Introduction
Neurotrophic factors (NTFs) are endogenously
produced as biological mediators that regulate neural growth,
differentiation and apoptosis in the development of the nervous
system (1–3). Nerve growth factor (NGF) is the most
well-known and best characterized member of the neurotrophin family
(1,4,5), which is
structurally similar to other growth factors including
brain-derived neurotrophic factor (BDNF) (1,6,7). NGF is produced by cells located in
several brain regions, and is expressed at the highest levels in
the hippocampus, cortex (1,5,8),
cerebellum and spinal cord (1,7,9). NGF, and receptors, tropomyosin receptor
kinase A (TrkA) and p75 nerve growth factor receptors, exhibit
pleiotropic effects in inducing cell differentiation, preventing
apoptosis and supporting cell survival in neuronal progenitor cells
and immature neurons of the central nervous system (CNS) and
peripheral nervous system (1,6,10,11). Previous studies have demonstrated that
NGF and TrkA may contribute an important role in the growth
modulation of human tumor cells (12). BDNF, the second member of the NTFs to
be identified, is widely distributed in the CNS (13–17), and
is produced primarily in the hippocampus, thalamus and cerebellum
(1,18). BDNF serves an essential role in
maintaining and modulating the physiological functions of neurons
and promoting the growth, survival and metastasis of a variety of
tumor cells through binding to its receptors, tropomyosin receptor
kinase B (TrkB) and p75 nerve growth factor receptors (19–24).
Previous studies have suggested that NTFs and their
receptors are associated with cell maturation and tumoral cell
apoptosis (1,25,26). In
contrast, a number of growth factors and signaling molecules appear
to represent specific markers for distinct histological types of
tumors and may be involved in the differentiation process of
neoplasms (1). In accordance with
these distinct mechanisms of action of NTFs, the aim of the present
study was to examine the expression of NGF and BDNF in
astrocytomas, and to investigate the association between NGF, BDNF,
pathological grading and location, as well as tumorigenesis of
astrocytomas.
Materials and methods
Tissue samples
A total of 70 clinical astrocytoma samples (39 male
and 31 female, age range 21–75 years; median age 47.3 years) were
collected from The First Affiliated Hospital of Chengdu Medical
College (Chengdu, China) from February 2006 to May 2013. All
procedures were performed according to an Institutional Review
Board (IRB)-approved protocol. These samples, based on their
pathological grades, were classified as 10 cases of grade I, 24 of
grade II, 26 of grade III and 10 of grade IV. Conversely, grouped
according to the location of astrocytomas, there were 20 samples
from the temporal lobe, 16 from the frontal lobe, 18 from the
parietal lobe and 16 from the cerebellum. In addition, 15 normal
brain specimens were obtained as controls from the normal brain
tissues (7 samples from the temporal lobe, 6 from the frontal lobe,
1 from the parietal lobe and 1 from the cerebellum) of patients who
underwent surgery for cerebral trauma. The expression of NGF and
BDNF was examined in different locations in astrocytomas, not in
normal brain tissues, because it is relatively difficult to obtain
the samples of normal brain tissues from locations other than the
temporal lobe and frontal lobe.
The samples were obtained from patients during
surgery, fixed in 10% neutralized formalin for 12 h at room
temperature within 24 h of being obtained and subsequently
dehydrated in graded ethanol, embedded in paraffin and then sliced
into serial 4 µm sections. Each specimen was cut into 3 sections,
which were used for immunohistochemistry, stained with hematoxylin
and eosin together with a negative control (PBS).
Reagents
A rabbit anti-human NGF polyclonal antibody (cat.
no. MFCD07370460; 1:200 dilution) and a rabbit anti-human BDNF
polyclonal antibody (cat. no. SAB2108004; 1:200 dilution) were
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Streptavidin-Peroxidase kit (cat. no. kit-9710) and
diaminobenzidine (DAB) chromogenic reagent kit (DAB-2031) were
purchased from Maixin New Biology (Fuzhou, Fujian, China).
Immunohistochemistry
The sections were deparaffinized in xylene,
rehydrated using serial dilutions of ethanol and washed three times
in 0.1 M PBS for 5 min each time. Antigen retrieval was performed
for 15 min at 100°C in citrate buffer (10 mmol/l; pH 6.0) in a
microwave oven. The sections were then washed three times in 0.1 M
PBS for 5 min each, and incubated at room temperature in 0.2%
hydrogen peroxide for 20 min to block the action of any endogenous
peroxidase. Following a further three 5 min washes in 0.1 M PBS,
normal goat serum was added for 20 min at 37°C prior to incubation
overnight at 4°C in one of the primary antibody solutions
(polyclonal neurotrophin antisera for NGF and BDNF). Subsequently,
the sections were washed in 0.1 M PBS three times for 5 min each
prior to incubation with labeled streptavidin-biotin for 30 min at
room temperature according to the manufacturer's protocol in Maixin
New Biology. Thereafter, they were washed in 0.1 M PBS again, and
DAB chromogenic reagent was added in order to observe the
distribution of NGF and BDNF according to the manufacturer's
protocol (Maixin New Biology). Finally, all sections were mounted,
dehydrated, placed on coverslips and observed under a light
microscope at magnification, ×200.
The tumor specimens were used as the experimental
group (grade I–IV groups) with the normal brain tissue used as the
control group. Instead of using the primary antibody as a negative
control, PBS was used for both experimental and normal groups.
Microscopic examination
Each immunohistochemistry section was observed under
a light microscope (XS-212; Nanjing Jiangnan Novel Optics, Co.,
Ltd., Nanjing, China). The number of positively stained cells was
determined from 10 fields of view at magnification, ×400 and the
mean positive cell numbers were calculated independently by two
pathologists from the Department of Pathology at The First
Affiliated Hospital of Chengdu Medical College. Subsequently, the
total cell number in the same fields of view was determined and the
mean number of cells for each section was calculated.
Expression rate of positive cells=(number of
positive cells/number of total cells) ×100%.
Statistical analysis
The data and the categorical variables were analyzed
and compared using analysis of variance (ANOVA) followed by a
Student-Newman-Keuls post-hoc test, using the SPSS software package
for Windows (version 11.5; SPSS, Inc., Chicago, IL, USA). Data are
expressed as the mean ± standard deviation and P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of NGF and BDNF in normal
brain tissues and in astrocytomas of distinct pathological
grades
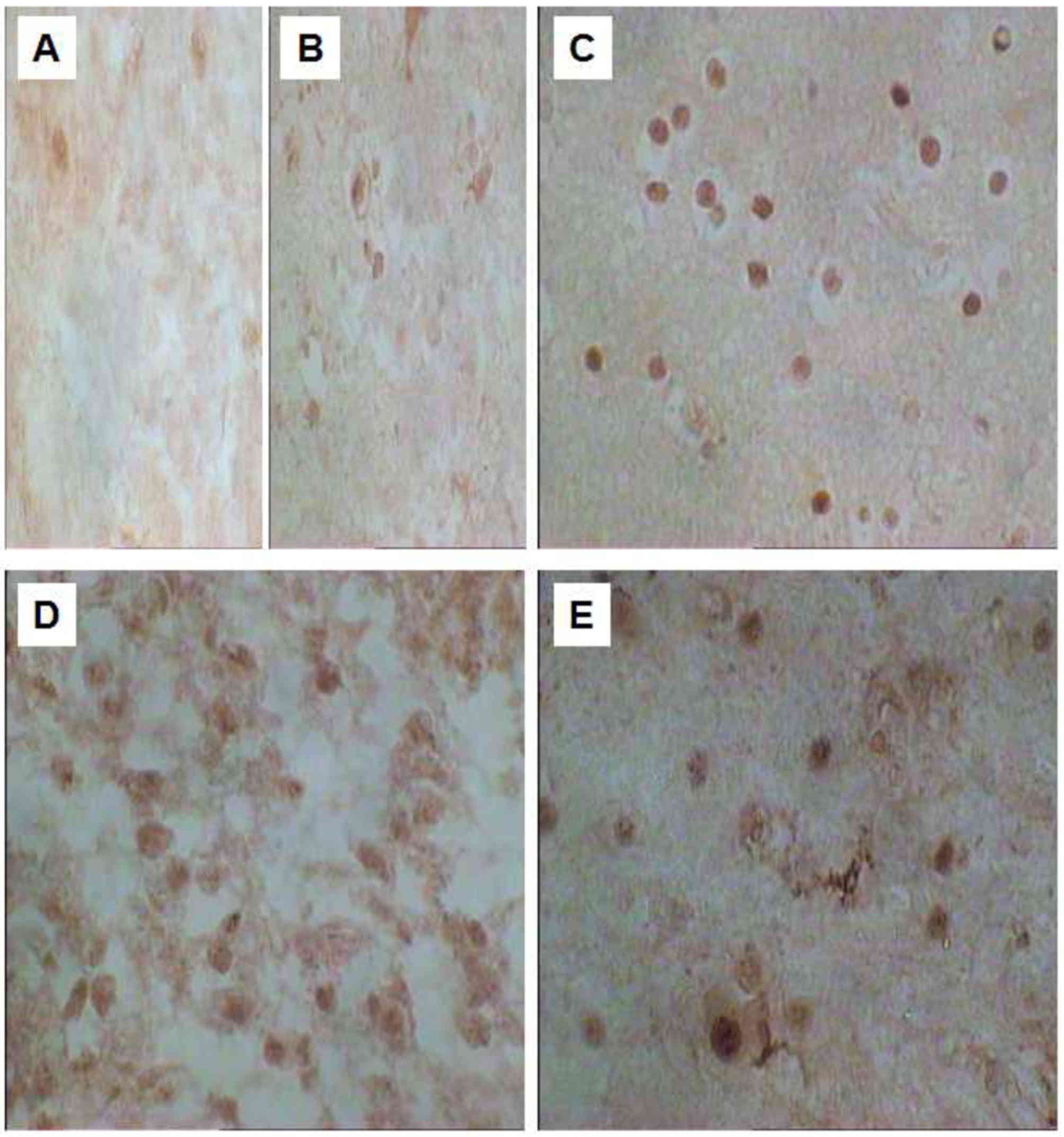
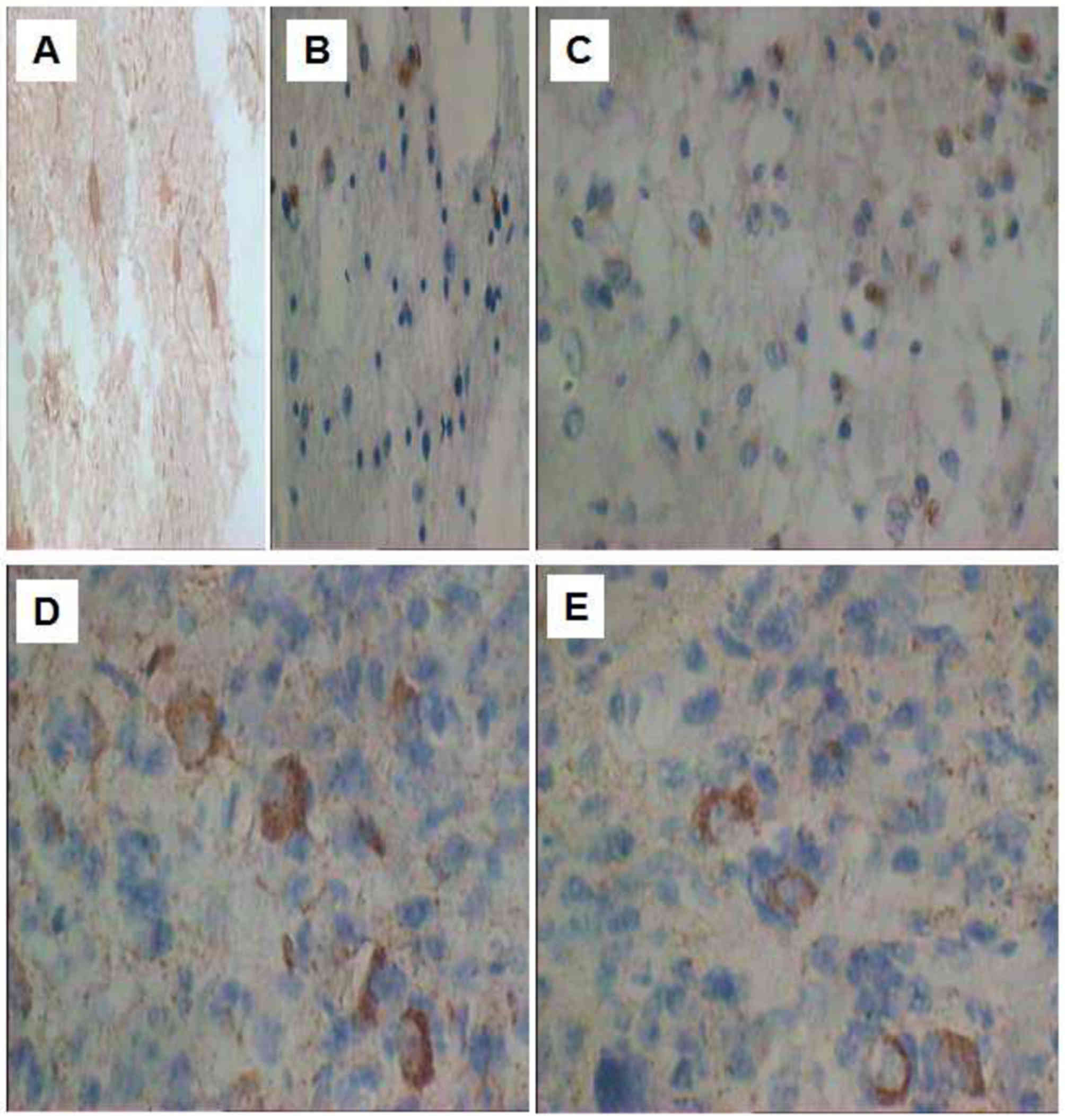
The immunopositive products of NGF (Fig. 1) and BDNF (Fig. 2) were observed in the normal brain
tissues and in the astrocytomas. The positive expression rate of
NGF in astrocytomas was significantly increased compared with that
in normal brain tissue (P<0.05). Furthermore, there was a
statistically significant difference (P<0.05) in the expression
rate of NGF- and BDNF-positive cells between distinct pathological
grades of astrocytomas, with the highest expression observed in
grade III (Table I; P<0.05).
 | Table I.Expression of NGF and BDNF in normal
brain tissue and astrocytomas of distinct pathological grades. |
Table I.
Expression of NGF and BDNF in normal
brain tissue and astrocytomas of distinct pathological grades.
| Grade | Cases | NGF expression,
% | BDNF expression,
% |
|---|
| Normal tissues | 15 |
7.06±0.95a |
5.98±0.49a |
| I | 10 |
16.56±4.56a |
11.24±2.05a |
| II | 26 |
32.45±10.07a |
18.23±4.96a |
| III | 24 |
40.91±26.40a |
21.44±3.35a |
| IV | 10 |
24.71±8.28a |
15.39±2.01a |
The staining of NGF-positive cells was observed in
the cytoplasm and nucleus, but primarily in the nucleus (Fig. 1). However, the staining of
BDNF-positive cells was primarily observed in the cytoplasm
(Fig. 2).
Expression of NGF and BDNF in
astrocytomas from distinct locations
NGF and BDNF were expressed in astrocytomas from all
locations; however, the expression rate of positive cells was
statistically different (P<0.05) between distinct locations. The
sources may be ranked in decreasing order of expression rate from
the temporal lobe, parietal lobe and cerebellum to the frontal lobe
(Table II; P<0.05).
 | Table II.Expression of NGF and BDNF from
distinct astrocytoma locations. |
Table II.
Expression of NGF and BDNF from
distinct astrocytoma locations.
| Region | Cases | NGF expression,
% | BDNF expression,
% |
|---|
| Temporal lobe | 18 |
42.57±13.57a |
24.19±7.12a |
| Parietal lobe | 16 |
35.62±9.47a |
20.09±4.56a |
| Cerebellum | 16 |
28.67±6.47a |
16.17±4.29a |
| Frontal lobe | 20 |
21.45±7.56a |
16.17±4.29a |
Discussion
NTFs serve a crucial role in a variety of distinct
types of brain cell and in their differentiation processes
(1,7–9). Previous
studies have suggested that NTFs may be associated with certain
biochemical and molecular mechanisms of carcinogenesis and growth
signaling pathways, undergoing deregulation in numerous human
malignancies, including neuroectodermal brain tumors, testicular
germ cell tumors and prostate cancer (1,27–30). However, little has been known
previously regarding the association between the expression of NGF
and BDNF, pathological grading, and region of astrocytomas. The
results of the present study demonstrated that NGF and BDNF levels
were increased in astrocytomas compared with the controls, and were
associated with the pathological grading and region of
astrocytomas.
The results of the present study revealed that NGF
and BDNF were overexpressed in astrocytomas and were associated
with the location, generation, progression and pathological grade
of the astrocytoma. Goustin et al (31) demonstrated that the amount of serum
required in the process of tumor cell growth was decreased and
there was a ubiquitous decreasing demand for growth factors from
extrinsic sources in numerous types of cancer cell. Specifically,
the decrease may be restored via activating the autocrine pathway,
altering the synthesis of growth factor receptors and activating
the post-receptor machinery (31).
The results of the present study demonstrated that the expression
of NGF and BDNF were significantly upregulated gradually from
normal tissues to grade I, II and III astrocytomas. This suggested
that an autocrine pathway may be activated in the process of
astrocytoma tumorigenesis and the proliferation of cancer cells was
accelerated by synthesizing autocrine growth factors. However, the
expression of NGF and BDNF in grade IV astrocytomas decreased
suddenly, which was significantly different from in grade I, II and
III astrocytomas. This may have resulted from the tumor cells no
longer requiring the activation of the NGF receptor pathway.
Therefore, it is hypothesized that the overexpression of NGF and
BDNF may be the early and middle events in astrocytoma
tumorigenesis.
In normal brain, the in situ hybridization
experiment verified that NGF mRNA is expressed in pyramidal cells,
granular cells in the hippocampus, stellate cells, oligodendrocytes
and certain cortical neurons. NGF is produced by cells located in a
number of brain regions, and is expressed in the highest amounts in
the hippocampus and cortex (5,8). BDNF is
produced primarily in the hippocampus, thalamus and cerebellum
(18). The results of the present
study identified that the expression of NGF and BDNF exhibited a
statistically significant gradual decrease in the temporal lobe
near the hippocampus, parietal lobe near the cerebral cortex,
cerebellum and frontal lobe respectively. This phenomenon suggested
that hippocampal and cortical tissues are activated to synthesize
and release NGF and BDNF in the formation of astrocytomas.
NGF and BDNF are able to promote the survival and
differentiation of neurons and contribute a key role in the
reparative process of injured nerve cells. Multiple previous
studies have also demonstrated that NGF and BDNF and their
receptors are associated with tumor biology, behavior and
progression (1,29,32),
although the underlying molecular mechanism of tumor progression
remains unclear. Zhu et al (33) identified that NGF and TrkA were
notably overexpressed in pancreatic cancer tissues compared with in
normal pancreatic tissue, and increased NGF/TrkA expression was
associated with the invasion and the pain caused by the tumor,
although it was not associated with the degree of tumor
differentiation or grading. Furthermore, NGF is notably
downregulated in esophageal carcinoma tissues and is associated
with the degree of tumor differentiation as well as its
pathological grades. Low TrkA expression was associated with
advanced tumor stage (34). In
addition, NGF mRNA and protein are expressed in breast cancer, but
not in normal breast tissue, and may be inhibited by
NGF-neutralizing antibodies or a TrkA-blocking agent such as K-252a
(35). A study by Breit et al
(36) demonstrated that TrkA was
associated with the differentiation, generation and angiogenesis of
neuroblastoma (NB). Another study demonstrated that the TrkA gene
is a good prognostic marker in NB and inhibited NB hyperplasia,
induced benign differentiation and affected the growth of blood
vessels of NB (37). NGF and TrkA
were also observed to be decreased, whereas p75 was increased in
childhood low-grade astrocytomas and ependymoma tissue (1). Similarly, BDNF and TrkB are expressed in
multiple myeloma cell lines (38).
BDNF was also identified to induce cell migration and cell
proliferation in cultured human ovarian cancer cells (39). In addition, the level of BDNF in serum
was significantly associated with tumor size in patients with
hepatocellular carcinoma (19). These
studies and the results of the present study revealed that NGF and
BDNF, with receptors, participate in tumorigenesis.
The results of the present study indicate that NGF
and BDNF overexpression in astrocytomas and their positive cell
rate may be ranked in decreasing order from grade III, II, IV and
I, and, finally, normal brain tissue. This suggests that NGF and
BDNF are involved in the process of astrocytoma tumorigenesis, and
the overexpression of NGF and BDNF may occur at early and middle
stages of astrocytoma formation. The results of the present study
also identified that the closer to the hippocampal and cortical
areas, the higher the expression rate of positive cells for NGF and
BDNF in astrocytomas. This may be that the astrocytoma stimulates
astrocyte cells to secrete NGF and BDNF during the process of
astrocytoma formation, and the hippocampal and cortical regions
produce growth factors including NGF and BDNF.
In conclusion, the results of the present study
suggest that NGF and BDNF are overexpressed in astrocytomas, with
associations with the pathological grade as well as the location of
the astrocytomas.
Acknowledgements
The authors would like to thank Professor Yuan Chun
Fan and Dr Liang Jiang, from the Department of Pathology at The
First Affiliated Hospital of Chengdu Medical College (Chengdu,
China) for their technical assistance and helpful discussion.
References
|
1
|
Chiaretti A, Aloe L, Antonelli A, Ruggiero
A, Piastra M, Riccardi R, Tamburrini G and Di Rocco C: Neurotrophic
factor expression in childhood low-grade astrocytomas and
ependymomas. Childs Nerv Syst. 20:412–419. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aloe L, Tirassa P and Bracci-Laudiero L:
Nerve growth factor in neurological and non-neurological diseases:
Basic findings and emerging pharmacological prospectives. Curr
Pharm Des. 7:113–123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Drago J, Kilpatrick TJ, Koblar SA and
Talman PS: Growth factor: Potential therapeutic applications in
neurology. J Neurol Neurosurg Psychiatry. 57:1445–1450. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levi-Montalcini R: The nerve growth factor
35 years later. Science. 237:1154–1162. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thoenen H, Bandtlow C and Heumann R: The
physiological function of nerve growth factor in the central
nervous system: Comparison with the periphery. Rev Physiol Biochem
Pharmacol. 109:145–178. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barde YA: Neurotrophins: A family of
proteins supporting the survival of neurons. Prog Clin Biol Res.
390:45–56. 1994.PubMed/NCBI
|
|
7
|
Sofroniew MV, Howe CL and Mobley WC: Nerve
growth factor signaling, neuroprotection, and neural repair. Annu
Rev Neurosci. 24:1217–1281. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Korsching S, Auburger G, Heumann R, Scott
J and Thoenen H: Levels of nerve growth factor and its mRNA in the
central nervous system of the rat correlate with cholinergic
innervation. Embo J. 4:1389–1393. 1985.PubMed/NCBI
|
|
9
|
Connor B and Dragunow M: The role of
neuronal growth factors in neurodegenerative disorders of the human
brain. Brain Res Brain Res Rev. 27:1–39. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levi-Montalcini A and Angeletti PU: Nerve
growth factor. Physiol Rev. 48:534–569. 1968.PubMed/NCBI
|
|
11
|
Chao MV and Hempstead BL: p75 and Trk: A
two-receptor system. Trends Neurosci. 18:321–326. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Z, Yang Y, Gong A, Wang C, Liang Y
and Chen Y: Localization of NGF and TrkA at mitotic apparatus in
human astrocytoma cell line u251. Biochem Biophys Res Commun.
337:68–74. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Friedman WJ, Ibáñez CF, Hallböök F,
Persson H, Cain LD, Dreyfus CF and Black IB: Differential actions
of neurotrophins in the locus corelueus and basal forebrain. Exp
Neurol. 119:72–78. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mamounas LA, Blue ME, Siuciak JA and Altar
CA: Brain-derived neurotrophic factor promotes the survival and
sprouting of serotonergic axons in rat brain. J Neurosci.
15:7929–7939. 1995.PubMed/NCBI
|
|
15
|
Siuciak JA, Altar CA, Wiegand SJ and
Lindsay RM: Antinociceptive effect of brain-derived neurotrophic
factor and neurotrophin-3. Brain Res. 633:326–330. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sklair-Tavron L and Nestler EJ: Opposing
effects of morphine and the neurotrophins, NT-3, NT-4, and BDNF, on
locus coeruleus neurons in vitro. Brain Res. 702:117–125. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Juric DM, Miklic S and Carman-Krzan M:
Monoaminergic neuronal activity up-regulates BDNF synthesis in
cultured neonatal rat astrocytes. Brain Res. 1108:54–62. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baloh RH, Enomoto H, Johnson EM Jr and
Milbrandt J: The GDNF family ligands and receptors-implications for
neural development. Curr Opin Neurobiol. 10:103–110. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang ZF, Ho DW, Lau CK, Tam KH, Lam CT, Yu
WC, Poon RT and Fan ST: Significance of the serum brain-derived
neurotrophic factor and platelets in hepatocellular carcinoma.
Oncol Rep. 16:1237–1243. 2006.PubMed/NCBI
|
|
20
|
Tapia-Arancibia L, Rage F, Givalois L and
Arancibia S: Physiology of BDNF: Focus on hypothalamic function.
Front Neuroendocrinol. 25:77–107. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsumoto K, Yamamoto K, Karasawa Y, Hino
N, Nakamura A, Takahashi M, Araki H, Okuyama S, Choshi T, Sugino E,
et al: Possible involvement of induction of brain-derived
neurotrophic factor in the neuroprotective effect of a
5-phenylpyrimidine derivative. Biochem Pharmacol. 66:1019–1023.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Renné C, Willenbrock K, Küppers R,
Hansmann ML and Bräuninger A: Autocrine and paracrine-activated
receptor tyrosine kinases in classic hodgkin lymphoma. Blood.
105:4051–4059. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pearse RN, Swendeman SL, Li Y, Rafii D and
Hempstead BL: A neurotrophin axis in myeloma: TrkB and BDNF promote
tumor-cell survival. Blood. 105:4429–4436. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Douma S, Van Laar T, Zevenhoven J,
Meuwissen R, Van Garderen E and Peeper DS: Suppression of anoikis
and induction of metastasis by the neurotrophic receptor TrkB.
Nature. 430:1034–1039. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grotzer MA, Janss AJ, Fung K, Biegel JA,
Sutton LN, Rorke LB, Zhao H, Cnaan A, Phillips PC, Lee VM and
Trojanowski JQ: TrkC expression predicts good clinical outcome in
primitive neuroectodermal brain tumors. J Clin Oncol. 18:1027–1035.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Segal RA, Goumnerova LC, Kwon YK, Stiles
CD and Pomeroy SL: Expression of the neurotrophin receptor TrkC is
linked to a favorable outcome in medulloblastoma. Proc Natl Acad
Sci USA. 91:pp. 12867–12871. 1994; View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chiappa SA, Chin LS, Zurawel RH and Raffel
C: Neurotrophins and Trk receptors in primitive neuroectodermal
tumor cell lines. Neurosurgery. 45:1148–1155. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Colucci-D'Amato GL, D'Alessio A, Califano
D, Cali G, Rizzo C, Nitsch L, Santelli G and de Franciscis V:
Abrogation of nerve growth factor-induced terminal differentiation
by ret oncogene involves perturbation of nuclear translocation of
ERK. J Biol Chem. 275:19306–19314. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Devouassoux-Shisheboran M, Mauduit C,
Tabone E, Droz JP and Benahmed M: Growth regulatory factors and
signalling proteins in testicular germ cell tumours. APMIS.
111:212–224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eggert A, Sieverts H, Ikegaki N and
Brodeur GM: P75 mediated apoptosis in neuroblastoma cells is
inhibited by expression of TrkA. Med Pediatr Oncol. 35:573–576.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goustin AS, Leof EB, Shipley GD and Moses
HL: Growth factors and cancer. Cancer Res. 46:1015–1029.
1986.PubMed/NCBI
|
|
32
|
Nishio S, Morioka T, Hamada Y, Fukui M and
Nakagawara A: Immunohistochemical expression of tyrosine kinase
(Trk) receptor proteins in mature neuronal cell tumors of the
central nervous system. Clin Neuropathol. 17:123–130.
1998.PubMed/NCBI
|
|
33
|
Zhu Z, Friess H, diMola FF, Zimmermann A,
Graber HU, Korc M and Büchler MW: Nerve growth factor expression
correlates with perineural invasion and pain in human pancreatic
cancer. J Clin Oncol. 17:2419–2428. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu ZW, Friess H, Wang L, Di Mola FF,
Zimmermann A and Büchler MW: Down-regulation of nerve growth factor
in poorly differentiated and advanced human esophageal cancer.
Anticancer Res. 20:125–132. 2000.PubMed/NCBI
|
|
35
|
Dollé L, ElYazidi-Belkoura I, Adriaenssens
E, Nurcombe V and Hondermarck H: Nerve growth factor overexpression
and autocrine loop in breast cancer cells. Oncogene. 22:5592–5601.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Breit S, Ashman K, Wilting J, Rössler J,
Hatzi E, Fotsis T and Schweigerer L: The N-myc oncogene in human
neuroblastoma cells: Down-regulation of an angiogenesis inhibitor
identified as activin. Cancer Res. 60:4596–4601. 2000.PubMed/NCBI
|
|
37
|
Zhang J, Zhang J, Li A and Fan Y: Role of
nerve growth factor receptor in neuroblastoma angiogenesis. Chinese
J Contemporary Pediatrics. 6:93–98. 2004.
|
|
38
|
Hu Y, Sun CY, Wang HF, Guo T, Wei WN, Wang
YD, He WJ, Wu T, Tan H and Wu TC: Brain-derived neurotrophic factor
promotes growth and migration of multiple myeloma cells. Cancer
Genet Cytogenet. 169:12–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qiu L, Zhou C, Sun Y, Di W, Scheffler E,
Healey S, Kouttab N, Chu W and Wan Y: Crosstalk between EGFR and
TrkB enhances ovarian cancer cell migration and proliferation. Int
J Oncol. 29:1003–1011. 2006.PubMed/NCBI
|