Introduction
Chemotherapy serves an important and indispensable
role in leukemia therapy. However, chemotherapy-induced multidrug
resistance (MDR) frequently induces therapy failure or tumor
recurrence (1–3). MDR involves various mechanisms
including, adenosine triphosphate-binding cassette (ABC)
transporters, P-glycoprotein (P-gp), multidrug-resistance-related
protein (MRP) and breast-cancer-resistance protein (BCRP)
overexpression, which function to efflux certain molecules out of
the cells (4–8). In addition, mechanisms of acquired MDR
in leukemia also include abnormal drug metabolism and the presence
of leukemia stem cells (LSC). LSCs are particularly of interest and
considered to serve an important role in leukemia resistance and
relapse (4). Numerous previous
studies have confirmed that the rare LSC sub-population in leukemia
has self-renewal abilities, uncommitted proliferative capacities,
relative dormancy or quiescence, high expression of ABC
transporters and good DNA-damage repair ability (9–12). In
addition, LSCs have strong resistance to almost all
chemotherapeutics (2,5). However, the role of LSCs and the
mechanism underlying transmission of super-drug resistance to
daughter cells remains unclear. Additionally, the specific markers
of LSCs remain uncertain. Previous studies have demonstrated that
LSC populations are heterogeneous and may be defined as cluster of
differentiation (CD)34+, CD38−, human
leukocyte antigen D related, CD90−, CD117−
and CD123+ phenotypes (3,13–17). Previous studies demonstrated that
CD123 was aberrantly expressed on acute myeloid leukemia
CD34+CD38− cells but was not detected on
healthy CD34+CD38− cells. Therefore, the
level of CD123 expression appears to be leukemic-specific and may
be a criterion for identifying LSCs from abnormal hematopoietic
stem cells (13,14).
Previous studies have revealed that the majority of
MDR leukemia cells have increased sensitivity to
As2O3, rather than cross-resistance (3,4). One
potential explanation for this is that As2O3
may not be a substrate of P-gp and may inhibit P-gp activity
(2,18–22). The
present study selected HL-60 human promyelocyte leukemia cells for
doxorubicin (ADM)-resistance by long-term exposure to intermittent
and continuous stepwise increments of ADM, and characterized the
distinguishing features of acquired MDR, particularly in terms of
sensitivity to As2O3 and the role of
LSCs.
Materials and methods
Materials
ABCB1, ABCC1, ABCG2 and β-actin
primers were synthesized by Takara Bio, Inc. (Otsu, Japan). SYBR
Premix Ex Taq and Prime Script RT reagents were obtained from
Takara Bio, Inc. The following antibodies were used: Mouse
anti-β-actin antibody (cat no. 3598-100; dilution, 1:1,000;
BioVision, Inc., Milpitas, CA, USA), rabbit anti-P-gp antibody
(cat. no. BA1351-2; dilution, 1:500), anti-BCRP antibody (cat. no.
BA2307-2; dilution, 1:500), and anti-MRP1 antibody (cat. no.
BA0567; dilution, 1:500) from Boster Biological Technology, Wuhai,
China, PE-IgG1 (cat. no. GM4993), ECD-IgG1 (cat. no. A99022),
PEcy5-IgG1 (cat. no. 85-15-4714-71), PEcy5-CD34 (cat. no.
CD3458118) and ECD-CD38 (cat. no. 14-0389-82) and PE-CD123 (cat.
no. 12-1239), FITC-BCRP (cat. no. lv1506735) antibodies
(eBioscience; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
P-gp (MRK16; cat. no. Mc-012; Kamiya Biomedical Co., Tukwila, WA,
USA). The chemotherapeutic drugs used were ADM (Shenzhen Wanle
Pharmaceutical Co., Ltd., Shenzhen, China), daunorubicin (Zhejiang
Haizheng Pharmaceutical Co., Ltd, Zhejiang, China), cisplatin and
etoposide (Qilu Pharmaceutical Co., Ltd., Jinan, China),
5-fluorouracil (Haipu Pharmaceutical Co. Ltd., Shanghai, China),
vincristine hydrochloride (Guangdong Lingnan Pharmaceutical Co.,
Ltd. Guangzhou, China), cytarabine (Haipu Pharmaceutical Co., Ltd.)
and As2O3 (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany).
Transmission electron microscope
observation of cell ultrastructure change
Cells were harvested and washed with 0.1 M PBS. The
Cell pellets were immersed in 2.5% glutaraldehyde and incubated at
4°C for 48 h. Following cell fixation, the sample was treated with
1% osmium tetroxide at room temperature for 1 h and then dehydrated
in acetone for 30 min. The sample was then embedded in embedding
resin, followed by cutting into ultrathin sections with a microtome
and lead-uranium double staining and then observed and photographed
under a transmission electron microscope.
Scanning electron microscopy
analysis
A total of 1×106 cells were washed with
PBS and quickly fixed in precooled 2.5% glutaraldehyde
(Sigma-Aldrich; Merck KGaA) at 4°C overnight. Following three
washes in 0.1 M PBS, the cells were fixed with ice-cold 1% osmium
tetroxide (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China)
for 1.5–2 h, followed by sequential 15 min incubations in 50, 70
and 80% ethanol at 4°C. Subsequently, fixing steps in 90 and 100%
ethanol at room temperature, and gradually permeabilizing steps in
a series of buffers that consisted of dehydrating agent
acetone/isoamyl acetate (1:1) mixed with epoxy resin at a ratio of
2:1, 1:1 and 1:3, followed by 100% resin, for 0.5–1 h each
incubation. Finally, the cells were soaked in tert-butyl alcohol
twice for 10 min each time, dried with a vacuum pump for 24 h and
platinum sputter-coated for 90 sec. The prepared specimen was
observed under a scanning electron microscope.
Cell culture and incubation
HL-60 human promyelocyte leukemia cells were
purchased from American Type Culture Collection (Manassas, VA,
USA). The cells were maintained in RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) and
cultivated at 37°C in a 5% CO2 incubator.
Selection of ADM-resistant cells
HL-60 human leukemia cells were selected for
ADM-resistance by long-term exposure to intermittent, repeated and
continuous stepwise increments of ADM concentrations from 0.01–40
mg/l. If the cells survived a given ADM concentration and
proliferated at a similar rate to parental HL-60 cells, the
concentration of treatment was increased. The procedure was
repeated intermittently and repeatedly until the cells were stably
able to tolerate 40 mg/l ADM. This procedure was used to generate a
stable drug-resistant leukemia subline, named HL-60/RS.
In vitro drug sensitivity
analysis
HL-60 cells tolerant to various concentrations of
ADM were collected for determination of the half-maximal inhibitory
concentrations (IC50) of ADM and for cytotoxicity
assays. A total of 1×105 cells/ml were plated in 96-well
plates and cultured at 37°C with corresponding concentrations of
the aforementioned chemotherapeutics (ADM 1, 2, 4, 6, 8, 10 mg/l,
cisplatin 1, 5, 10, 20, 40 mg/l, etoposide 0.5, 1, 2, 4, 8, 10
mg/l, 5-fluorouracil 0.5, 1, 2, 4, 8, 16 mg/l, vincristine
hydrochloride 0.5, 1, 2, 4, 8, 10 mg/l and cytarabine 0.5, 1, 2, 4,
8, 10 mg/l, all dissolved with 0.9% saline solution and
As2O3, dissolved in sodium hydroxide as a
storage concentration of 10 mmol/l whose pH value was adjusted with
1 mol/l HCL to 7.2–7.4 and diluted to 2 and 5 µmol/l before using)
for 24–72 h. Absorbance was quantified using a Powerwave X plate
reader (Omega Bio-Tek, Inc., Norcross, GA, USA) by MTT assay. A
total of 5 mg/ml MTT was added to each well and incubated at 37°C
for 4 h, then 100 µl 10% SDS was added to each well and incubated
at 37°C overnight; absorbance was then detected at 570 nm
wavelength.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was extracted using a
TRIzol® kit (Invitrogen; Thermo Fisher Scientific,
Inc.). cDNA was derived from the RNA as PCR template using the
Prime Script reverse transcriptase kit (Takara Bio, Inc.).
Temperature protocol: 70°C for 30 min, 37°C for 15 min, 95°C for 5
min. For PCR, cDNA (2 µl) was mixed with SYBR Premix Ex Taq (Takara
Bio, Inc.) and the following relevant primers for PCR were used:
β-actin forward, 5′-TGCTCCTCCTGAGCGCAAGTA-3′ and reverse,
5′-CCACATCTGCTGGAAGGTGGA-3′; P-gp forward,
5′CCCATCATTGCAATAGCAGG3′ and reverse, 5′GTTCAAACTTCTGCTCCTGA3′;
MRP1 forward, 5′TGCAGAAGGCGGGGAGAACCTC3′ and reverse,
5′GTCGTCCGTTTCCAGGTCCACG3′; BCRP2 forward,
5′GCTGCAAGGAAAGATCCAAGT3′ and reverse, 5′TAGTTGTTGCAAGCCGAAGAG3′,
which were designed and synthesized by Takara Bio, Inc. The
conditions included an initial denaturing step at 95°C for 10 sec,
then 40 cycles of denaturing at 95°C for 5 sec and annealing at
60°C for 30 sec. The relative expression of each mRNA was
calculated by comparison to β-actin mRNA using the
2−ΔΔCq method (4).
Western blot analysis
The cells were lysed using a
radioimmunoprecipitation assay (RIPA) protein extraction reagent
(cat no. P0013B; Beyotime Institute of Biotechnology, Haimen,
China), supplemented with phenylmethylsulfonyl fluoride (PMSF) (0.1
mmol/ml PMSF 10 µl in 1 ml RIPA). Total protein concentration was
determined using a BCA protein quantitative kit (cat no. P0010s;
Beyotime Institute of Biotechnology). Proteins (30 µg per lane)
were separated by 10% SDS-PAGE, transferred to a polyvinylidene
fluoride membrane and blocked with 5% skimmed milk at room
temperature for 1 h. Membranes were washed with PBS-Tween-20 (PBST)
three times for 5 min. The membranes were probed with primary
antibodies (anti-P-gp, anti-MRP1 or anti-BCRP, with the same
conditions as above) at 4°C overnight. The membrane was washed with
PBST three times for 5 min. IRDye800CW-(goat anti-mouse; cat. no.
926-32210; dilution, 1:10,000; LI-COR Biosciences, Lincoln, NE,
USA) or IRDye680DX-conjugated secondary antibodies (goat
anti-rabbit; cat. no. 926-32221; dilution, 1:10,000; LI-COR
Biosciences, Lincoln, NE, USA) was added to the membrane and
agitated for 1 h at room temperature prior to washing three times
with PBST for 5 min, using the anti-β-actin antibody as the
control. The blots of antibody-coated protein bands in immunoblots
were quantitated and visualized using an Odyssey double-color
infrared-laser imaging system (Odyssey v1.2 software; LI-COR
Biosciences Inc., Lincoln, NE, USA).
ADM accumulation/efflux assay
In order to determine the intracellular uptake of
ADM, the sensitive and resistant HL-60 cells were incubated with 30
mg/l ADM at 37°C for 30 min. Following two washes with PBS and
re-suspension in 200 µl PBS, intracellular uptake of ADM in
106 cells was determined immediately using a MoFlo XDP
Cell Sorter (Beckman Coulter, Inc., Brea, CA, USA) with excitation
at 488 nm and emission at 525 nm wavelengths. The other
106 cells were re-suspended in PBS at 37°C for a further
60 min and assessed by flow cytometry again (18). The intracellular positive rate and
fluorescence intensity of ADM were determined using FlowJo v7.6.3
software (FlowJo, LLC, Ashland, OR, USA).
LSC detection
Parental HL-60 and ADM-induced HL-60 cells were
co-incubated with PEcy5-CD34 (5 µl), ECD-CD38 (5 µl) and PE-CD123
(10 µl) in 100 µl binding buffer and/or fluorescein isothiocyanate,
FITC-BCRP (1 µl) antibodies, with PEcy5-, ECD-, PE- and FITC-murine
IgG1 (with the same corresponding volume as aforesaid) as isotype
controls, respectively. The relative proportions of LSCs,
CD34+CD38−CD123+ and
BCRP+CD34+CD38−CD123+
subsets in the cell population were determined by flow cytometry,
as aforementioned.
Colony-formation assay
A total of 103 cells/ml were seeded in
half-solid RPMI 1640 media with 20% fetal bovine serum and 0.9%
methylcellulose. Following 5- and 10-day incubation at 37°C in 5%
CO2, the total number of colonies, defined as a mass of
>40 cells, within each well was counted under a light microscope
(original magnification, ×100). Colony-formation rate=total number
of colonies/1,000/well ×100%); three representative fields were
imaged.
Statistical analysis
Data were analyzed using SPSS version 17.0 (SPSS,
Inc., Chicago, IL, USA). Data are presented as the mean ± standard
deviation. Multiple comparisons between the three groups was
performed using two-way analysis of variance followed by a
Newman-Keuls post hoc test. The intragroup comparisons were
performed using paired Student's t-test. All experiments were
repeated at least three times. Error bars represent the standard
error of the mean and P<0.05 was considered to indicate a
statistically significant difference.
Results
Establishment of multidrug-resistant
HL-60 cells
Human promyelocytic leukemia HL-60 cells were
selected for the MDR phenotype by stepwise increments of ADM
treatment until the cells were able to survive and proliferate
normally at a concentration of 40 mg/l ADM. The resulting cells
were 85.68-fold more resistant to ADM compared with the parental
HL-60 cells, as revealed by the IC50 (mg/l) presented in
Fig. 1A. Additionally,
drug-resistance was maintained following culture in the absence of
ADM for 3 months or refrigeration for 6 months. These results
demonstrated that following a long induction period imitating
clinical chemotherapy, HL-60 cells acquired a stable drug-resistant
phenotype, and therefore a stable MDR leukemia subline was
established.
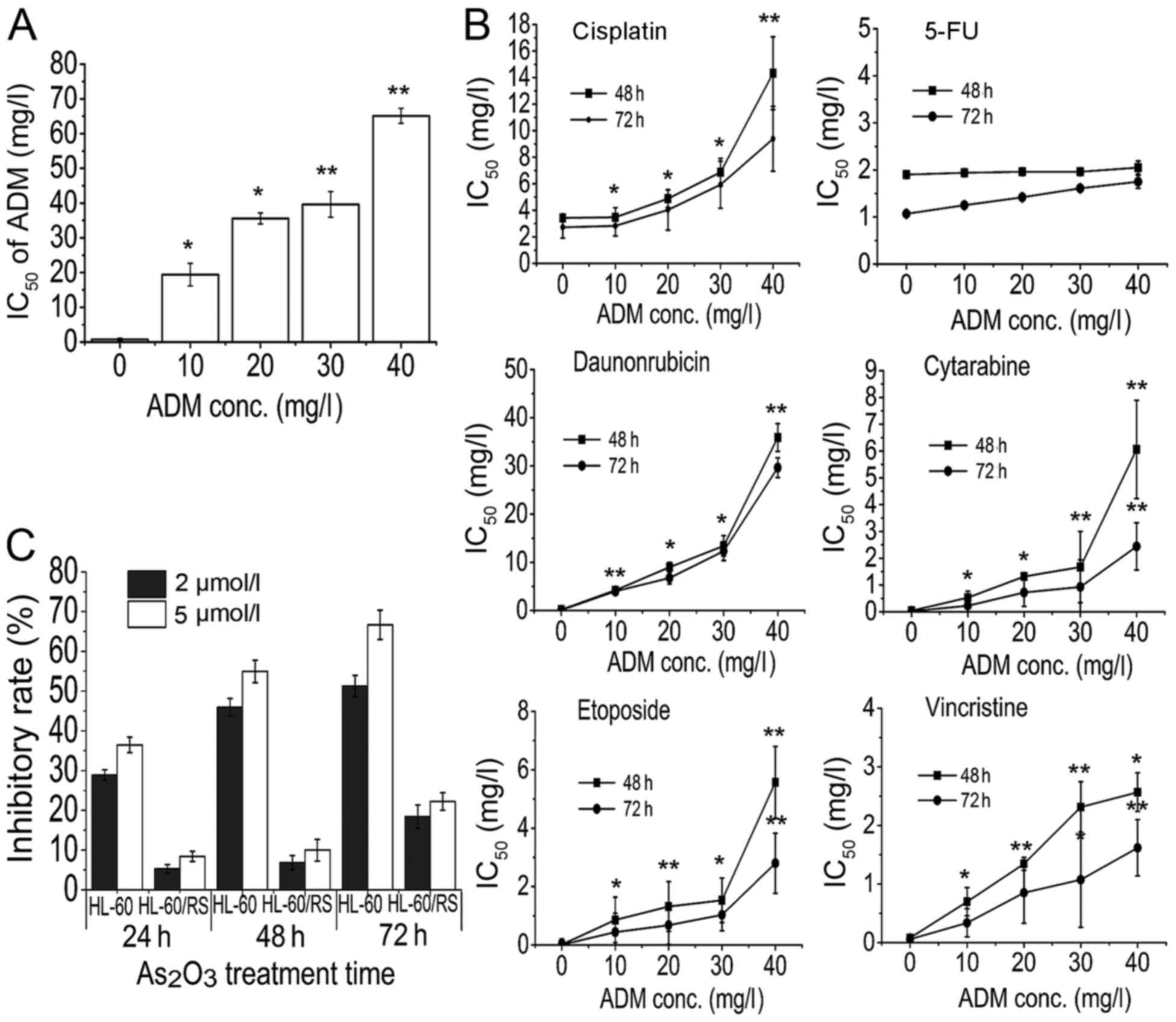 | Figure 1.HL-60 cells acquire multidrug
resistance. HL-60 cells resistant to 10–40 mg/l ADM were treated
with various chemotherapeutic drugs. Inhibitory rate and
IC50 values were determined by MTT assay. HL-60 cells
resistant to 40 mg/l ADM were defined as HL-60/RS cells. (A) HL-60
cells became increasingly resistant to ADM at increasing
concentrations of ADM and increasing exposure times. (B)
ADM-resistant HL-60 cells were cross-resistant to other
chemotherapeutic drugs, including cisplatin, daunorubicin,
cytarabine, vincristine and etoposide, but not 5-fluorouracil. (C)
HL-60/RS cells acquired high As2O3
resistance. *P<0.05, **P<0.01 compared with HL-60 cells.
IC50, half-maximal inhibitory concentration; ADM,
doxorubicin; conc., concentration; 5-FU, 5-fluorouracil. |
Resistance and cross-resistance of
ADM-selected HL-60 cells
The sensitivity of ADM-induced HL-60 cells to
chemotherapeutic agents including ADM was analyzed during and at
terminal induction. HL-60 cells became increasingly resistant to
ADM at increasing concentrations of ADM and increasing exposure
times (Fig. 1A). Additionally,
ADM-resistant HL-60 cells were also cross-resistant to other
chemotherapeutics including cisplatin, daunorubicin, cytarabine,
vincristine, arsenic trioxide and etoposide, but not 5-fluorouracil
(Fig. 1B). These results confirmed
that HL-60/RS cells possessed high resistance to ADM and other
numerous chemotherapeutics.
Sensitivity of ADM-selected HL-60
cells to arsenic trioxide
A previous study revealed that the majority of MDR
leukemia cells did not develop cross-resistance, but conversely
demonstrated higher sensitivity to As2O3
(4). However, the established
HL-60/RS sub-line was strongly cross-resistant to
As2O3, with a 12.89-fold increase in
resistance compared with HL-60 cells. Therefore, HL-60/RS cells
acquired the unique feature of high
As2O3-resistance (Fig. 1C).
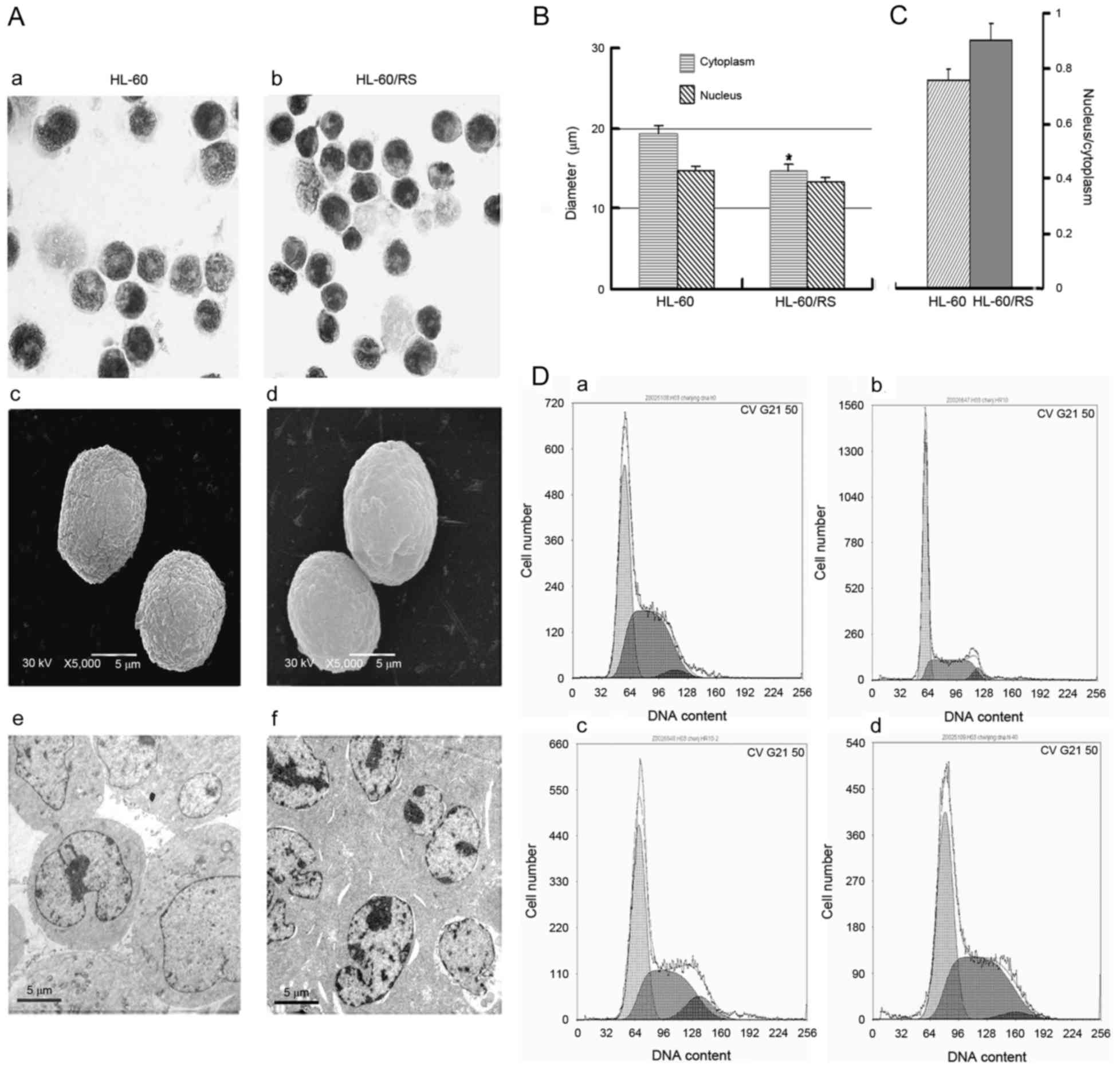
Morphology and cell cycle distribution
of HL-60/RS cells
Drug-resistant HL-60/RS and parental sensitive HL-60
cells were similar in size and had similar morphological
phenotypes. The nuclei in HL-60/RS cells were uniformly round or
oval (Fig. 2A) and exhibited an
increased relative nucleus/cytoplasmic ratio compared with the
parental cells (Fig. 2B and C).
Sensitive HL-60 cells included more mature, band or
polymorphonuclear (segmented)-like cells compared with HL-60/RS
cells, indicating that HL-60/RS cells were less mature compared
with the parental HL-60 cells. Although both cell lines appeared
round, HL-60/RS cells had a smooth surface, whereas the surface of
parental HL-60 cells appeared rough and more bulging under scanning
electron microscopy (Fig. 2A).
Electron microscopy revealed morphologic ultrastructural changes in
HL-60/RS cells, including denser cytoplasm and nuclear chromatin
and increased heterochromatin, compared with untreated, parental
cells (Fig. 2A).
The cell cycle distribution was analyzed in HL-60/RS
cells, which were exposed to 40 mg/l ADM and subsequently cultured
continuously in ADM-free conditions. At the early stage (7 days),
the distribution of total DNA content in
G0/G1 was markedly higher compared with the
parental HL-60 cells, but lower in S and G2/M phases.
The cell cycle distribution became similar in both cell types with
increasing incubation times (for 15–60 days) and cell proliferation
(Fig. 2D).
Expression of drug transporter genes
in ADM-selected resistant HL-60 cells
In cells tolerant to the highest induced dose of 40
mg/l ADM, relative ABCB1, ABCC1 and ABCG2 mRNA
expression levels reached 92.5, 45.4 and 75.0-fold of that in
sensitive HL-60 cells (Fig. 3B),
respectively. The expression of P-gp and BCRP proteins increased
simultaneously with increased tolerance to ADM, as determined by
flow cytometry. The percentage of P-gp+ cells reached
~100% in cells tolerant to 5 mg/l ADM (Fig. 3A). RT-qPCR demonstrated marked
increases in ABCB1, ABCC1 and ABCG2 mRNA
expression with increasing tolerance to ADM and extended induction
times (Fig. 3Ba). The levels of P-gp,
MRP1 and BCRP expression in sensitive parental HL-60 cells and
cells tolerant to 30 and 40 mg/l ADM cells (HL-60/RS) was analyzed
by western blotting, and the findings were consistent with the gene
expression results (Fig. 3Bb). These
results revealed that long-term and intermittent ADM exposure of
HL-60 cells resulted in overexpression of the drug transporters
P-gp, MRP and BCRP, which may represent the major mechanism of
drug-resistance in HL-60 cells.
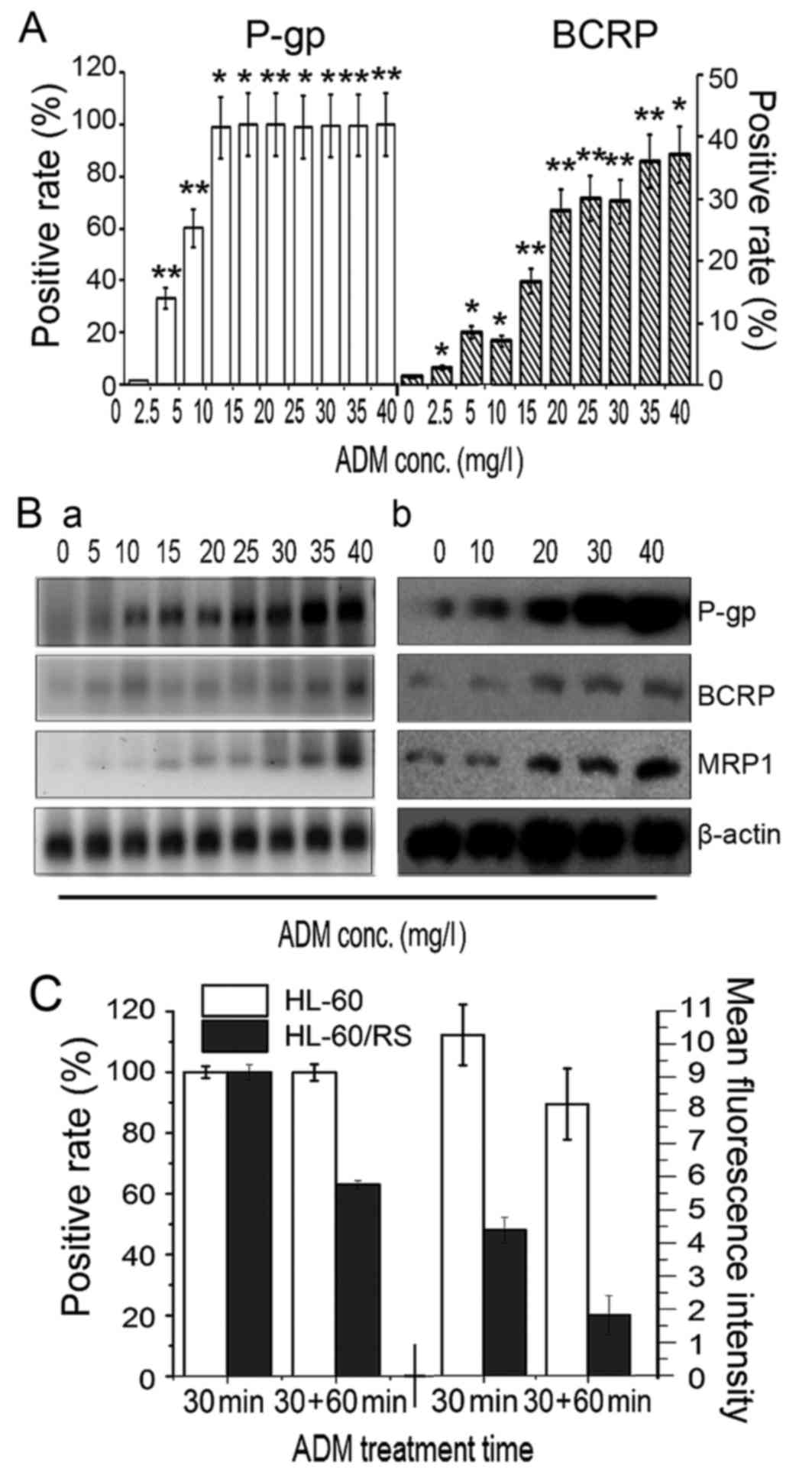 | Figure 3.Membrane transporter expression and
ADM accumulation-efflux function. (A) The number of
P-gp+ and BCRP+ cells increased gradually in
ADM-induced HL-60 cells with stepwise increases in ADM doses. (B)
Levels of P-gp, MRP1 and BCRP (a) gene mRNA
expression were determined by reverse transcription-quantitative
polymerase chain reaction, and (b) levels of proteins expression
were evaluated by western blotting, in HL-60 parental cells (ADM, 0
mg/l) and in cells tolerant to various doses of ADM. (C)
Accumulation and efflux of HL-60 cells and HL-60/RS cells. The
cells were incubated in 30 mg/l ADM medium at 37°C for 30 min and
ADM content was assessed by the number of fluorescence-positive
cells and mean fluorescence intensity. Subsequently, the cells were
further incubated for 60 min and re-assessed. *P<0.05,
**P<0.01 compared with HL-60 cells. ADM, doxorubicin; conc.,
concentration; P-gp, P-glycoprotein; BCRP, breast-cancer-resistance
protein. |
Function of drug transporters in
ADM-selected resistant HL-60 cells
ADM has spontaneous fluorescence that can be used to
analyze intercellular residual ADM (18). HL-60 cells and HL-60/RS cells were
exposed to 30 mg/l ADM-containing medium for 30 min. Almost all
cells of both lines demonstrated ADM fluorescence. However, the
cellular ADM fluorescence intensity in HL-60/RS cells was only
42.75% of the value of sensitive HL-60 cells. Following
re-incubation in ADM-free medium for a further 60 min, only 62.02%
of HL-60/RS cells retained ADM fluorescence, compared with ~100% of
the HL-60 cells, and the mean fluorescence intensity in HL-60/RS
cells remained only 21.01% of that in HL-60 cells (Fig. 3C).
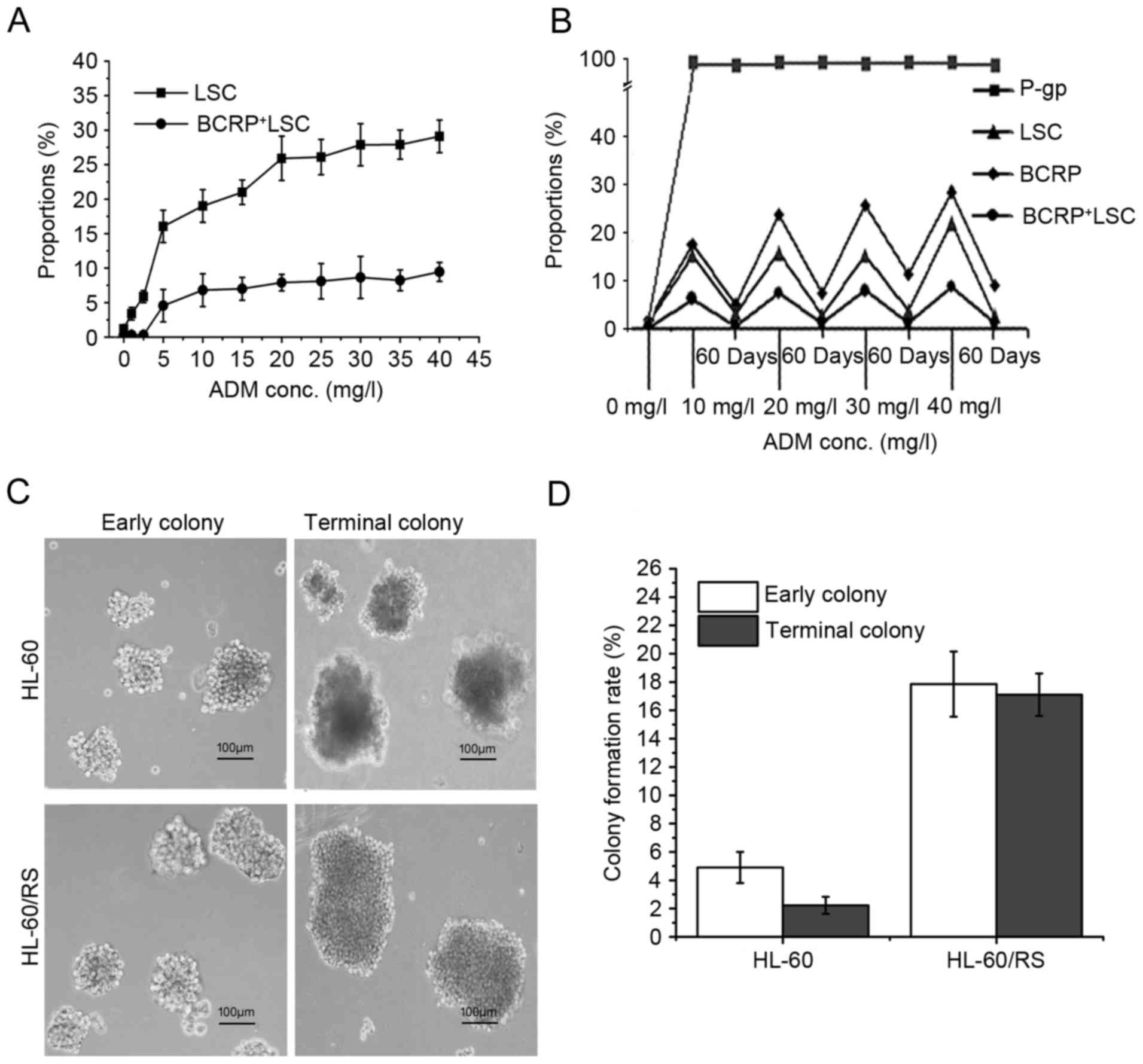
Dynamic changes in LSC proportion
during drug-resistance induction of HL-60 cells
Dynamic changes were observed in the proportions of
LSCs and BCRP+LSCs in ADM-selected HL-60 cells during
the enhancement of drug-resistance, with
CD34+CD38−CD123+ and
BCRP+CD34+CD38−CD123+
phenotypes, respectively. The relative proportion of LSCs in the
HL-60 cell population increased notably along with increments of
ADM-inducing concentrations and length of induction time. The
proportion of BCRP+LSC increased synchronously (Fig. 4A). In HL-60 cells tolerant to 40 mg/l
ADM, the proportions of LSCs and BCRP+LSCs were 24.25
and 23.63-fold higher compared with the sensitive parental HL-60
cells, respectively (Fig. 4B). These
levels gradually fell during culture in ADM-free medium, except for
P-gp, which remained stable near 100% (Fig. 4B). These findings suggested that LSCs
have a pivotal role in the acquisition of drug-resistance in
leukemia, and BCRP+ or P-gp+BCRP+
cells may be a specific hallmark of the multidrug-resistant LSC
subpopulation.
Colony-forming capacity
The self-renewal and unrestricted-proliferation
potentials of the cells were analyzed by using the methylcellulose
half-solid-medium colony-forming-culture (CFC) method. The early
and terminal colony-formation ratios of HL-60/RS cells were 3.64
and 7.67-fold than that of HL-60 cells, respectively. The early
colonies were morphologically small and loose but became bigger and
denser. Additionally, the majority of the late HL-60 colonies were
composed of dead and disintegrating cells (Fig. 4C). The colony formation rate in early
and late colonies were markedly higher in tolerant HL-60/RS cells
compared with the parental HL-60 cells (Fig. 4D).
Discussion
MDR is currently a major and unresolved impediment
to leukemia therapy (1–3,10).
Although numerous extensive studies have investigated leukemia
drug-resistance, regulatory pathways and intervention measures, the
underlying mechanisms remain to be fully understood and few
effective or clinically practical countermeasures have been
identified (23–25). The fundamental role of LSCs in
leukemia-MDR has recently attracted widespread attention. LSCs have
been suggested to be a core factor underlying leukemia drug
resistance and also the key cause of conventional chemotherapy
failure and relapse in leukemia (12,26,27).
Further studies investigating the characteristics of drug
resistance in leukemia and its cellular and molecular mechanisms
are required. In the present study, HL-60 human promyelocytic
leukemia cells were selected for ADM-resistance by imitating the
clinical chemotherapy process. Long-term, intermittent and
continuous stepwise increments of ADM concentrations were used to
establish a stable MDR leukemia subline (HL-60/RS). The expression
of drug-resistance-related genes/proteins and their functions as
wells as the LSC composition of the cell lines were examined in
relation to the degree of ADM resistance. Following 22 months of
ADM induction, the resistance of HL-60/RS cells to ADM had
increased 85.68-fold compared with parental HL-60 cells. These
ADM-resistant cells were also cross-resistant to other chemically
and functionally unrelated chemotherapeutics. In particular, the
ADM-induced cells achieved high
As2O3-resistance. Additionally, the present
study demonstrated that the rate of proliferation, cell morphology
and cell cycle distribution all differed between HL-60/RS and
parental HL-60 cells.
As2O3 is widely used in
chemotherapy for most types of leukemia and solid tumors.
As2O3 has good curative effects and little
cross-resistance with conventional chemotherapeutics (2,17–21). Previous studies have revealed that
conventional MDR leukemia cells exhibit increased sensitivity to
As2O3 instead of manifesting
cross-resistance. This is possibly because
As2O3 is not a P-gp substrate and may even
inhibit P-gp expression and activity (2–4,27). However, unlike other ADM-selected
P-gp-overexpressing MDR tumor cells, including K562/ADM cells
(2,3,27), the
HL-60/RS cells established in the present study demonstrated
cross-resistance to As2O3, which is a unique
feature in MDR cells.
The reason for strong resistance of HL-60/RS cells
to arsenic remains unclear, and the possible mechanisms are still
being investigated. It was previously reported that the presence of
mutations in the arsenic binding domain of promyelocytic
leukemia/retinoic acid receptor α (PML-RARA) induced arsenic
resistance in patients treated with As2O3
(23–25,28).
Previous studies in anti-arsenic organisms indicated that
overexpression of arsenic-related transporters and
glutathione-S-transferases, which mainly exported arsenic leading
to intracellular arsenic load reduction, were responsible for the
formation of resistance to arsenic (29–31).
Follow-up studies are currently proceeding in the laboratory of the
authors to investigate the underlying mechanisms of how MDR cells
become sensitive or resistant to As2O3. The
preliminary results confirmed that resistance to arsenic in MDR
cells was associated with factors, including the overexpression of
arsenic transporters.
There is growing evidence demonstrating that LSCs
are a main source of leukemia relapse and treatment resistance
(13,26,27,32,33).
LSCs are naturally resistant to cytotoxic drugs, which kill more
mature leukemia cells in the cell proliferation phase, due to their
quiescence/dormancy, strong self-DNA-damage-repair capability and
overexpression of ABC transporters in LSCs. Therefore, an
investigation of the key regulatory pathways of functional ABC
family members in drug-resistance of LSCs is required. In the
present study, during the process of ADM induction and
colony-forming ability, the proportion of LSCs increased with
increasing drug-resistance in HL-60 cells. The proportion of LSCs
in HL-60 cells gradually decreased when ADM-induced resistant HL-60
cells were cultured in ADM-free medium, stabilizing at ~10%, which
was ~10-fold higher compared with the parental HL-60 cells.
Notably, ~100% of LSCs expressed P-gp. However, only ~30% expressed
BCRP (BCRP+LSCs), which increased (when incubated in
ADM-free media for 7 days) or decreased (when incubated in ADM-free
media for 15–60 days), but more slowly compared with the total
number of LSCs, with increasing resistance to ADM. BCRP in LSCs or
leukemic CD34+CD38− stem cells were
preferentially expressed and may contribute to their resistant
phenotype (26,28,32).
Therefore, it was hypothesized that BCRP+ or
P-gp+BCRP+LSCs may represent the
multi-resistant LSC subset generated by long exposure to ADM, which
survived to produce resistant daughter leukemia cells. Repeated
stimulation by cytotoxic drugs may drive LSCs to become
multi-resistant and develop specific regulatory pathways, which may
in turn be transmitted to daughter cells and maintain a
drug-resistant leukemia cell population.
In conclusion, the present study established the
leukemia HL-60/RS sub-line with MDR characteristics. These
characteristics include overexpression of the main ABC transporter
members (P-gp, MRP1 and BCRP), an increased proportion of LSCs and
a phenotype of high As2O3-resistance, which
contrasts with the majority of classical MDR leukemia cells. These
findings suggested that the main mechanisms underlying MDR in
HL-60/RS cells are mediated by excess LSCs and high expression of
ABC transporters. Notably, the leukemia MDR cell line was highly
cross-resistant to As2O3. Furthermore, the
BCRP+ or P-gp+BCRP+ phenotype may
be a specific hallmark of a highly drug-resistant LSC
subpopulation. This leukemia MDR cell line may be a good model for
further studies that examine the mechanisms underlying
As2O3-resistance, particularly the
cross-resistance of conventional chemotherapeutics with
As2O3, and to investigate strategies to
reverse chemotherapy resistance in leukemia.
Acknowledgements
The authors thank Dr David Cushley of International
Science Editing (Shannon, Ireland) for language editing and
revision of this paper. The present study was supported by the
National Natural Science Foundation of China (grant nos. 81541025
and 81141053), the Fundamental Research Funds for the Central
Universities (grant no. lzujbky-2016-174), Science and Technology
Planning Project from Chengguan District, Lanzhou, Gansu Province,
China (grant no. 2015-3-8) and the Natural Science Fund of Gansu
(grant no. 1208RJZA183).
References
|
1
|
Matsumoto T, Jimi S, Hara S, Takamatsu Y,
Suzumiya J and Tamura K: Importance of inducible multidrug
resistance 1 expression in HL-60 cells resistant to gemtuzumab
ozogamicin. Leuk Lymphoma. 53:1399–1405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang QH, Dou HT, Xu P, Zhuang SC and Liu
PS: Tumor recurrence and drug resistance properties of side
population cells in high grade ovary cancer. Drug Res. 65:153–157.
2015.
|
|
3
|
Gao F, Dong W, Yang W, Liu J, Zheng Z and
Sun K: Expression of P-gp in acute myeloid leukemia and the
reversal function of As2O3 on drug resistance. Oncol Lett.
9:177–182. 2015.PubMed/NCBI
|
|
4
|
Chen J, Wei H, Xie B, Wang B and Cheng J
and Cheng J: Endoplasmic reticulum stress contributes to arsenic
trioxide-induced apoptosis in drug-sensitive and -resistant
leukemia cells. Leuk Res. 36:1526–1535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Figueiredo-Pontes LL, Pintão MC,
Oliveira LC, Dalmazzo LF, Jácomo RH, Garcia AB, Falcão RP and Rego
EM: Determination of P-glycoprotein, MDR-related protein 1, breast
cancer resistance protein, and lung-resistance protein expression
in leukemic stem cells of acute myeloid leukemia. Cytometry B Clin
Cytom. 74:163–168. 2008.PubMed/NCBI
|
|
6
|
Munić V, Kelnerić Z, Mikac L and Eraković
Haber V: Differences in assessment of macrolide interaction with
human MDR1 (ABCB1, P-gp) using rhodamine-123 efflux, ATPase
activity and cellular accumulation assays. Eur J Pharm Sci.
41:86–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakanishi T and Ross DD: Breast cancer
resistance protein (BCRP/ABCG2): Its role in multidrug resistance
and regulation of its gene expression. Chin J Cancer. 31:73–99.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Laterra J and Pomper MG: Hedgehog
pathway inhibitor HhAntag691 is a potent inhibitor of ABCG2/BCRP
and ABCB1/Pgp. Neoplasia. 11:96–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gutiérrez-González A, Belda-Iniesta C,
Bargiela-Iparraguirre J, Dominguez G, García Alfonso P, Perona R
and Sanchez-Perez I: Targeting Chk2 improves gastric cancer
chemotherapy by impairing DNA damage repair. Apoptosis. 18:347–360.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ding Q, Gu R, Liang J, Zhang X and Chen Y:
PI-103 sensitizes acute myeloid leukemia stem cells to
daunorubicin-induced cytotoxicity. Med Oncol. 30:3952013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saito Y, Kitamura H, Hijikata A,
Tomizawa-Murasawa M, Tanaka S, Takagi S, Uchida N, Suzuki N, Sone
A, Najima Y, et al: Identification of therapeutic targets for
quiescent, chemotherapy-resistant human leukemia stem cells. Sci
Transl Med. 2:17ra92010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moore N and Lyle S: Quiescent,
slow-cycling stem cell populations in cancer: A review of the
evidence and discussion of significance. J Oncol.
2011:pii:3960762011. View Article : Google Scholar
|
|
13
|
Pollyea DA, Gutman JA, Gore L, Smith CA
and Jordan CT: Targeting acute myeloid leukemia stem cells: A
review and principles for the development of clinical trials.
Haematologica. 99:1277–1284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou J and Chng WJ: Identification and
targeting leukemia stem cells: The path to the cure for acute
myeloid leukemia. World J Stem Cells. 6:473–484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Crews LA and Jamieson CH: Selective
elimination of leukemia stem cells: Hitting a moving target. Cancer
Lett. 338:15–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marques DS, Sandrini JZ, Boyle RT, Marins
LF and Trindade GS: Relationships between multidrug resistance
(MDR) and stem cell markers in human chronic myeloid leukemia cell
lines. Leukemia Res. 34:757–762. 2010. View Article : Google Scholar
|
|
17
|
Buda G, Orciuolo E, Maggini V, Galimberti
S, Barale R, Ross AM and Petrini M: MDR1 modulates apoptosis in
CD34+ leukemic cells. Ann Hematol. 87:1017–1018. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao D, Jiang Y, Dong X, Liu Z, Qu B,
Zhang Y, Ma N and Han Q: Arsenic trioxide reduces drug resistance
to adriamycin in leukemic K562/A02 cells via multiple mechanisms.
Biomed Pharmacother. 65:354–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perkins C, Kim CN, Fang G and Bhalla KN:
Arsenic induces apoptosis of multidrug-resistant human myeloid
leukemia cells that express Bcr-Abl or overexpress MDR, MRP, Bcl-2,
or Bcl-x(L). Blood. 95:1014–1022. 2000.PubMed/NCBI
|
|
20
|
Zhao H, Guo W, Peng C, Ji T and Lu X:
Arsenic trioxide inhibits the growth of adriamycin resistant
osteosarcoma cells through inducing apoptosis. Mol Biol Rep.
37:2509–2515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beauchamp EM, Ringer L, Bulut G, Sajwan
KP, Hall MD, Lee YC, Peaceman D, Ozdemirli M, Rodriguez O,
Macdonald TJ, et al: Arsenic trioxide inhibits human cancer cell
growth and tumor development in mice by blocking Hedgehog/GLI
pathway. J Clin Invest. 121:148–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei H, Su H, Bai D, Zhao H, Ge J, Wan B,
Yao X and Ma L: Arsenic trioxide inhibits p-glycoprotein expression
in multidrug-resistant human leukemia cells that overexpress MDR1
gene. Chin Med J. 116:1644–1648. 2003.PubMed/NCBI
|
|
23
|
Fung TK and So CW: Overcoming treatment
resistance in acute promyelocytic leukemia and beyond. Oncotarget.
4:1128–1129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tomita A, Kiyoi H and Naoe T: Mechanisms
of action and resistance to all-trans retinoic acid (ATRA) and
arsenic trioxide (As2O3) in acute promyelocytic leukemia. J
Hematol. 97:717–725. 2013.
|
|
25
|
Zhu HH, Qin YZ and Huang XJ: Resistance to
arsenic therapy in acute promyelocytic leukemia. N Engl J Med.
370:1864–1866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Felipe Rico J, Hassane DC and Guzman ML:
Acute myelogenous leukemia stem cells: From Bench to Bedside.
Cancer Lett. 338:4–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang F, Wang XK, Shi CJ, Zhang H, Hu YP,
Chen YF and Fu LW: Nilotinib enhances the efficacy of conventional
chemotherapeutic drugs in CD34+CD38- stem cells and ABC transporter
overexpressing leukemia cells. Molecules. 19:3356–3375. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lo-Coco F, Avvisati G, Vignetti M, Thiede
C, Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona
E, et al: Retinoic acid and arsenic trioxide for acute
promyelocytic leukemia. N Engl J Med. 369:111–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu S, Zhang YF, Carew MW, Hao WH, Loo JF,
Naranmandura H and Le XC: Multidrug resistance protein 1 (ABCC1)
confers resistance to arsenic compounds in human myeloid leukemic
HL-60 cells. Arch Toxicol. 87:1013–1023. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hemmingsson O, Nöjd M, Kao G and Naredi P:
Increased sensitivity to platinating agents and arsenite in human
ovarian cancer by downregulation of ASNA1. Oncol Rep. 22:869–875.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matulis SM, Morales AA, Yehiayan L, Lee
KP, Cai Y and Boise LH: Alterations in glutathione levels and
apoptotic regulators are associated with acquisition of arsenic
trioxide resistance in multiple myeloma. PLoS One. 7:e526622012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yi J, Chen J, Sun J and Wei HL: The
relationship between multi-drug resistance and proportion of
leukemia stem cells and expression of drug transporters in
drug-resistant leukemia K562/ADM cells. Zhonghua Yi Xue Za Zhi.
89:1741–1744. 2009.(In Chinese). PubMed/NCBI
|
|
33
|
Qiu S, Jia Y, Xing H, Yu T, Yu J, Yu P,
Tang K, Tian Z, Wang H, Mi Y, et al: N-Cadherin and Tie-positive
CD34+CD38−CD123+ leukemic stem cell populations can develop acute
myeloid leukemia more effectively in NOD/SCID mice. Leuk Res.
38:632–637. 2014. View Article : Google Scholar : PubMed/NCBI
|