
|
42
|
Doll JA, Stellmach VM, Bouck NP, Bergh AR,
Lee C, Abramson LP, Cornwell ML, Pins MR, Borensztajn J and
Crawford SE: Pigment epithelium-derived factor regulates the
vasculature and mass of the prostate and pancreas. Nat Med.
9:774–780. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Carnagarin R, Dharmarajan AM and Dass CR:
PEDF-induced alteration of metabolism leading to insulin
resistance. Mol Cell Endocrinol. 401:98–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cai J, Chen Z, Ruan Q, Han S, Liu L, Qi X,
Boye SL, Hauswirth WW, Grant MB and Boulton ME: γ-Secretase and
presenilin mediate cleavage and phosphorylation of vascular
endothelial growth factor receptor-1. J Biol Chem. 286:42514–42523.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pollina EA, Legesse-Miller A, Haley EM,
Goodpaster T, Randolph-Habecker J and Coller HA: Regulating the
angiogenic balance in tissues. Cell Cycle. 7:2056–2070. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Volpert OV, Zaichuk T, Zhou W, Reiher F,
Ferguson TA, Stuart PM, Amin M and Bouck NP: Inducer-stimulated Fas
targets activated endothelium for destruction by anti-angiogenic
thrombospondin-1 and pigment epithelium-derived factor. Nat Med.
8:349–357. 2002. View Article : Google Scholar : PubMed/NCBI
|