Introduction
The requirement for genetic testing for tumor
pathological diagnosis is increasing due to the association between
tumor genesis and genetic abnormalities, thus objective diagnosis
may be achieved by investigating such aberrations. Furthermore,
genetic diagnosis methods, including fluorescence in situ
hybridization (FISH) are important for determining the target drug
for individual patients. For example, in cases of breast cancer, it
is important to investigate the expression of human epidermal
growth factor receptor 2 (HER2) protein or determine HER2 genetic
amplification when considering administration of the molecular
targeting drug trastuzumab (1). As a
result, institutions are increasing adopting FISH for routine
pathological examinations. In addition, FISH is frequently used to
diagnose hematologic malignancy as important gene abnormalities
have been observed in affected patients (2). For example, the important chimaera gene
for oncogenesis, which has been observed in soft portions of
tumors, such as synovial sarcoma (2).
It is considered that the importance of FISH for examining solid
tumors may increase in the same manner.
When using FISH, various problems can be encountered
including those associated with reproducibility. The protocol for
detection is complicated and considerable difficulties with
obtaining stable results are faced when performing examinations for
HER2 administration. Furthermore, a formalin-fixed
paraffin-embedded (FFPE) technique is typically used for
pathological examinations. However, signal strength and rate of
detection with FISH have been reported to be affected by formalin
fixation time (3). Additionally, it
is difficult to distinguish target cells during observations with a
fluorescence microscope.
To address these problems, the present study aimed
to simplify the FISH protocol and develop a double-detection method
that includes fluorescence immunostaining of FFPE tissue sections.
In the present study, experiments were performed to validate this
novel method.
Materials and methods
Cases
FFPE sections from 32 cases (20 mammary gland and 12
stomach) that underwent an examination of HER2 at Tsuchiura Kyodo
General Hospital (Tsuchiura, Japan) between May and November in
2015 were used. All samples were biopsied. The samples that were
fixed for >48 h were excluded from the subject. The mean age of
the patients was 46 years (range, 32–68 years). All the mammary
gland samples were collected from female patients, while stomach
samples were collected from 7 males and 5 females. In addition,
FFPE sections of lymph nodes from 1 patient with Hodgkin's disease
were examined. Written informed consent was obtained from all of
the patients who provided specimens. Approval for the present study
was obtained from Tsuchiura Kyodo General Hospital Ethical Review
Board. All diagnoses of HER2 were obtained based on the HER2
guidelines of the Japanese Society of Pathology (4).
Simplified FISH method and FISH
combined with fluorescence immunostaining
FISH was performed with a Path-vision HER2 DNA kit
(Abbott Pharmaceutical Co., Ltd., Lake Bluff, IL, USA). For the
FISH method protocol, 4-µm dewaxed sections were washed with
xylene, rehydrated in a descending alcohol series and incubated in
antigen activation fluid (pH 9.0; cat no. 415211; Nichirei
Biosciences, Inc., Tokyo, Japan) for 30 min at 99°C, with the
incubation time extended to 45 min for surgical samples that
underwent extensive fixation, followed by cooling for 20 min.
Following drying, the FISH probe (from the kit) was added and the
section was incubated for 6 h in a moisture chamber at 42°C after
denaturing treatment for 5 min at 94°C. The sections were then
washed with Tris buffer (cat. no. 102189; LSI Medience Corp.,
Tokyo, Japan), including 0.3% Nonidet P-40 (cat. no. 25223-04;
Nacalai Tesque, Inc., Kyoto, Japan) at 42°C for 15 min, prior to
being air dried and cover-slipped in DAPI.
A novel double-detection method was developed using
a combination of FISH and fluorescence immunostaining. All
processes were performed in a chamber at 42°C. First, the section
was treated with antigen activation solution (pH 9.0; Nichirei
Bioscience) for 30 min and cooled for 20 min. The section was then
reacted with the primary HER2 antibody (clone CB11; dilution,
1:300; cat. no. NCL-L-CB11; Leica Microsystems GmbH, Wetzlar,
Germany) for 60 min, followed by incubation with a biotinylated
secondary antibody (cat. no. 426072; undiluted; Nichirei
Biosciences, Inc.) for 30 min and Alexa Fluor 488-labelled
streptavidin (cat. no. S11223; Thermo Fisher Scientific Inc.,
Waltham, MA, USA; dilution, 1:30) for a further 30 min.
Subsequently, re-fixation in 4% formalin was performed for 5 min at
room temperature; then, after 15 min washing with Tris buffer (LSI
Medience Corp.), including 0.3% Nonidet P-40 (Nacalai Tesque, Inc.)
and drying, FISH was performed using the aforementioned simplified
protocol.
Correlation between HER2 protein and
gene amplification
The association between HER2 protein and gene
amplification was investigated using 12 stomach and 20 mammary
biopsy specimens. The routine laboratory tests were scored, as
described previously (4). A FISH
examination was also performed in cases with a score of 2. In
addition, double-detection was performed using FISH and
fluorescence immunostaining for HER2 in all 32 cases to examine
HER2 protein overexpression, then the HER2/CEP17 ratio was
calculated, and compared the results with those of routine
laboratory testing.
Cytokeratin and HER2 genetic
double-detection
In a conventional FISH examination, it is difficult
to distinguish target cells under a fluorescence microscope. Our
double-detection method was performed with cytokeratin, a
representative marker of epithelial cells, to examine 3 biopsy
samples obtained from subjects with poorly differentiated gastric
cancer, with AE1/AE3 utilized as the cytokeratin antibody (cat no.
M3515; dilution, 1:300; Dako; Agilent Technologies, Inc. Santa
Clara, CA, USA).
Cluster of differentiation (CD)30+ IgH
chimaera gene double-detection in Hodgkin's disease
In Hodgkin's disease, Hodgkin's cells coexist with
non-tumor cells. Using our double-detection method, FISH was
performed along with identification of tumor cells. One FFPE
section of a Hodgkin's disease specimen was immunostained with an
anti-CD30 antibody (clone JCM182; dilution, 1:150; cat. no.
NCL-L-CD30-591; Leica Microsystems GmbH) to identify Hodgkin's
cells, after which it was reacted with an IgH break-apart probe
(cat. no. KBI 10729; Kreatech Biotechnology B.V., Amsterdam,
Netherland) and the condition of the IgH gene in those cells was
observed using fluorescence microscope at ×400 magnification.
Statistical analysis
In order to investigate the correlation between HER2
protein and gene amplification, the Spearman's rank correlation
test was performed using SPSS Statistical software 24.0 (IBM Corp.,
Armonk, NY, USA).
Results
Correlation between HER2 protein and
gene amplification
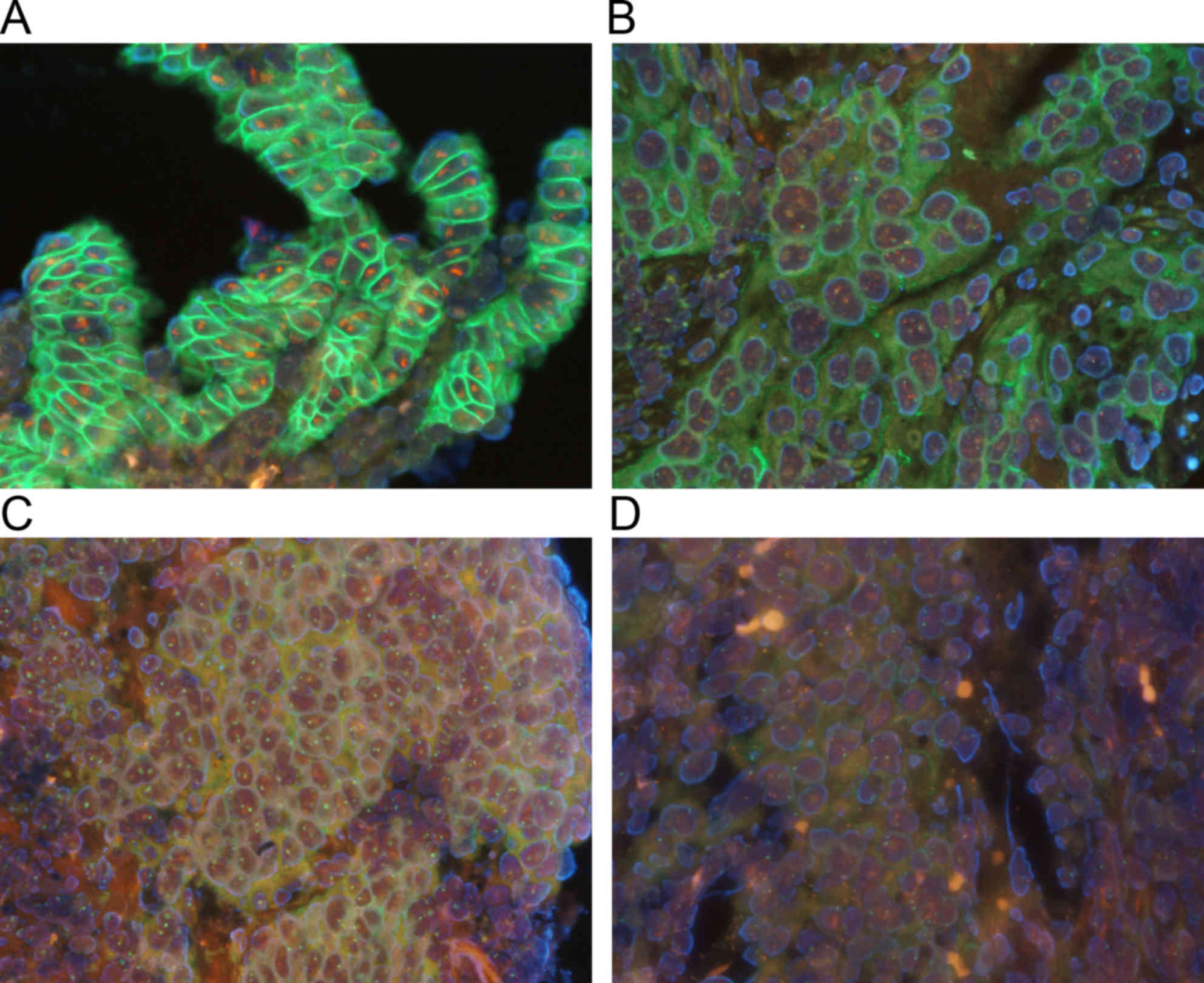
The results are summarized in Table I. HER2 protein and genes were
distinctly observed with the use of the double staining method
(Fig. 1). In the routine laboratory
tests, 8 cases were given an HER2 protein score of 2+. Those were
subjected to FISH, which identified 4 cases as positive.
Furthermore, 11 cases had an HER2 score of 3+, thus the total
number of positive cases was 15. Protein overexpression and gene
amplification were recognized in all of these positive cases with
our double-detection method. The mean HER2/CEP17 ratio for the 17
negative cases was 1.27, while that of the 15 positive cases was
5.98. Thus, these results demonstrated that double-detection
provided results equal to those obtained with routine laboratory
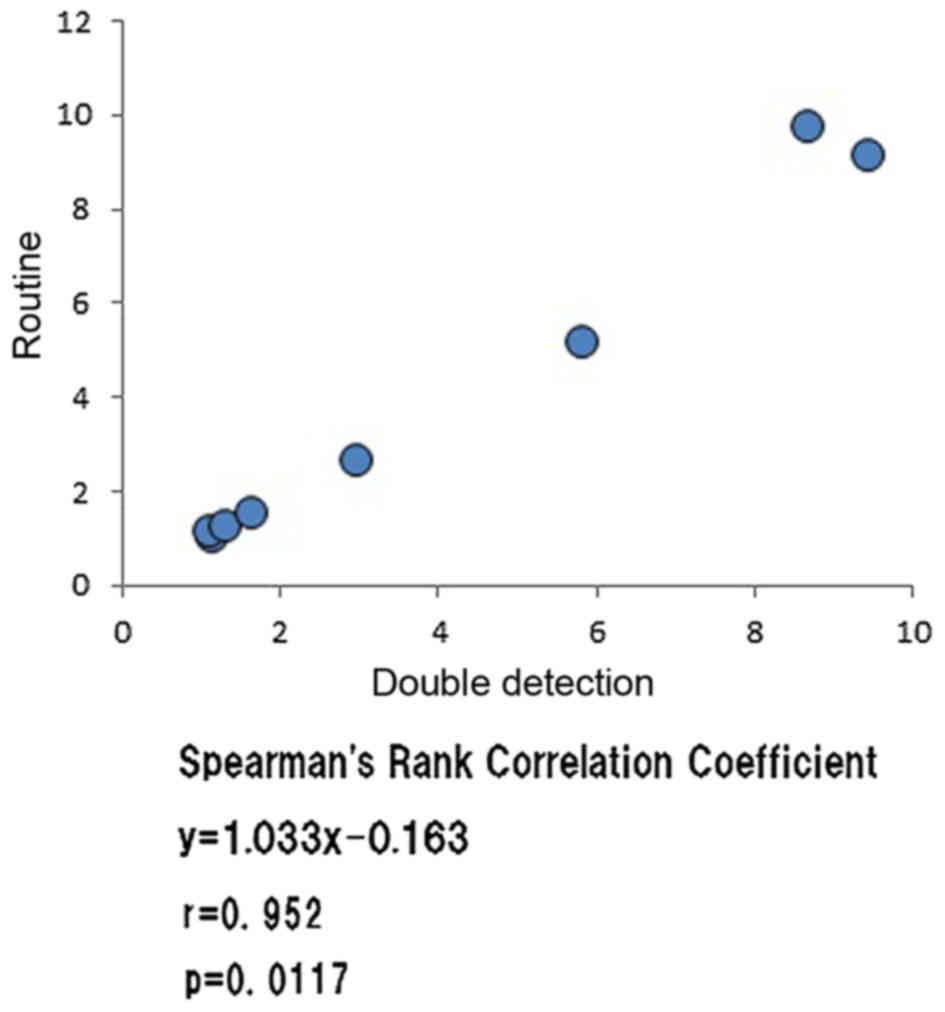
testing. A FISH examination was also performed in 8 cases that
underwent routine laboratory testing and the correlation with the
HER2/CEP17 ratio results obtained from the double-detection method
was investigated, which indicated a significant and positive
correlation (Fig. 2). Thus, a similar
FISH ratio using double-detection was obtained.
 | Table I.Results of routine testing and double
detection method. |
Table I.
Results of routine testing and double
detection method.
|
| Routine | Double-detection |
|
|
|---|
|
|
|
|
|
|
|---|
| Case no. | Immunostaining | Fish ratio | Immunostaining | Gene
amplification | Fish ratio | Specimen type | Organ |
|---|
| 1 | 0 | ND | − | − | 1.09 | Biopsy | Mammary gland |
| 2 | 0 | ND | − | − | 1.24 | Biopsy | Mammary gland |
| 3 | 0 | ND | + | − | 1.09 | Biopsy | Stomach |
| 4 | 0 | ND | + | − | 1.1 | Biopsy | Stomach |
| 5 | 0 | ND | + | − | 1.21 | Biopsy | Mammary gland |
| 6 | 0 | ND | + | − | 1.38 | Biopsy | Mammary gland |
| 7 | 0 | ND | + | − | 1.44 | Biopsy | Stomach |
| 8 | 1+ | ND | + | − | 1.02 | Biopsy | Mammary gland |
| 9 | 1+ | ND | + | − | 1.06 | Biopsy | Mammary gland |
| 10 | 1+ | ND | + | − | 1.3 | Biopsy | Mammary gland |
| 11 | 1+ | ND | + | − | 1.5 | Biopsy | Mammary gland |
| 12 | 1+ | ND | + | − | 1.53 | Biopsy | Mammary gland |
| 13 | 1+ | ND | + | − | 1.55 | Biopsy | Mammary gland |
| 14 | 2+ | 1.12 | ++ | − | 1.05 | Surgical | Mammary gland |
| 15 | 2+ | 1.08 | ++ | − | 1.17 | Biopsy | Stomach |
| 16 | 2+ | 1.29 | ++ | − | 1.25 | Biopsy | Mammary gland |
| 17 | 2+ | 1.63 | ++ | − | 1.55 | Surgical | Mammary gland |
| 18 | 2+ | 2.96 | ++ | + | 2.66 | Biopsy | Mammary gland |
| 19 | 2+ | 5.82 | ++ | + | 5.18 | Biopsy | Stomach |
| 20 | 2+ | 9.43 | +++ | + | 9.13 | Biopsy | Stomach |
| 21 | 2+ | 8.67 | ++ | + | 9.75 | Biopsy | Mammary gland |
| 22 | 3+ | ND | ++ | + | 2.67 | Biopsy | Stomach |
| 23 | 3+ | ND | +++ | + | 2.45 | Biopsy | Mammary gland |
| 24 | 3+ | ND | +++ | + | 2.54 | Surgical | Mammary gland |
| 25 | 3+ | ND | ++ | + | 3.25 | Biopsy | Stomach |
| 26 | 3+ | ND | ++ | + | 4.41 | Biopsy | Stomach |
| 27 | 3+ | ND | +++ | + | 6.57 | Biopsy | Stomach |
| 28 | 3+ | ND | ++ | + | 7.36 | Surgical | Mammary gland |
| 29 | 3+ | ND | ++ | + | 7.56 | Biopsy | Stomach |
| 30 | 3+ | ND | +++ | + | 8.2 | Surgical | Mammary gland |
| 31 | 3+ | ND | +++ | + | 8.41 | Biopsy | Stomach |
| 32 | 3+ | ND | +++ | + | 9.57 | Biopsy | Mammary gland |
Cytokeratin and HER2 genetic
double-detection
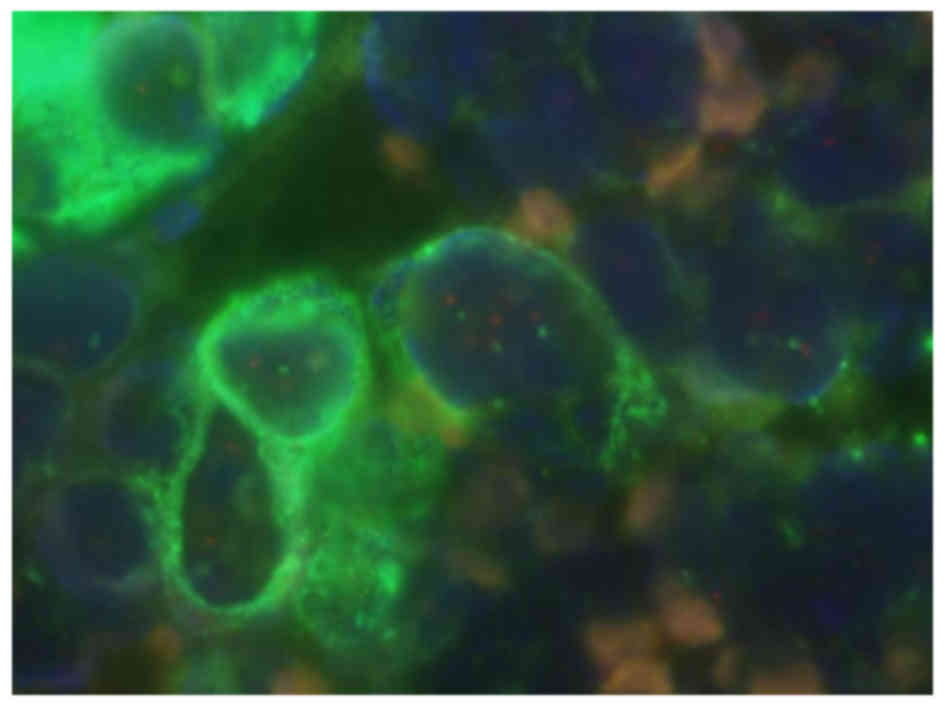
The results of the investigation in 3 cases of
poorly differentiation gastric cancer demonstrated distinct
validation of the presence of epithelial cells and observation of
the HER2 gene in all cases. Since the HER2 protein was negative in
all of these cases, FISH was not performed. Furthermore, gene
amplification was not identified even with the double-detection
method (Fig. 3). Thus, the method may
precisely observe objective cellular genetic conditions.
CD30+IgH chimaera gene
double-detection in Hodgkin's disease
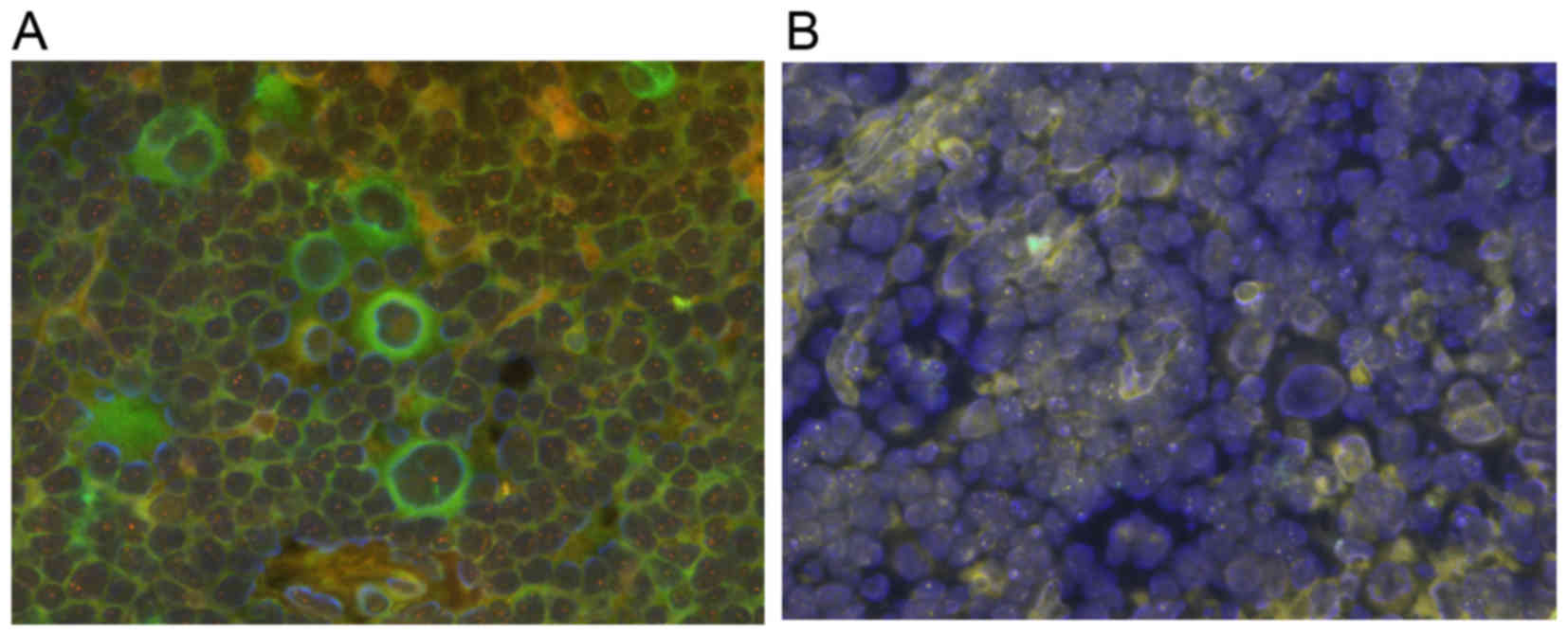
Using the double-detection method, Hodgkin's cells
were identified using the CD30 antibody and the genetic condition
was confirmed with an IgH probe (Fig.
4A). However, it was difficult to distinguish Hodgkin's cells
with a simple FISH examination (Fig.
4B).
Discussion
In the present study, a novel double-detection
method was developed to detect proteins and the genetic condition
of isologous FFPE sections used for routine pathological
examinations. The protocol is simple and easy, and similar to that
used for double immunostaining. Thus, the present double-detection
method may be applied as a part of routine laboratory testing.
In the present study with clinical samples, the
results of our double-detection method were nearly the same as
compared with routine laboratory tests, confirming its
applicability. It is well known that the protein expression of HER2
is associated with gene amplification in gastric and breast cancer
(5). When examining tissue sections,
it is sometimes difficult to identify the target cells among
various observed cells, though cytokeratin is useful to easily
identify epithelial cells. Similarly, Hodgkin's cells were
identified using CD30 in the present study. With our
double-detection method, the genetic condition of targeted cells
was observed.
Previous reports in other fields, including
hematologic malignancy have presented double-detection methods
(6). In 1992, Weber-Matthiesen et
al (7,8) developed a method called Fluorescence
Immuno-phenotyping and Interphase Cytogenetics as a Tool of the
Investigation of Neoplasm. However, based on images presented in
those reports, it was considered that improvements in the technique
used for detection were required and it has yet to become a
universal method. An examination using FFPE has also been reported
(9). In that report, the images were
low quality due to excessive proteolytic enzyme treatment and it is
considered that improvements in the technique used for detection
with that method are also necessary. With our method, heat
treatment alone was used. Protease is usually used for pretreatment
of ISH. The optimal treatment time of protease is associated with
the formalin fixation time of a sample (10). Tissue samples in a routine laboratory
test vary in terms of optimal protease treatment time, because the
fixation time is not constant. This may be the reason why results
of ISH vary. The methods used in the present study did not use
protease, but used detergent instead. As a result, dispersion of
signal intensity is suppressed. In the present study, observation
was easy in comparison with previous reports.
Similar studies have used a visible light detection
system. In 2005, Downs-Kelly et al (11) examined HER2 protein and gene
double-detection, while Ni et al (12) in 2007 and Reisenbichler et al
(13) in 2012 presented the same
method. Those authors concluded that the techniques employed with
that method allowed for observation of gene and protein
expressions, and examinations of both in detail. In 2012, Nitta
et al (14) reported a method
for detection of HER2 protein and gene expression using an
automatic immunohistochemistry system, termed gene protein assay,
which utilizes pigments, including DAB for visualization. Detection
under visible light has numerous advantages. For example, there is
no need for a fluorescence microscope or specially equipped
darkroom and the preparations are permanently preserved. In
contrast, our method using a fluorescence microscope has some
merits. First, the choice of the target allows for the use of
various probes, making it useful for a variety of applications.
Second, an expensive detection system is not necessary. Therefore,
our method is useful for pathological diagnosis using FFPE
sections.
Another merit of our method is its simple protocol,
though high quality FFPE sections are important to obtain good
results. As for preparing routine FFPE sections, the methods and
formalin fixation times are not uniform. It is well known that FFPE
with an inferior condition results in incorrect immunostaining or
FISH results. In the present study, good results were obtained with
mammary gland needle biopsy specimens and gastric endoscopic biopsy
specimens, for which a long period was not needed for fixation of
the specimens.
In conclusion, the present study reported a novel
and simple method of double-detection with FISH, and fluorescence
immunostaining for use with FFPE sections. With this method,
various genetic aberrations and protein overexpression were
observed in isologous sections. Since the protocol is similar to
that of double immunostaining, the method may be easily applied in
a clinical laboratory setting.
References
|
1
|
Wolff AC, Hammond ME, Schwartz JN, Hagerty
KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer
A, et al: American Society of Clinical Oncology/College of American
Pathologists guideline recommendations for human epidermal growth
factor receptor 2 testing in breast cancer. J Clin Oncol.
25:118–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kawai A, Woodruff J, Healey JH, Brennan
MF, Antonescu CR and Ladanyi M: SYT-SSX gene fusion as a
determinant of morphology and prognosis in synovial sarcoma. N Engl
J Med. 338:153–160. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moatamed NA, Nanjangud G, Pucci R, Lowe A,
Shintaku IP, Shapourifar-Tehrani S, Rao N, Lu DY and Apple SK:
Effect of ischemic time, fixation time, and fixative type on
HER2/neu immunohistochemical and fluorescence in situ hybridization
results in breast cancer. Am J Clin Pathol. 136:754–761. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Japanese Society of Pathology, . Guideline
for HER2 examination of gastric cancer and breast cancer. 1st.
Kanehara Press Co.; Tokyo: 2015
|
|
5
|
Owens MA, Horten BC and Da Silva MM: HER2
amplification ratios by fluorescence in situ hybridization and
correlation with immunohistochemistry in a cohort of 6556 breast
cancer tissues. Clin Breast Cancer. 5:63–69. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abaza HM, Youssef SR, Saad AA, Kamal GM,
Hegazy MG, Ibrahim RI and Annaka LM: Detection of 14q32
rearrangements in multiple myeloma, using simultaneous FISH
analysis combined with immunofluorescence. Hematol Oncol Stem Cell
Ther. 8:56–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weber-Matthiesen K, Winkemann M,
Müller-Hermelink A, Schlegelberger B and Grote W: Simultaneous
fluorescence immunophenotyping and interphase cytogenetics: A
contribution to the characterization of tumor cells. J Histochem
Cytochem. 40:171–175. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weber-Matthiesen K, Deerberg J,
Müller-Hermelink A, Schlegelberger B and Grote W: Rapid
immunophenotypic characterization of chromosomally aberrant cells
by the new FICTION method. Cytogenet Cell Genet. 63:123–125. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nomoto J, Hiramoto N, Kato M, Sanada M,
Maeshima AM, Taniguchi H, Hosoda F, Asakura Y, Munakata W,
Sekiguchi N, et al: Deletion of the TNFAIP3/A20 gene detected by
FICTION analysis in classical Hodgkin lymphoma. BMC Cancer.
12:4572012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mostegl MM, Richter B, Dinhopl N and
Weissenböck H: Influence of prolonged formalin fixation of tissue
samples on the sensitivity of chromogenic in situ hybridization. J
Vet Diagn Invest. 23:1212–1216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Downs-Kelly E, Pettay J, Hicks D, Skacel
M, Yoder B, Rybicki L, Myles J, Sreenan J, Roche P, Powell R, et
al: Analytical validation and interobserver reproducibility of
EnzMet GenePro: A second-generation bright-field metallography
assay for concomitant detection of HER2 gene status and protein
expression in invasive carcinoma of the breast. Am J Surg Pathol.
29:1505–1511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ni R, Mulligan AM, Have C and O'Malley FP:
PGDS, a novel technique combining chromogenic in situ hybridization
and immunohistochemistry for the assessment of ErbB2 (HER2/neu)
status in breast cancer. Appl Immunohistochem Mol Morphol.
15:316–324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reisenbichler ES, Horton D, Rasco M, Andea
A and Hameed O: Evaluation of dual immunohistochemistry and
chromogenic in situ hybridization for HER2 on a single section. Am
J Clin Pathol. 137:102–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nitta H, Kelly BD, Padilla M, Wick N,
Brunhoeber P, Bai I, Singh S, Ranger-Moore J, Bieniarz C, Tsuda H
and Grogan TM: A gene-protein assay for human epidermal growth
factor receptor 2 (HER2): Brightfield tricolor visualization of
HER2 protein, the HER2 gene, and chromosome 17 centromere (CEN17)
in formalin-fixed, paraffin-embedded breast cancer tissue sections.
Diagn Pathol. 7:602012. View Article : Google Scholar : PubMed/NCBI
|