Introduction
Gastric cancer is one of the leading causes of
cancer-associated mortality in the world (1). It is challenging to cure by treatments
involving surgery, chemotherapy and radiation therapy, unless it is
identified at an early stage (2).
Although significant progress has been achieved in its systemic
treatment, metastatic gastric cancer remains a major therapeutic
challenge for oncologists (3,4). Therefore, it is necessary to identify
new therapeutic agents with low toxicity and high selectivity to
kill cancer cells and suppress tumor metastasis (4,5).
Serine protease inhibitors (serpins) are a family of
proteins that inhibit chymotrypsin-like serine proteinases and
control activated proteinases, and several are involved in the
regulation of cell death (6–8). Maspin is a member of the serpin family
and is present in normal mammary epithelial cells, but it is
downregulated or absent in numerous tumor cell lines (9–11). Maspin
is found in the extracellular matrix and at the plasma membrane and
has been shown to act at the cell surface to block cell motility
and inhibit invasion of breast and prostate cancer cells (6,9).
Celastrus orbiculatus Thunb., a Chinese
traditional herb, has been used against inflammatory diseases for
thousands of years in China. Previous studies have revealed that
C. orbiculatus extract (COE) exhibits anticarcinogenic
potentials, including the induction of cell apoptosis, inhibition
of cell proliferation and inhibition of angiogenesis (12–14). COE
was also revealed to inhibit migration and invasion of human
colorectal carcinoma cells (15). The
present study investigated whether COE effects maspin expression
synergistically, with the aim of clarifying the mechanisms.
Materials and methods
Chemicals and reagents
MTT and dimethyl sulfoxide (DMSO) were acquired from
Sigma-Aldrich (EMD Millipore, Billerica, MA, USA). Fetal bovine
serum (FBS) and RPMI-1640 medium were purchased from Gibco (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Matrigel was acquired
from BD Biosciences (San Jose, CA, USA). Antibodies against
extracellular regulated protein kinase (ERK; cat no. 4695,
1:1,000), phospho-ERK (cat no. 4370; 1:2,000), p38 (cat no. 2371;
1:1,000), phospho-p38 (cat no. 4511; 1:1,000), Akt(cat no. 4691;
1:1,000), phospho-Akt (cat no. 4058; 1:1,000), mechanistic target
of rapamycin (mTOR; cat no. 2983; 1:1,000), phospho-mTOR (cat no.
5536; 1:1,000), caspase-3 (cat no. 9664; 1:1,000) and β-actin (cat
no. 4970; 1:1,000) were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). B-cell lymphoma-2 (Bcl-2; cat no. 39869;
1:1,000) and Bcl-2-like 12 (cat no. 37451; 1:10,000) were purchased
from Epitomics (Burlingame, CA, USA). Antibodies against Maspin
(cat no. B1910; 1:1,000), Blc-2-associated X protein (Bax; cat no.
J3113, 1:1,000) and Maspin small interfering RNA (siRNA; cat no.
sc-35859) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Sofast was purchased from Sunma Biotechnology
Co., Ltd. (Xiamen, China). The enhanced chemiluminescent (ECL)
substrate for detection of horseradish peroxidase (HRP) kit was
acquired from GE Healthcare Life Sciences (Chalfont, UK). Other
reagents were acquired from Beyotime Institute of Biotechnology
(Jiangsu, China).
Cell lines
The human gastric carcinoma cell line MGC-803 was
obtained from the Cell Bank of Shanghai Institutes for Biological
Sciences (Shanghai, China). MGC-803 cells were cultured in
RPMI-1640 containing 10% FBS and incubated at 37°C in a 5%
CO2 atmosphere.
Plant material
The stems of C. orbiculatus plants (production batch
no. 070510) were purchased from Guangzhou Zhixin Pharmaceutical
Co., Ltd. (Guangzhou, China) in 2007. The preparation and
characterization of COE was from the Department of Chinese Materia
Medica Analysis, China Pharmaceutical University (Nanjing, China).
The chemical constituents of COE were described previously
(15). The resultant COE micropowder
was diluted in DMSO to 1% and was further diluted with RPMI-1640
medium to different concentrations (10, 20, 40, 80, 160, 320 mg/ml)
prior to use. The final concentration of DMSO did not exceed 0.1%
in the cell medium (4).
Transfection of siRNA
Cells were transfected with siRNA and Sofast
according to the manufacturer's protocol. Briefly, cells were
seeded onto a 6-well plate at a density of 3×105
cells/well with antibiotics-free medium (Gaithersburg, MD, USA), 12
h prior to the transfection. Sofast (8 µl) in 92 µl serum-free
RPMI-1640 medium was mixed with siRNA (10 µM) in 90 µl serum-free
RPMI-1640 medium. The mixture was incubated at room temperature for
20 min to form a complex. After 20 min, the 200 µl transfection
mixtures were added to each well with 1.8 ml RPMI-1640 medium
containing 10% FBS at a final concentration (0.5 µM). Following 24
h transfection, cells were collected for RNA and protein
isolation.
MTT assay
The MGC-803/maspin− cells were seeded
onto a 96-well plate and treated with COE at various concentrations
(0, 10, 20, 40, 80, 160 and 320 µg/ml) in triplicate to evaluate
the effect of COE on cell viability. Following incubation with the
drug for 24 h, MGC-803/maspin− cells were
re-supplemented with 200 µl culture medium containing 10% MTT dye,
and incubated for 4 h (37°C, 5% CO2). Cells were then
suspended in 100 µl DMSO. The relative cell viability was
determined by a microplate reader (Implen GmbH, München, Germany)
at an absorbance of 490 nm.
Flow cytometry
Apoptotic cells were detected by the Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
detection kit (MACS Technology; Miltenyi Biotec GmbH, Bergisch
Gladbach, Germany). Cells were digested with trypsin EDTA-free
following treatment with different concentrations (0, 20, 40 and 80
µg/ml) of COE for 24 h. Following centrifugation (300 × g) at 4°C
for 5 min, 1×106 cells were collected and washed twice
with cold PBS. Cells were resuspended in 500 µl 1X binding buffer
and then incubated for 15 min at room temperature in the dark
following 5 µl Annexin V-FITC and 5 µl PI additions. For each
analysis, 10,000 events were recorded.
Cell invasion and migration
assays
A Transwell membrane (Costar; Corning Incorporated,
Corning, NY, USA) was used for cell invasion and migration assays,
according to the manufacturer's protocol. Following treatment with
various concentrations of negative group (wild-type MGC-803 cells),
COE group (20, 40 and 80 µg/ml) and 5-fluorouracil (5-FU) positive
group (32 µg/ml) for 24 h, cells were seeded in the upper part of a
Matrigel-coated invasion chamber in a serum-free medium. Medium
containing 20% FBS was applied to the lower chamber. After 24 h,
the cells remaining in Matrigel were removed by scraping, while the
cells that invaded through Matrigel were fixed and stained by using
0.5% crystal violet (Beyotime Institute of Biotechnology) in
methanol for 30 min. Images were captured under a fluorescence
microscope at magnification, ×400 (Nikon Corporation, Tokyo, Japan)
and invading cells were quantified by manually counting 5 fields of
view. Migration assays followed in the same procedure, but with no
Matrigel coating on the polycarbonate membrane. Each experiment was
repeated three times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
To quantitatively determine the mRNA expression
levels of maspin in the MGC-803, RT-qPCR was used. Total RNA was
isolated from MGC-803 cells using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) under RNase-free conditions. DNA
was synthesized by a RT reaction kit (Takara Biotechnology Co.,
Ltd., Dalian, China). Subsequently, RT-qPCR was performed with a
LightCycler 96 real-time PCR system using the SYBR Premix Ex Taq
kit (Takara Biotechnology Co., Ltd.) in 96-well reaction plates
(Axygen Scientific, Inc., Union City, CA, USA). All the
aforementioned methods were performed by following the
manufacturers' protocols. Primers were purchased from Takara
Biotechnology Co., Ltd. and their primer sequences were as follows:
Maspin forward, 5′-CATCCTACTACCCAAGGATGTGGAG-3′ and reverse,
5′-TTGGCATTGGCCATGGTG-3′; β-actin forward,
5′-GTGGGCCGCTCTAGGCACCAA-3′ and reverse,
5′-CTCTTTGATGTCACGCACGATTTC-3′. Data were analyzed using the
comparative Cq method (2−∆∆Cq) (16).
Western blot analysis
Expression of Bcl-2, Bax, caspase-3, MAPK (p38,
p-p38, ERK and p-ERK) and phosphoinositide 3-kinase (PI3K)/Akt/mTOR
signaling protein (Akt, p-Akt, mTOR and p-mTOR) levels in
MGC-803/maspin− cells was examined by western blot
analysis. Cells were centrifuged (8000 × g, 15 min, 4°C), washed
with cold PBS and lysed on ice for 30 min in lysates (Beyotime
Institute of Biotechnology) containing 100 µg/ml
phenylmethanesulfonyl fluoride. Protein concentrations were
determined by NanoPhotometer pearl (P-330-31; Implen GmbH). Total
protein (20–70 µg) was electrophoresed on 8–12% SDS-PAGE and then
transferred to a nitrocellulose membrane. Following incubation with
5% non-fat dried milk for 2 h at room temperature, Primary
antibodies were diluted with 5% skimmed milk powder solution and
incubated with the membranes overnight at 4°C, then washed three
times with Tris-buffered saline (pH 6.8) with Tween-20 (0.1%)
(TBST), incubated with the secondary antibody (sheep anti-rabbit
IgG-HRP; cat no. HA1001; 1:2,000; Hangzhou HuaAn Biotechnology Co.,
Ltd. Hangzhou, China) at room temperature for 2 h and washed three
times with TBST. The ECL reagent was used to visualize the positive
bands on the membrane.
Statistical analysis
All experiments were performed at least 3 times. The
experimental results are presented as the mean ± standard
deviation. Statistical analysis was performed by the unpaired
Student's t-test using GraphPad Prism 5.0 statistical analysis
software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of COE on maspin in MGC-803
cells
To investigate the effect of COE on maspin in
MGC-803 cells, maspin siRNA was transiently transfected into
MGC-803 cells using Sofast kit. At 24 h post-transfection,
expression of maspin mRNA and protein was assessed by RT-qPCR and
western blotting, respectively. As shown in Fig. 1A, compared with wild-type MGC-803
cells, maspin protein was knocked down in the
MGC-803/maspin− cells. Following treatment with COE,
maspin protein was increased in a dose-dependent manner in
MGC-803/maspin− cells. Maspin mRNA assay by RT-qPCR had
the same results, as shown in Fig.
1B. The MTT assay showed that COE inhibited
MGC-803/maspin− cell growth (Fig. 1C). The cell viability had no
significant affect when the concentration of COE was <80 µg/ml.
Therefore, concentrations <80 µg/ml of COE were chosen for
further experiments. Phase-contrast images of cells from the same
fields were captured at 24 h after transfection. Representative
images of MGC-803/maspin− cells revealed that viability
was decreased following treatment with COE.
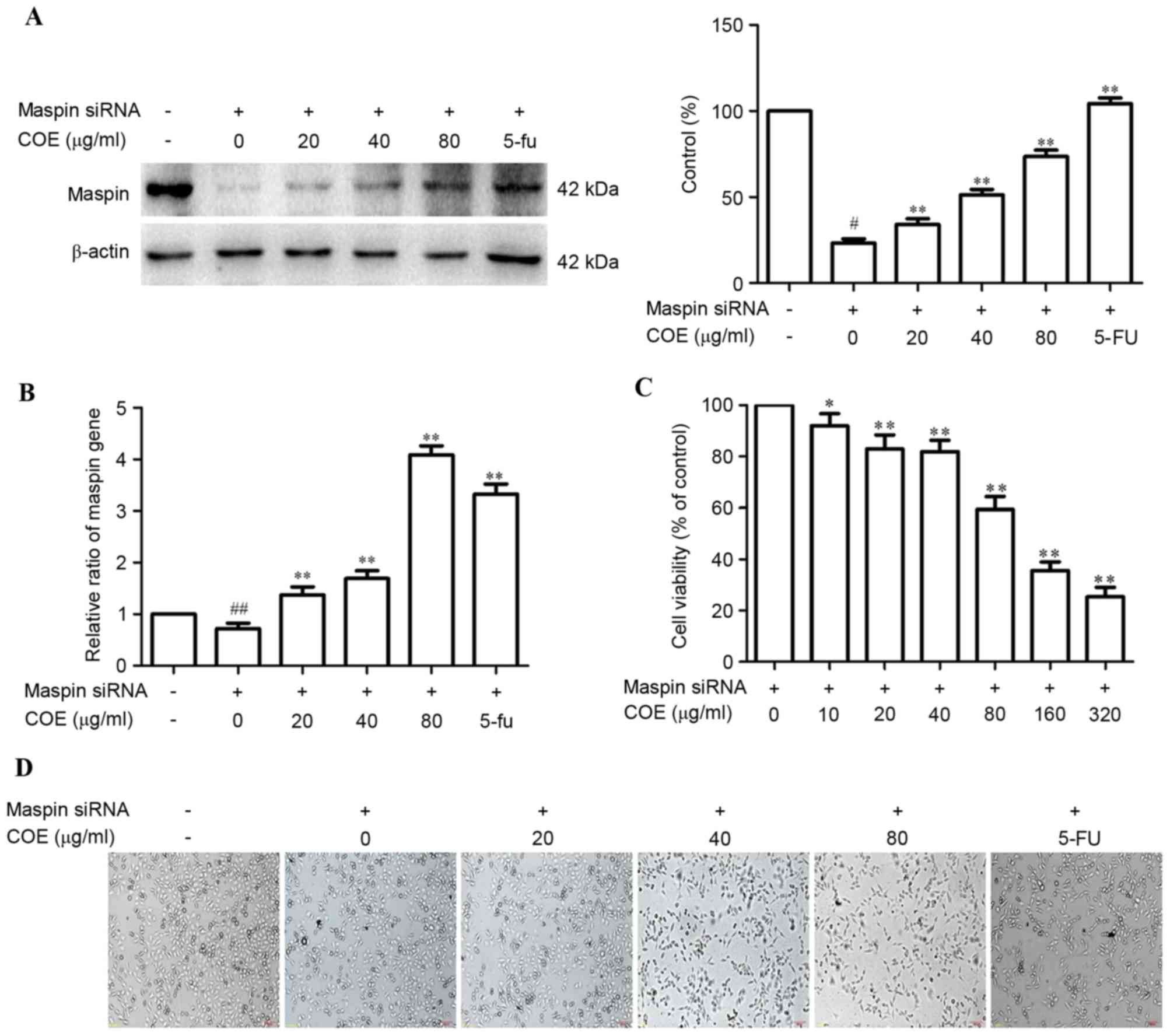 | Figure 1.Effects of COE on maspin expression in
MGC-803 cells. (A) COE increases the maspin protein in MGC-803
cells in a dose-dependent manner. (B) The effect of COE on maspin
mRNA expression in MGC-803 cells was assessed by quantitative
polymerase chain reaction. (C) The MTT assay was used to measure
the viability of MGC-803/maspin− cells following
treatment with COE (0, 10, 20, 40, 80, 160 and 320 µg/ml) for 24 h.
(D) Morphological changes between wild-type MGC803 cells and maspin
siRNA-transfected MGC803 cells treated with COE (0, 20, 40 and 80
µg/ml) for 24 h (magnification, ×100). The values are represented
as the mean ± standard deviation of at least three independent
experiments. #P<0.05 and ##P<0.01
compared with the control. *P<0.05 and **P<0.01 compared with
maspin siRNA-transfected cells. COE, Celastrus orbiculatus extract;
siRNA, small interfering RNA. |
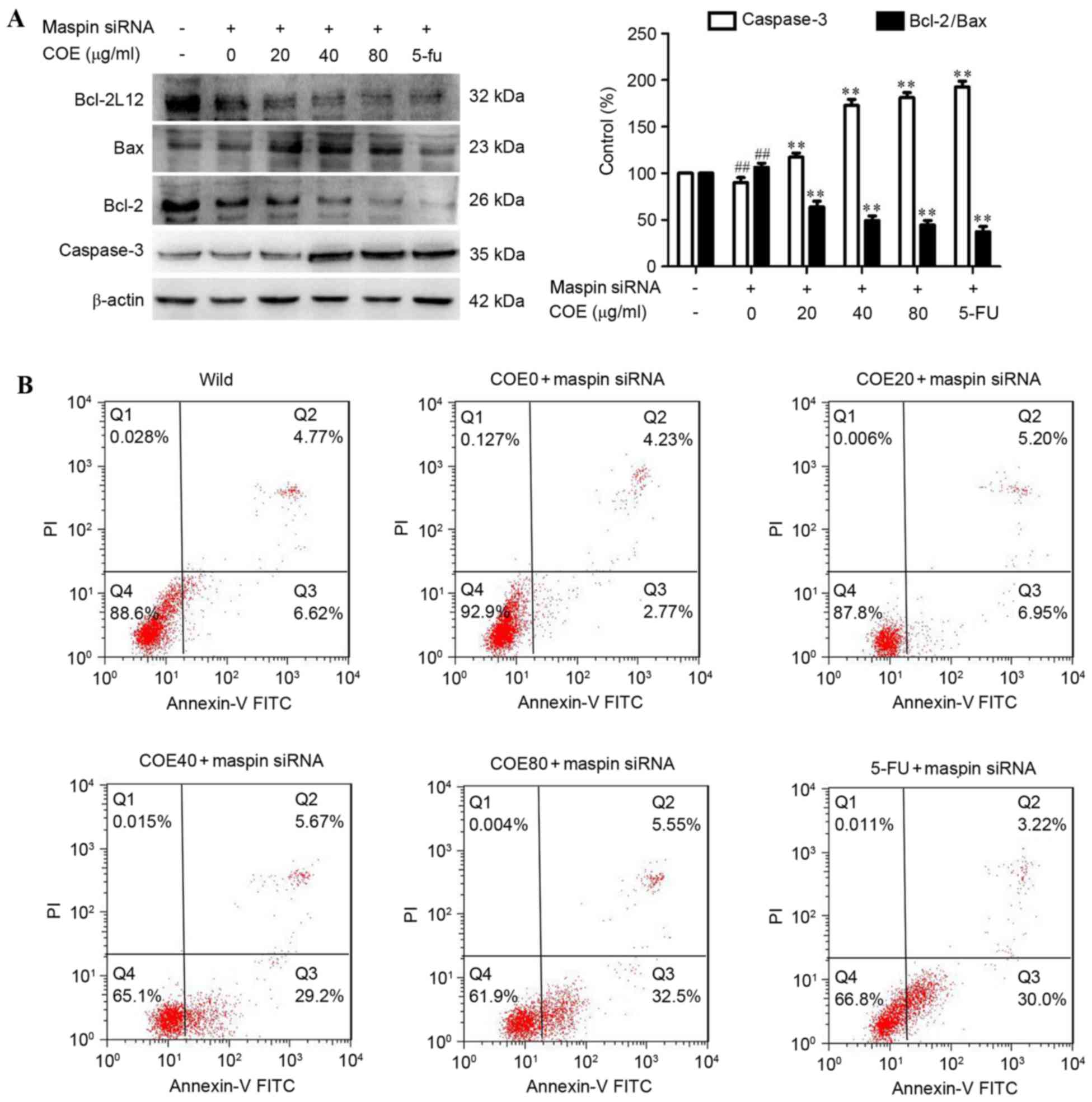
COE induces apoptosis in
MGC-803/maspin− cells
The effect of COE on apoptosis in
MGC-803/maspin− cells was then investigated by western
blot analysis and flow cytometry. As shown in Fig. 2A, following treatment with COE,
expression of caspase-3 was increased, but the expression of
Bcl-2/Bax was significantly reduced in a dose-dependent manner in
MGC-803/maspin− cells. It was revealed that COE induced
apoptosis in MGC-803/maspin− cells in a dose-dependent
manner. The MGC-803 cells were labeled with PI and Annexin V. Cells
in early apoptosis were Alexa PI-negative and Fluor 488-Annexin
V-positive, and cells in late apoptosis were PI and Alexa Fluor
488-Annexin V-positive. The number of apoptotic cells (early and
late apoptosis) reduced to 7.0% in MGC-803/maspin−
cells, and the number of apoptotic cells was 11.39% in wild-type
MGC-803 cells. COE promoted the apoptosis of
MGC-803/maspin− cells in a dose-dependent manner
(Fig. 2B).
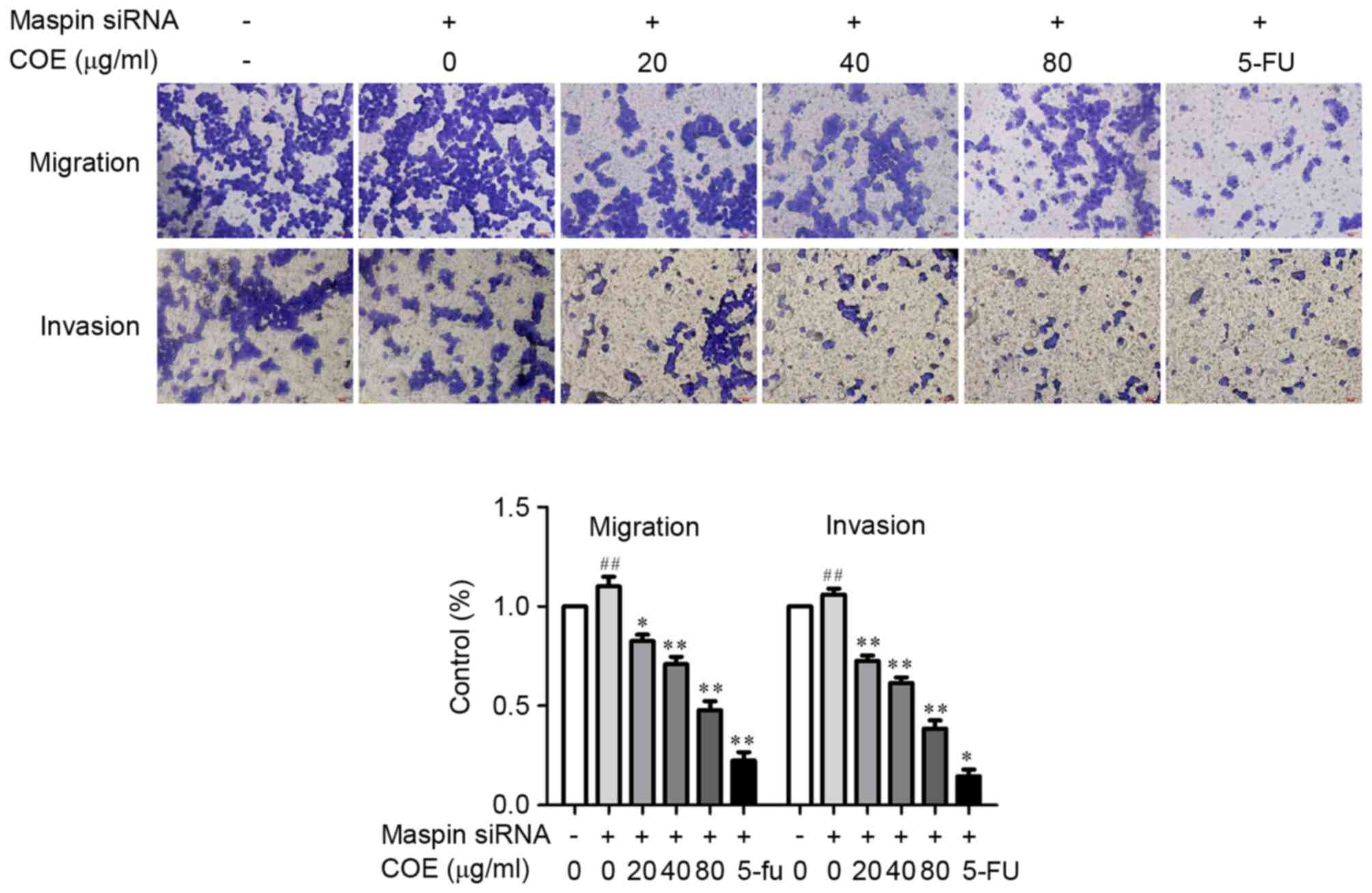
COE inhibits invasion and migration
through PI3K/Akt/mTOR and MAPK signaling pathways in
MGC-803/maspin− cells
The effect of COE on invasion and migration in
MGC-803/maspin− cells was investigated by Transwell
assay and western blotting. Following the treatment with COE, the
Transwell chamber was observed under the fluorescence microscope at
magnification, ×400 (Nikon Corporation, Tokyo, Japan) and invading
cells were quantified by manually counting 5 fields of view. The
number of cells that invaded to the lower chamber was significantly
reduced in a dose-dependent manner (Fig.
3). These results revealed that COE decreased the invasion and
migration of MGC-803/maspin− cells in a dose-dependent
manner, suggesting COE has an inhibitory effect on the metastatic
process of MGC-803/maspin− cells. As shown in Fig. 4, the phosphorylation of Akt, p-mTOR
and ERK was significantly inhibited (P<0.01) by COE compared
with the control group. By contrast, the total protein levels of
p38 and mTOR were not markedly changed following COE treatment. The
total protein level of p-p38 was increased in the
MGC-803/maspin− cells dose-dependently. In conclusion,
these data revealed that COE inhibits the invasion and migration of
MGC-803/maspin− cells, possibly through the
PI3K/Akt/mTOR and MAPK signaling pathways.
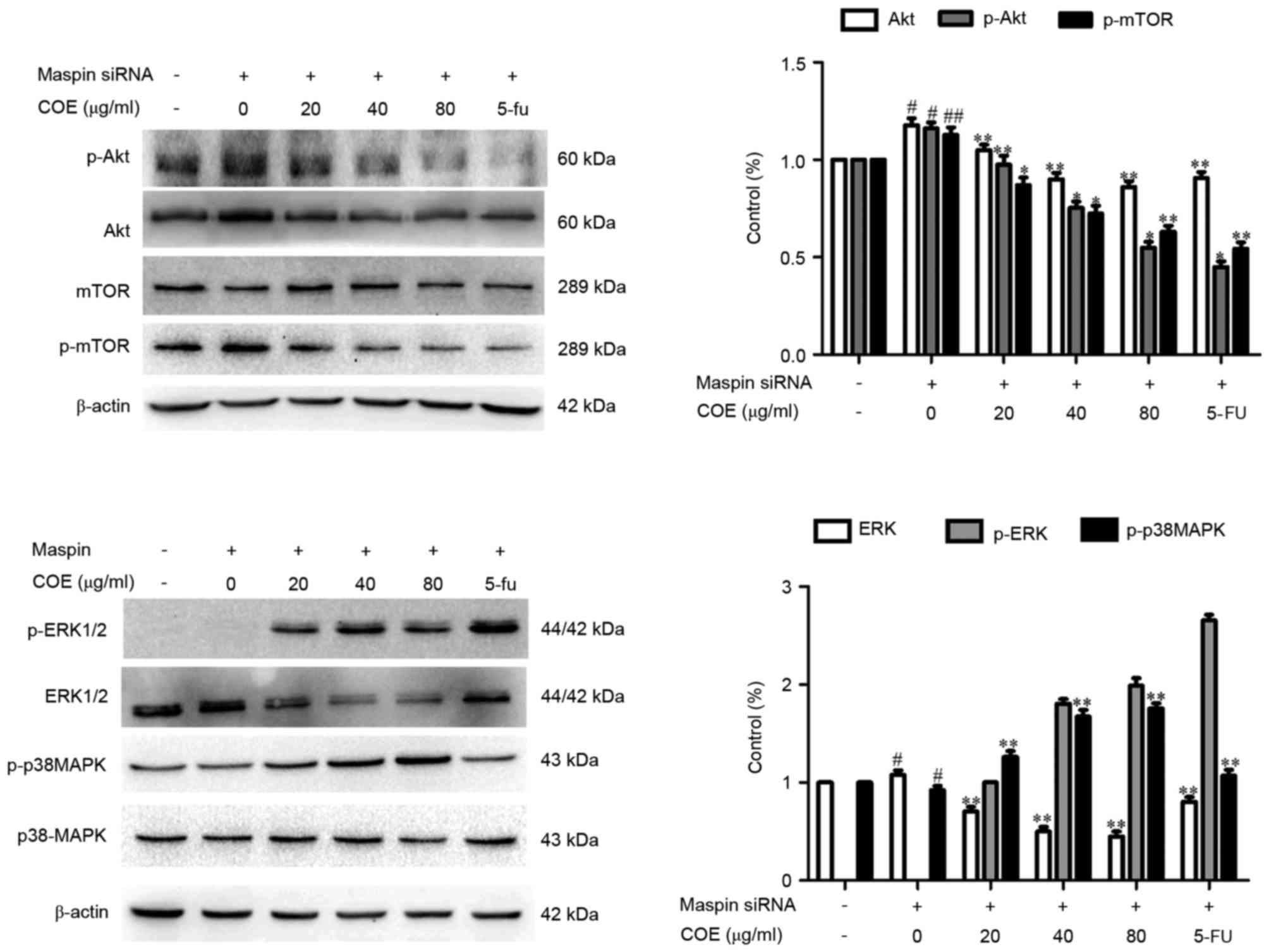 | Figure 4.Effects of COE on the MAPK and
phosphoinositide 3-kinase/Akt/mTOR signaling pathways. The activity
of p-p38 and p38, p-Akt and Akt, p-ERK and ERK and p-mTOR and mTOR
was examined by western blot analysis. The relative density of the
aforementioned proteins was normalized to β-actin, which was
determined by densitometric analysis. The values are presented as
the mean ± standard deviation of at least three independent
experiments. #P<0.05; ##P<0.01,
compared with the control. *P<0.05; **P<0.01, compared to
maspin siRNA-transfected cells. mTOR, mechanistic target of
rapamycin; COE, Celastrus orbiculatus extract; MAPK,
mitogen-activated protein kinases; ERK, extracellular
signal-regulated kinase; siRNA, small interfering RNA. |
Discussion
Traditional Chinese Medicine (TCM) is widely used as
a therapy in patients with cancer worldwide, to suppress tumor
proliferation, induce apoptosis and prevent complications (17). TCM also serves an important role in
reducing the side effects and improving the quality of life of
conventional treatments in patients with cancer (17–20). In
previous years, bioactive component extracts from TCM were
identified as therapeutic agents, and were effective in the
prevention of potential cancers (4,13,21). An ethyl acetate extract of C.
orbiculatus was revealed to have a significant ability against
various human tumor cell lines to inhibit proliferation and induce
apoptosis (20,22–24).
Suppressor gene maspin expression is reduced or
absent in numerous tumor cells (6).
Increasing the level of maspin expression in tumor cells may
promote tumor cell apoptosis and inhibit invasion and metastasis,
inhibit tumor angiogenesis and even increase the sensitivity of
tumor cells to chemotherapy (6–10).
Apoptosis is a defensive mechanism of the body to eliminate
malignant cells, and it has a significant role in preventing
cancer. Notably, the predominant function of numerous antitumor
drugs is to induce apoptosis in tumor cells via various
apoptosis-associated signaling pathways (25). In the present study, western blot
analysis demonstrated that COE can reduce the expression of Bcl-2
protein, and increase the expression of Bax and caspase-3 total
protein, decreasing Bcl-2/Bax. COE performs a pro-apoptotic role
through Bcl-2, Bax and caspase-3-mediated signaling pathways.
There may be other factors that may affect the
invasion and metastasis of tumor cells, but each process requires
migration ability. The Transwell assay is a common method of
detecting migration of tumor cells in vitro; the number of
cells in the small compartment at the bottom of the polycarbonate
film reflects cell migration. The present results reveal that
compared with wild-type MGC803 cells, the number of cells that
migrated through the Transwell chamber increased significantly in
MGC803 cells with low expression of the tumor suppressor gene
maspin. With increasing COE concentration, the ability to inhibit
gastric cancer cell migration was more evident. COE may enhance the
effect of the tumor suppressor gene maspin to inhibit tumor cell
migration.
The invasion and metastasis of tumor cells is
coordinately regulated by a variety of cytokines and signaling
pathways. ERK1/2, MAPK and PI3K/Akt/mTOR signaling pathways are
major signal transduction pathways in the regulation of tumor
invasion and metastasis (26,27). Therefore, the effect of the tumor
suppressor gene maspin and COE on the key protein molecular
expression and phosphorylation levels of the MAPK and PI3K/Akt/mTOR
signaling pathways was analyzed. It was identified that COE
significantly inhibited the phosphorylation of Akt and ERK, while
promoting the expression of p-P38MAPK. Therefore, COE targeting of
maspin to inhibit metastasis of gastric cancer cells may occur
through MAPK and PI3K/Akt/mTOR signaling pathways. The present
study also demonstrated that TCM inhibiting tumor invasion and
metastasis may be a multi-target, multi-channel integrated action.
The antitumor mechanisms should be assessed from cells, molecules
and systematic study of animal models.
In the present study, the results demonstrated that
COE increased the expression level of maspin to inhibit the
proliferation of gastric cancer cells and induce apoptosis in a
concentration-dependent manner. Furthermore, COE targeting maspin
to inhibit the migration and invasion of MGC803 cells may be
through MAPK and PI3K/Akt/mTOR signaling pathways. In terms of the
underlying mechanisms, the results of the present study
demonstrated that COE affects maspin expression synergistically to
induce apoptosis and inhibit invasion and migration in gastric
cancer cells by regulating apoptosis-associated proteins and
inhibiting MAPK and PI3K/Akt/mTOR signaling pathways.
In conclusion, COE may be a potential therapy
against gastric cancer. Nevertheless, all experiments were
performed in vitro, and in vivo studies are required
for additional investigation. The present findings reveal that COE
has a promising prospect in treating metastatic gastric cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81403232 and
81274141), the National Natural Science Foundation of Jiangsu
Province (grant nos. BK2012686 and BK 20171290) and the Doctoral
Fund of the Ministry of Education of China (grant no.
20133250120003).
References
|
1
|
Wu HH, Lin W and Tsai KW: Advances in
molecular biomarkers for gastric cancer: miRNAs as emerging novel
cancer markers. Expert Rev Mol Med. 16:e12014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim SJ, Wang YG, Lee HW, Kang HG, La SH,
Choi IJ, Irimura T, Ro JY, Bresalier RS and Chun KH: Up-regulation
of neogenin-1 increases cell proliferation and motility in gastric
cancer. Oncotarget. 5:3386–3398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kanat O and O'Neil BH: Metastatic gastric
cancer treatment: A little slow but worthy progress. Med Oncol.
30:4642013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu YD, Liu YQ, Qian YY, Zhang H, Li GQ
and Yang L: Extracts of Celastrus orbiculatus exhibit
anti-proliferative and anti-invasive effects on human
gastricadenocarcinoma cells. Chin J Integr Med. Nov 10–2014.(Epub
ahead of print). View Article : Google Scholar
|
|
5
|
Wang S, Zhong Z, Wan J, Tan W, Wu G, Chen
M and Wang Y: Oridonin induces apoptosis, inhibits migration and
invasion on highly-metastatic human breast cancer cells. Am J Chin
Med. 41:177–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tarakji B, Ashok N, Sheirawan MK, Altamimi
MA, Alenzi F, Azzeghaiby SN, Baroudi K and Nassani MZ: Maspin as a
tumour suppressor in salivary gland tumour. J Clin Diagn Res.
8:ZE05–ZE07. 2014.PubMed/NCBI
|
|
7
|
Bodenstine TM, Seftor RE, Seftor EA,
Khalkhali-Ellis Z, Samii NA, Monarrez JC, Chandler GS, Pemberton PA
and Hendrix MJ: Internalization by multiple endocytic pathways and
lysosomal processing impact maspin-based therapeutics. Mol Cancer
Res. 12:1480–1491. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lovato A, Lionello M, Staffieri A,
Blandamura S, Tealdo G, Giacomelli L, Staffieri C and Marioni G: A
higher angiogenin expression is associated with a nonnuclear maspin
location in laryngeal carcinoma. Clin Exp Otorhinolaryngol.
8:268–274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bagheri M, Eghtedari M, Bagheri M,
Geramizadeh B and Talebnejad M: Expression of Maspin and Ezrin
proteins in periocular basal cell carcinoma. Dermatol Res Pract.
2014:5965642014.PubMed/NCBI
|
|
10
|
Dzinic SH, Chen K, Thakur A, Kaplun A,
Bonfil RD, Li X, Liu J, Bernardo MM, Saliganan A, Back JB, et al:
Maspin expression in prostate tumor elicits host anti-tumor
immunity. Oncotarget. 5:11225–11236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lonardo F, Guan H, Dzinic S and Sheng S:
Maspin expression patterns differ in the invasive versus lepidic
growth pattern of pulmonary adenocarcinoma. Histopathology.
65:757–763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang WM, Liu YQ and Dai XJ: Apoptosis of
Hela cell induced by Celastrus orbiculatus Thunb extract and
primary mechanisms. Chin-German J Clin Oncol. 10:666–668. 2011.
View Article : Google Scholar
|
|
13
|
Qian YY, Zhang H, Hou Y, Yuan L, Li GQ,
Guo SY, Hisamits T and Liu YQ: Celastrus orbiculatus extract
inhibits tumor angiogenesis by targeting vascular endothelial
growth factor signaling pathway and shows potent antitumor activity
in hepatocarcinomas in vitro and in vivo. Chin J Integr Med.
18:752–760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma H, Qian YY, Zhang H, Xue JI, Yaodong
ZHU, Pingfang CUI and Yanqing LIU: Celastrus orbiculatus extract
could inhibit human colorectal carcinoma HT-29 cells metastasis via
suppression of the mTOR signaling pathway. Life Sci J.
10:1704–1710. 2013.
|
|
15
|
Zhang H, Qian Y, Liu Y, Li G, Cui P, Zhu
Y, Ma H, Ji X, Guo S and Tadashi H: Celastrus orbiculatus extract
induces mitochondrial-mediated apoptosis in human hepatocellular
carcinoma cells. J Tradit Chin Med. 32:621–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hong M, Wang N, Tan HY, Tsao SW and Feng
Y: MicroRNAs and Chinese medicinal herbs: New possibilities in
cancer therapy. Cancers (Basel). 7:1643–1657. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Su CX, Wang LQ, Grant SJ and Liu JP:
Chinese herbal medicine for cancer-related fatigue: A systematic
review of randomized clinical trials. Complement Ther Med.
22:567–579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen CM, Lin LZ and Zhang EX: Standardized
treatment of Chinese medicine decoction for cancer pain patients
with opioid-induced constipation: A multi-center prospective
randomized controlled study. Chin J Integr Med. 20:496–502. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo FR, Ding J, Chen HX, Liu H, Fung MC,
Koehler M, Armand JP, Jiang L, Xu X, Zhang G, et al: Breakthrough
cancer medicine and its impact on novel drug development in China:
Report of the US Chinese anti-cancer association (USCACA) and
Chinese society of clinical oncology (Csco) joint session at the
17th CSCO annual meeting. Chin J Cancer. 33:620–624.
2014.PubMed/NCBI
|
|
21
|
Zhu Y, Liu Y, Qian Y, Dai X, Yang L, Chen
J, Guo S and Hisamitsu T: Antimetastatic effects of Celastrus
orbiculatus on humangastric adenocarcinoma by inhibiting
epithelial-mesenchymal transition and NF-κB/snail signaling
pathway. Integr Cancer Ther. 14:271–281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu Y, Liu Y, Qian Y, Dai X, Yang L, Chen
J, Guo S and Hisamitsu T: Research on the efficacy of Celastrus
Orbiculatus in suppressing TGF-β1-induced epithelial-mesenchymal
transition by inhibiting HSP27 and TNF-α-induced NF-κB/Snail
signaling pathway in human gastric adenocarcinoma. BMC Complement
Altern Med. 14:4332014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang M, Zhang X, Xiong X, Yang Z, Sun Y,
Yang Z, Hoffman RM and Liu Y: Efficacy of the Chinese traditional
medicinal herb Celastrus orbiculatus Thunb on human hepatocellular
carcinoma in an orthothopic fluorescent nude mouse model.
Anticancer Res. 32:1213–1220. 2012.PubMed/NCBI
|
|
24
|
Jeon H: Anti-metastatic effects of
celastrus orbiculatus extract in B16F10 melanoma cells. Nat Prod
Sci. 17:135–141. 2011.
|
|
25
|
Jin X and Shi YI: Isobavachalcone induces
the apoptosis of gastric cancer cells via inhibition of the Akt and
Erk pathways. Exp Ther Med. 11:403–408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nguyen KC, Willmore WG and Tayabali AF:
Cadmium tellunde quantum dots cause oxidative stress leading to
extnnsic and intrinsic apoptosis in hepatocellular carcinoma HepG2
cells. Toxicology. 306:114–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu R, Duan L, Ye L, Wang H, Yang X, Zhang
Y, Chen X, Zhang Y, Weng Y, Luo J, et al: S100A9 promotes the
proliferation and invasion of HepG2 hepatocellular carcinoma cells
via the activation of the MAPK signaling pathway. Int J Oncol.
42:1001–1010. 2013. View Article : Google Scholar : PubMed/NCBI
|