Introduction
Malignant glioma, the most frequent type of
malignant brain tumor in adults, is associated with an extremely
poor prognosis due to the rapid growth and highly invasive nature
that favor its infiltration into surrounding normal brain
parenchyma and facilitates recurrence following therapy (1,2). Glioma is
a major therapeutic challenge due to numerous factors, including
the tendency to adapt resistance against antiangiogenic agents, and
intratumoural cellular heterogeneity that varies over the course of
the disease and its treatment (3). To
improve the poor prognosis for patients with glioma, it is
important to understand the molecular mechanisms underlying and
supporting tumor cell survival and invasion.
Mounting evidence has demonstrated that microRNAs
(miRs), small non-coding RNAs of ~18–24 nucleotides in length,
regulate various biological processes, including cancer progression
and metastasis, by mediating the posttranscriptional silencing of
specific target mRNAs. A total of >1,500 miRs have been
identified in the human genome, which collectively control an
estimated 30% of human genes (4).
Each miRNA appears to modulate tens to hundreds of target genes to
coordinate cellular signaling pathways. It has been demonstrated
that miRs may regulate the proliferation and apoptosis of tumor
cells and act as oncogenes or tumor suppressor genes. Previously,
it was reported that miR-509-3p functions as a tumor suppressor,
potentially serving prominent roles in the development of various
types of cancer, including renal cell carcinoma, breast cancer,
acute lymphoblastic leukemia, lung cancer and hepatoma (5–9). However,
although there is a large body of research regarding the molecular
mechanisms of malignant glioma, the functions of miR-509-3p and its
target genes in regulating malignant glioma progression require
further investigation.
Materials and methods
Cell culture and transfection
The U251 human glioma cell line purchased from the
Cell Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China) was maintained in RPMI-1640 medium supplemented
with 10% fetal bovine serum (FBS; both Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C in 5% CO2. A
miR-509-3p mimic and a mimic control were purchased from Shanghai
GenePharma Co., Ltd. (Shanghai, China). Human X-linked inhibitor of
apoptosis (XIAP) short interfering (si) RNA and scramble siRNA were
purchased from Shanghai GenePharma Co., Ltd. The oligonucleotide
sequences were as follows: miR-509-3p mimic forward,
5′-UGAUUGGUACGUCUGUGGGUAGTT-3′ and reverse,
5′-CUACCCACAGACGUACCAAUCATT-3′; mimic control forward,
5′-UUGUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′; si-XIAP forward,
5′-GUGGUAGUCCUGUUUCAGCTT-3′ and reverse,
5′-GCUGAAACAGGACUACCACTT-3′; scramble siRNA forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′. The U251 cells were transfected using
Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. At 48 h, the cells were
harvested for further analysis.
Tissue samples and patient data
Tumor and non-tumor tissue samples from 120 patients
(69 males and 51 females) with glioma who underwent surgical
resection were obtained from the Second Affiliated Hospital of
Xinjiang Medical University (Ürümqi, China) between February 2010
and December 2014. The mean age was 50.13 years (range, 31–67
years). The tissue samples were immediately aliquoted into separate
tubes, frozen on dry ice and stored at −80°C until analysis.
Follow-up data were obtained from a review of the patients' medical
records. None of the patients had received radiotherapy or
chemotherapy prior to surgical resection. The present study was
approved by the Ethics Committee of Xinjiang Medical University.
The study was performed in accordance with the regulations of the
Institutional Review Board of Xinjiang Medical University. Written
informed consent was obtained prior to surgery from all enrolled
patients.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA, including miRNA, of cell lines and tissue
samples was isolated using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc., USA), according to the
manufacturer's protocol. The first-strand cDNA was synthesized from
1 µg of total RNA using PrimeScript RT reagent kit with gDNA Eraser
(Takara Bio, Inc., Otsu, Japan). Quantitative PCR of XIAP and GAPDH
was performed using SYBR Premix Ex Taq II (Takara, Bio, Inc.). The
reaction protocol involved heating for 10 sec at 95°C, followed by
40 cycles of amplification (5 sec at 95°C and 30 sec at 60°C).
Subsequent to reverse transcribing with TaqMan™ MicroRNA Reverse
Transcription Kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.), a TaqMan microRNA assay kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) was used to determine the expression level
of miR-509-3p and U6. U6 small nuclear RNA was used as an internal
control. The reaction protocol involved heating for 20 sec at 95°C,
followed by 40 cycles of amplification (3 sec at 95°C and 30 sec at
60°C). The relative expression level (as fold change) of the target
gene (2−∆∆Cq) was normalized to the endogenous U6 or
GAPDH reference (∆Cq) (10). Primer
sequences were as follows: miR-509-3p forward,
5′-TGATTGGTACGTCTGTGGGTAG-3′ with a universal reverse primer from
the TaqMan kit; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-ACGCTTCACGAATTTGCGT-3′; XIAP forward, 5′-ACCGTGCGGTGCTTTAGTT-3′
and reverse, 5′-TGCGTGGCACTATTTTCAAGATA-3′; GAPDH forward,
5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse, 5′-AGGGGCCATCCACAGTCTTC-3′.
Three independent experiments were performed to analyze the
relative gene expression and each sample was analyzed in
triplicate.
Cell proliferation and colony
formation assays
A Cell Counting Kit-8 (CCK-8; Beyotime Institute of
Biotechnology, Shanghai, China) was used according to the
manufacturer's protocol. Cells were seeded into 96-well plates at a
density of 3,000 cells in 100 µl medium per well, in quadruplicate.
Absorbance at 450 nm was evaluated 1 h after the addition of 10 µl
CCK-8 reagent per well, to determine the number of viable cells.
Cell viability was evaluated as the percentage of viable cells
relative to vehicle-treated cells. For the colony formation assay,
1,000 cells were seeded in a six-well plate. Following a 14-day
incubation, surviving colonies were counted using 0.1% crystal
violet staining for 20 min at room temperature. Triplicate
independent experiments were performed.
Apoptosis assay
A total of 5×105 cells were harvested and
stained with Annexin-V (allophycocyanin) and propidium iodide (1
mg/ml) for 15 min at 4°C in the dark. Data were acquired with a
fluorescence-activated cell sorting Canto II flow cytometer (BD
Biosciences, San Jose, USA) and analyzed with the Diva 6.1.3
software (BD Biosciences). Triplicate independent experiments were
performed.
Migration and invasion assay
A total of 2×104 cells were seeded into
the upper chambers of Transwell chambers (Corning Incorporated,
Corning, NY, USA) in 200 µl RPMI-1640 medium; 500 µl RPMI-1640
supplemented with 10% FBS was added into the lower wells as the
chemo-attractant. For the invasion assay, the chambers were also
coated with Matrigel (BD Biosciences). At 24 h, the filters were
stained with crystal violet. Cell migration and invasion were
assessed by counting the number of cells that had penetrated
through the filter under the light microscope (Leica Microsystems
GmbH, Wetzlar, Germany) at magnification, ×200 in 10 random fields.
The experiments were repeated at least three times.
Western blot
Cells were lysed with RIPA lysis buffer (Beyotime
Institute of Biotechnology, Shanghai, China). The protein
concentration was determined by Enhanced BCA Protein Assay Kit
(Beyotime Institute of Biotechnology, Shanghai, China). The protein
samples were denatured by boiling for 10 min. Protein samples (20
µg per lane) were loaded onto SDS-PAGE (10%) gel for
electrophoresis and transferred to a PVDF membrane (Millipore, MD,
USA). The membrane was incubated with a primary rabbit monoclonal
antibody against human XIAP (1:1,000; Cell Signaling Technology,
Massachusetts, USA) or a rabbit monoclonal antibody against human
β-actin (1:1,000; Cell Signaling Technology, Massachusetts, USA) at
4°C overnight and then incubated for 1 h with a goat anti-rabbit
(1:5,000; Cell Signaling Technology, Massachusetts, USA) secondary
antibody. Signals were detected by enhanced chemiluminescence
detection reagents (Pierce, IL, USA). Three independent experiments
were performed for each analysis and the gels have been run under
the same experimental conditions.
Statistical analysis
All statistical analyses were performed using SPSS
version 17.0 (SPSS, Inc., Chicago, IL, USA) software. Data are
presented as the mean ± standard deviation and were analyzed using
the Student's t-test. The paired t-test was used for paired
samples. The Kaplan–Meier method in combination with log-rank test
was performed to evaluate the overall survival between subgroups.
Pearson's test was adapted to determine the correlation between
miR-509-3p and XIAP expression. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-509-3p was frequently
downregulated in glioma
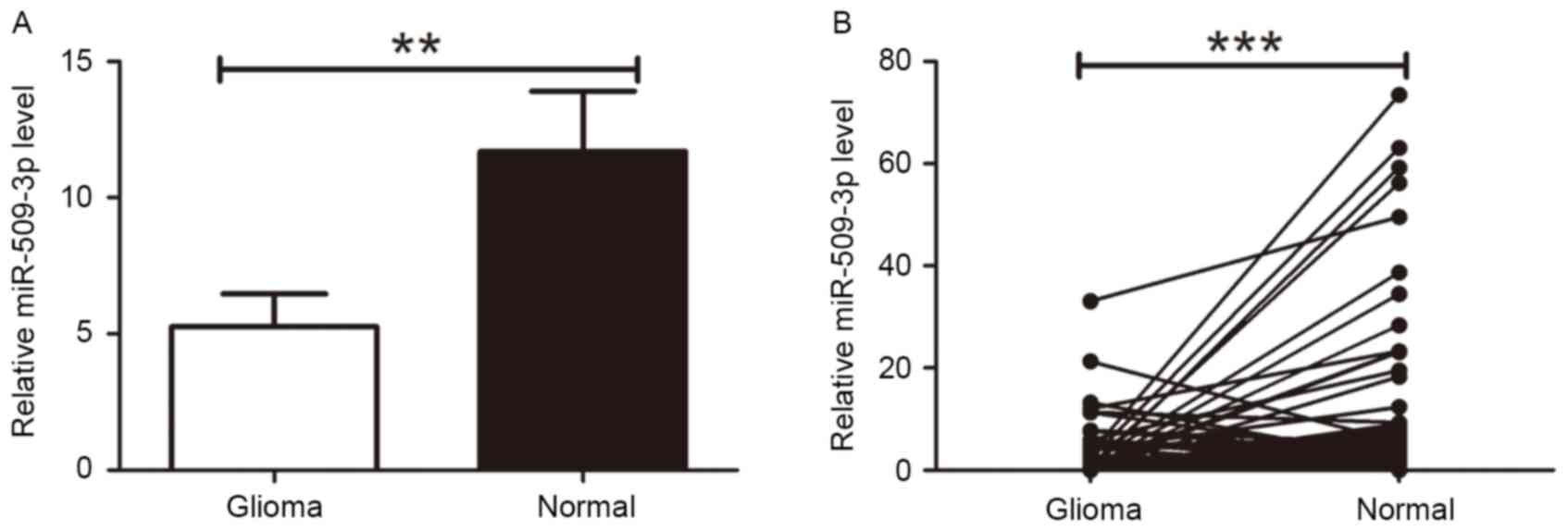
To determine the expression level of miR-509-3p in
glioma tissues, RT-qPCR was performed for all 120 tumor and 66
non-tumor tissue samples. The RT-qPCR analysis revealed that
miR-509-3p expression in tumor tissue was downregulated compared
with non-cancer tissue (P=0.0061; Fig.
1A). Furthermore, 66 paired tumor tissues and corresponding
neighboring non-cancer tissues were compared, which further
demonstrated the downregulation of miR-509-3p in tumor tissue
(P=0.0004; Fig. 1B). These results
suggested that miR-509-3p may act as a tumor suppressor in human
glioma.
miR-509-3p expression is associated
with disease progression
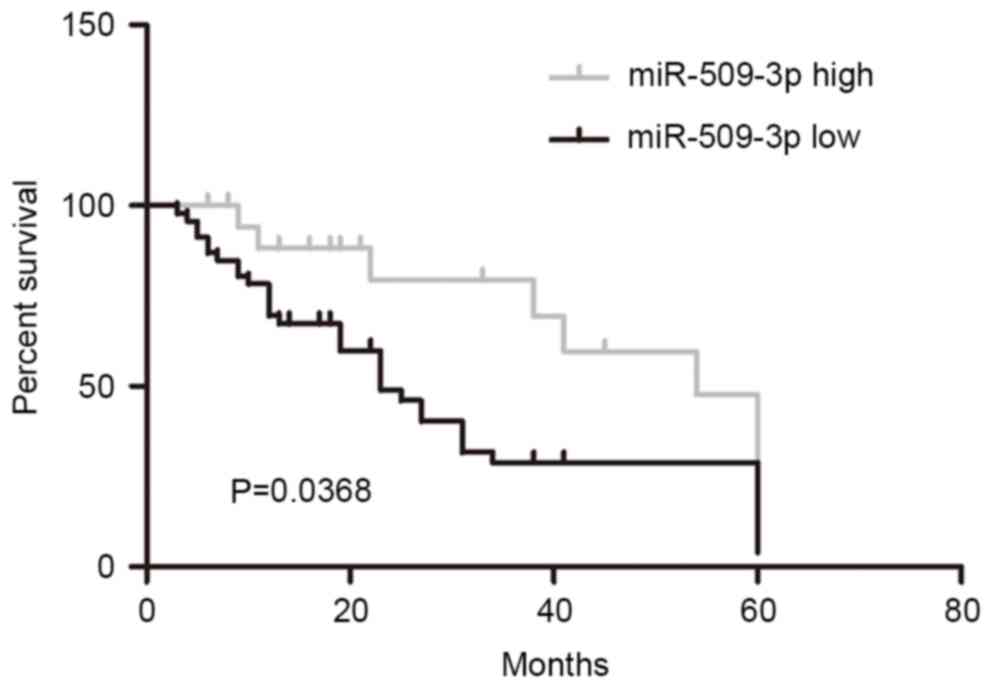
In order to investigate the prognostic role of
miR-509-3p downregulation in malignant glioma, patients were
divided into high and low-miR-509-3p groups according to the median
value of miR-509-3p expression (median value of the 120 giloma
specimens, 0.88) from all 120 cases. Kaplan-Meier analysis revealed
that the downregulation of miR-509-3p was significantly associated
with a poorer outcome for patients with glioma (Fig. 2; P=0.0368). These results suggested
that miR-509-3p may serve a role in glioma progression.
miR-509-3p inhibited cell
proliferation by inducing cell apoptosis in vitro
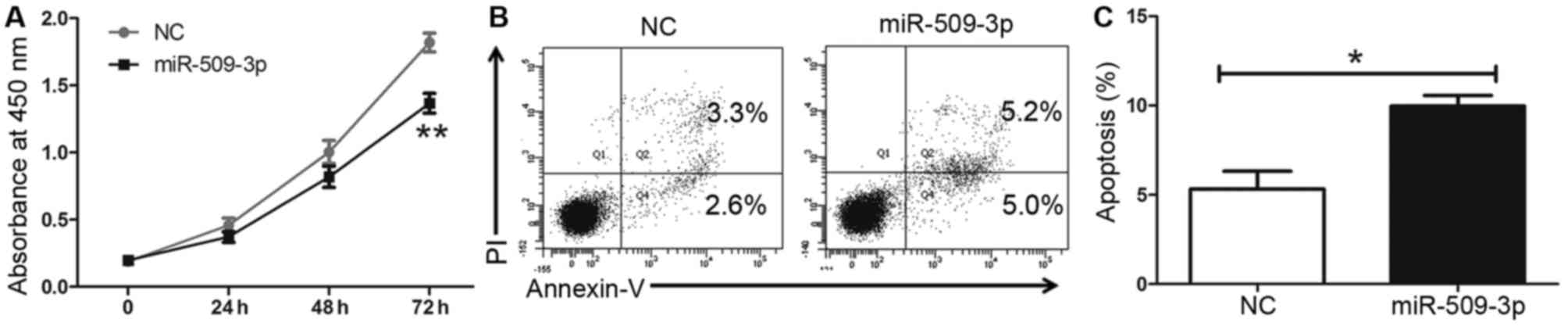
To further assess the tumor suppressive role of
miR-509-3p in glioma cells, an miR-509-3p mimic was used. The CCK8
results indicated that miR-509-3p upregulation may inhibit cell
proliferation in U251 cells compared with a mimic control (Fig. 3A; P=0.006). Furthermore, it was
revealed that the upregulation of miR-509-3p may promote apoptosis
compared with the control (Fig. 3B and
C; P=0.0252). These results suggested that miR-509-3p may
inhibit cell proliferation by promoting apoptosis in glioma
cells.
Upregulation of miR-509-3p suppressed
cell motility and invasiveness in vitro
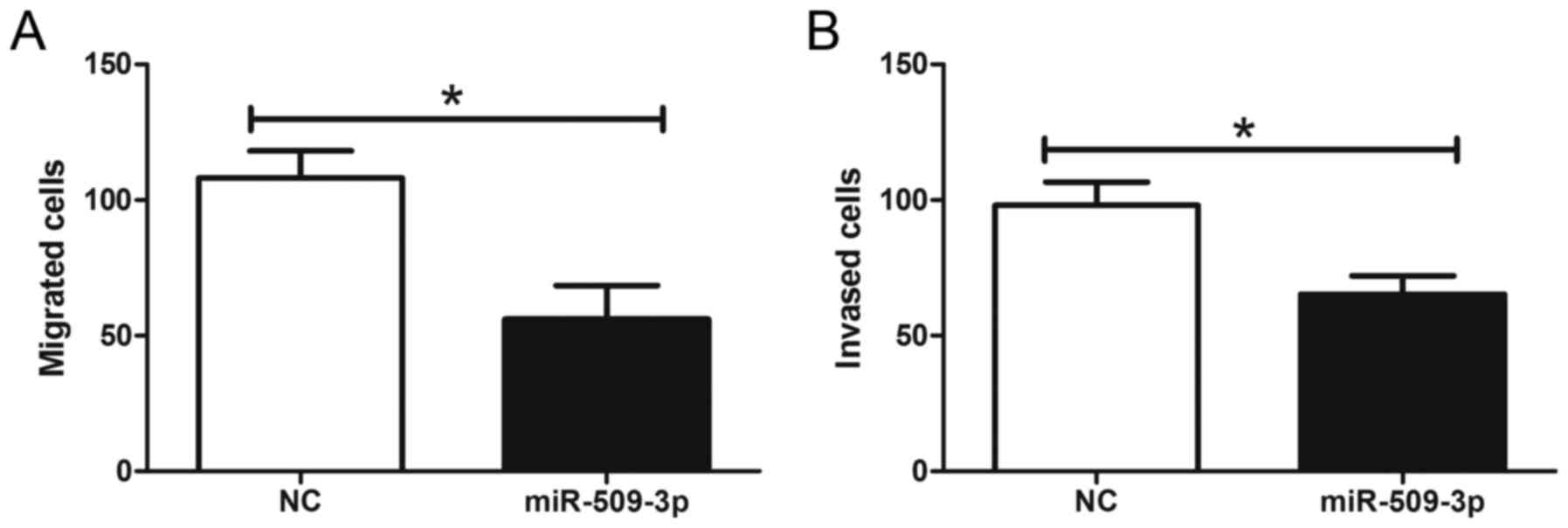
To further elucidate the effect of miR-509-3p on the
metastatic potential of human glioma, glioma cells were transfected
with an miR-509-3p mimic and then analyzed for their metastatic
potential by Transwell assays. The results revealed that miR-509-3p
overexpression significantly reduced cell migration and invasion
compared with the transfection control (Fig. 4; Pmigration=0.0311,
Pinvasion=0.0422). These results demonstrated that the
upregulation of miR-509-3p may inhibit the metastatic potential of
human glioma.
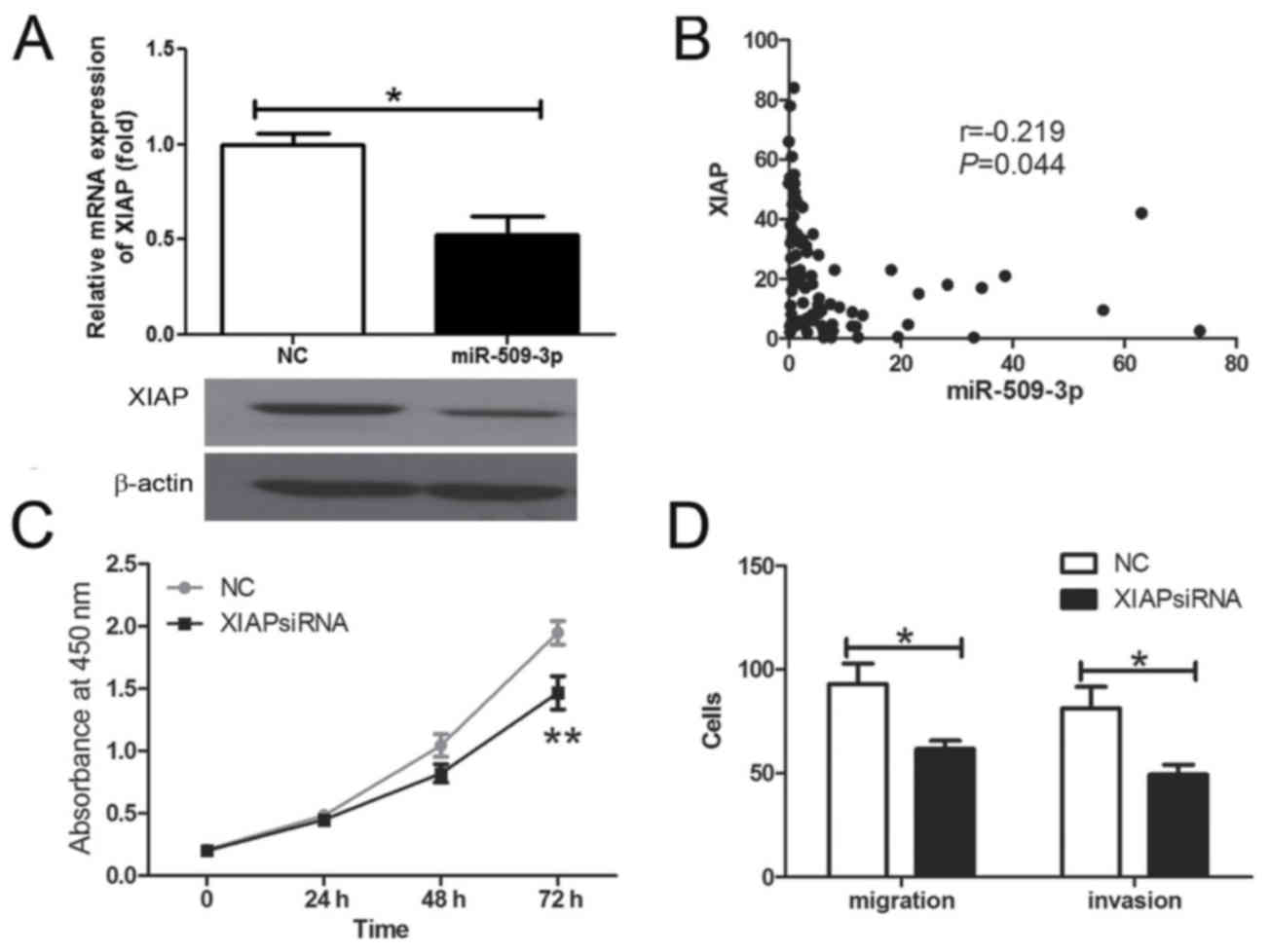
XIAP is a functional target of
miR-509-3p in human glioma
The present study further investigated whether XIAP
may have been regulated by mir-509-3p in human glioma cells. The
results demonstrated that the XIAP mRNA and protein expression were
inhibited by the overexpression of miR-509-3p (Fig. 5A; P=0.0157). Further analysis revealed
that the expression of miR-509-3p was inversely associated with
XIAP mRNA expression level in human glioma tissue samples (Fig. 5B; P=0.044). The present study
demonstrated that XIAP silencing may inhibit cell proliferation
(Fig. 5C; P=0.023), migration and
invasiveness (Fig. 5D;
Pmigration=0.0441, Pinvasion=0.0497) in
vitro. These results revealed that XIAP has the opposite effect
on cell proliferation and migration in comparison with miR-509-3p,
indicating that it may be a functional target for miR-509-3p in
human glioma cells.
Discussion
An increasing number of miRNAs have been implicated
in numerous critical biological processes, including tumor
development and progression. For example, it was reported that
miR-515-5p overexpression inhibited cell migration by
downregulating microtubule affinity regulating kinase 4 mRNA
expression levels in breast and lung cancer (11). Kouhkan et al (12) demonstrated that miR-129-1, a potential
tumor suppressor, induced cell cycle arrest by negatively
regulating insulin like growth factor 2 mRNA binding protein 3 and
mitogen-activated protein kinase 1 in glioblastoma multiforme
cells. Tan et al (13)
demonstrated that miR-1229 overexpression promoted cell
proliferation and tumorigenicity and activated Wnt/β-catenin
signaling in breast cancer. Edmonds et al (14) revealed that miR-31 expression
initiated lung tumorigenesis and promoted mutant KRAS-driven lung
cancer. Raffel and Trumpp (15)
demonstrated that miR-126, as a regulator of phosphatidylinositol
3-kinase-protein kinase B-mechanistic target of rapamycin and
cyclin-dependent kinase (CDK) 3 signaling, was driving leukemic
stem cell self-renewal and chemotherapy resistance. In addition,
>50% of human microRNA genes are located in cancer-associated
genomic regions or fragile sites (16).
The present study revealed that miR-509-3p was
significantly downregulated in malignant glioma tissues samples
compared with in normal tissues samples. Furthermore, the results
demonstrated that the overexpression of miR-509-3p inhibited the
proliferation and motility of malignant glioma cells. These data
are in accord with the in vitro data reported by Yoon et
al (6), in which the
overexpression of miR-509-3p induced G1 cell-cycle arrest, and
inhibited colony formation and migration via the downregulation of
CDK2, ras-related C3 botulinum toxin substrate 1 and
phosphatidylinositol-4-phosphate 3-kinase catalytic subunit type 2
α. The present study also demonstrated that relatively low levels
of miR-509-3p expression were significantly associated with poor
outcomes in glioma. These results suggest that glioma tumorigenesis
may be associated with the decreased expression level of
miR-509-3p.
XIAP is the most potent member in the family of
inhibitors of apoptosis; it is able to inhibit caspase-3 and −7 by
binding them to its XIAP baculovirus IAP repeat (BIR)2 domain, and
caspase-9 by binding it to its BIR3 domain (17). It has been reported that XIAP
expression is elevated in numerous types of cancer (18–21). Thus,
the downregulation of XIAP is recognized as a potential anticancer
approach (22–24). In epithelial ovarian cancer,
miR-509-3p, a downregulated miRNA, can directly target the XIAP via
its 3′-untranslated region (UTR) (25).
In general, miRs function by binding to the 3′-UTRs
of target genes. The present study aimed to investigate whether
miR-509-3p targeted XIAP in glioma cells. The present study
revealed that miR-509-3P negatively regulated XIAP expression, and
that miR-509-3p and XIAP were inversely correlated in human glioma
tissue samples. The results further confirmed that the
downregulation of XIAP may significantly attenuate the
proliferation, migration and invasion abilities of glioma cells.
However, in the present study, only one glioma cell line was
considered; this may represent a study limitation, and further
study is required.
In conclusion, the present study demonstrated that
miR-509-3p was downregulated in glioma tissue samples compared with
normal brain tissue, and that low expression levels of miR-509-3p
were associated with poor glioma outcomes. XIAP was previously
identified as a potential target for miR-509-3p, and miR-509-3p was
demonstrated to function as a negative regulator of XIAP in the
present study. Collectively, miR-509-3p, considered as a
tumor-suppressor gene, inhibits cell proliferation and invasion by
targeting XIAP in glioma, which may provide a novel insight into
tumorigenesis and the basis for the development of miRNA-targeting
therapies against glioma.
Acknowledgements
The present study was supported by grants from
Autonomous Region Natural Science Foundation of Xinjiang (grant no.
2017D01C247).
References
|
1
|
Ostrom QT, Gittleman H, Farah P, Ondracek
A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 15
Suppl 2:S1–S56. 2013. View Article : Google Scholar
|
|
2
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:1–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnson BE, Mazor T, Hong C, Barnes M,
Aihara K, McLean CY, Fouse SD, Yamamoto S, Ueda H, Tatsuno K, et
al: Mutational analysis reveals the origin and therapy-driven
evolution of recurrent glioma. Science. 343:189–193. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Griffiths-Jones S, Grocock RJ, van Dongen
S, Bateman A and Enright AJ: miRBase: microRNA sequences, targets
and gene nomenclature. Nucleic Acids Res. 34(Database Issue):
D140–D144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan YS, Kim M, Kingsbury TJ, Civin CI and
Cheng WC: Regulation of RAB5C is important for the growth
inhibitory effects of MiR-509 in human precursor-B acute
lymphoblastic leukemia. PLoS One. 9:e1117772014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoon S, Han E, Choi YC, Kee H, Jeong Y,
Yoon J and Baek K: Inhibition of cell proliferation and migration
by miR-509-3p that targets CDK2, Rac1, and PIK3C2A. Mol Cells.
37:314–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su Z, Chen D, Zhang E, Li Y, Yu Z, Shi M,
Jiang Z, Ni L, Yang S, Gui Y, et al: MicroRNA-509-3p inhibits
cancer cell proliferation and migration by targeting the
mitogen-activated protein kinase kinase kinase 8 oncogene in renal
cell carcinoma. Mol Med Rep. 12:1535–1543. 2015.PubMed/NCBI
|
|
8
|
Xing F, Sharma S, Liu Y, Mo YY, Wu K,
Zhang YY, Pochampally R, Martinez LA, Lo HW and Watabe K: miR-509
suppresses brain metastasis of breast cancer cells by modulating
RhoC and TNF-α. Oncogene. 34:4890–4900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Cui M, Cai X, Sun B, Liu F, Zhang
X and Ye L: The oncoprotein HBXIP up-regulates SCG3 through
modulating E2F1 and miR-509-3p in hepatoma cells. Cancer Lett.
352:169–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pardo OE, Castellano L, Munro CE, Hu Y,
Mauri F, Krell J, Lara R, Pinho FG, Choudhury T, Frampton AE, et
al: miR-515-5p controls cancer cell migration through MARK4
regulation. EMBO Rep. 17:570–584. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kouhkan F, Mobarra N, Soufi-Zomorrod M,
Keramati F, Hosseini Rad SM, Fathi-Roudsari M, Tavakoli R,
Hajarizadeh A, Ziaei S, Lahmi Ret, et al: MicroRNA-129-1 acts as
tumour suppressor and induces cell cycle arrest of GBM cancer cells
through targeting IGF2BP3 and MAPK1. J Med Genet. 53:24–33. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan Z, Zheng H, Liu X, Zhang W, Zhu J, Wu
G, Cao L, Song J, Wu S, Song L and Li J: MicroRNA-1229
overexpression promotes cell proliferation and tumorigenicity and
activates Wnt/β-catenin signaling in breast cancer. Oncotarget.
7:24076–24087. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edmonds MD, Boyd KL, Moyo T, Mitra R,
Duszynski R, Arrate MP, Chen X, Zhao Z, Blackwell TS, Andl T and
Eischen CM: MicroRNA-31 initiates lung tumorigenesis and promotes
mutant KRAS-driven lung cancer. J Clin Invest. 126:349–364. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raffel S and Trumpp A: miR-126 Drives
Quiescence and Self-Renewal in Leukemic Stem Cells. Cancer Cell.
29:133–135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vogler M, Walczak H, Stadel D, Haas TL,
Genze F, Jovanovic M, Bhanot U, Hasel C, Möller P, Gschwend JE, et
al: Small molecule XIAP inhibitors enhance TRAIL-induced apoptosis
and antitumor activity in preclinical models of pancreatic
carcinoma. Cancer Res. 69:2425–2434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paschall AV, Zimmerman MA, Torres CM, Yang
D, Chen MR, Li X, Bieberich E, Bai A, Bielawski J, Bielawska A and
Liu K: Ceramide targets xIAP and cIAP1 to sensitize metastatic
colon and breast cancer cells to apoptosis induction to suppress
tumor progression. BMC Cancer. 14:242014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moreno-Martínez D, Nomdedeu M,
Lara-Castillo MC, Etxabe A, Pratcorona M, Tesi N, Díaz-Beyá M,
Rozman M, Montserrat E, Urbano-Ispizua A, et al: XIAP inhibitors
induce differentiation and impair clonogenic capacity of acute
myeloid leukemia stem cells. Oncotarget. 5:4337–4346. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yabal M, Müller N, Adler H, Knies N, Groß
CJ, Damgaard RB, Kanegane H, Ringelhan M, Kaufmann T, Heikenwälder
M, et al: XIAP restricts TNF- and RIP3-dependent cell death and
inflammasome activation. Cell Rep. 7:1796–1808. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren Y, Han X, Yu K, Sun S, Zhen L, Li Z
and Wang S: microRNA-200c downregulates XIAP expression to suppress
proliferation and promote apoptosis of triple-negative breast
cancer cells. Mol Med Rep. 10:315–321. 2014.PubMed/NCBI
|
|
22
|
Li G, Chang H, Zhai YP and Xu W: Targeted
silencing of inhibitors of apoptosis proteins with siRNAs: A
potential anti-cancer strategy for hepatocellular carcinoma. Asian
Pac J Cancer Prev. 14:4943–4952. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li XQ, Ke XZ and Wang YM: Treatment of
malignant melanoma by downregulation of XIAP and overexpression of
TRAIL with a conditionally replicating oncolytic adenovirus. Asian
Pac J Cancer Prev. 13:1471–1476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li S, Sun J, Yang J, Zhang L, Wang L, Wang
X and Guo Z: XIAP expression is associated with pancreatic
carcinoma outcome. Mol Clin Oncol. 1:305–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen W, Zeng W, Li X, Xiong W, Zhang M,
Huang Y, Zhou L and Jiang S: MicroRNA-509-3p increases the
sensitivity of epithelial ovarian cancer cells to cisplatin-induced
apoptosis. Pharmacogenomics. 17:187–197. 2016. View Article : Google Scholar : PubMed/NCBI
|