Introduction
Bladder urothelial carcinoma (BUC) is a common
malignant bladder cancer, the fourth leading cause of cancer in
males, accounting for ~4% of the cancer-related deaths (1). Despite the surgical treatment of
transurethral resection (TUR)and postoperative recovery pathways,
the incidence of bladder recurrence within 5 years could be up to
20–75% worldwide (2). BUC is staged
via the tumor-node-metastasis (TNM) system, which describes the
degree of invasion (Tis-T4) (3).
Approximately, 75% of the BUC patients present non-muscle-invasive
bladder cancer (NMIBC; stage Ta-1). Surgical removal by TUR of
bladder tumor is still the major treatment of NMIBC; yet, half of
NMIBC patients treated with TUR have a recurrence of the disease
and 5–25% progressed to muscle-invasive bladder cancer (MIBC, stage
T2 and above) after repeated recurrences (4). Patients with MIBC are at high risk of
local invasion and distant metastasis, despite radical cystectomy
and pelvic lymph-node dissection, up to 50% of patients still
develop tumours at distant sites and exhibit a less favorable
prognosis with a 5-year survival of <50% (5,6). Hitherto,
a number of factors have been reported as potential targets and/or
prognostic markers, such as MAP4K1, Karyopherin α 2 (KPNA2),
thymosin β4 (Tβ4), Her2/neu, and microRNAs (7–12).
However, substantiated pathological or clinical tests to predict
the response are yet lacking. Herein, we sought to identify the
responsive factors, including cell cycle checkpoint kinase 1 (CHK1)
and p53, which are reported less in BUC with respect to their
advantages.
CHK1 is an enzyme, essential for preventing mitosis
in response to DNA damage, primarily responding to replication fork
interference in the S-phase and DNA damage in the G2 phase
(13,14). CHK1 kinase regulates the cell cycle
progression from S to M phase following disruption of DNA
replication or some types of DNA damage and has also been reported
to play a role in the S phase in undisturbed cells (15,16). Some
studies indicated that CHK1-deficient tumor cells exhibit multiple
defects, such as the loss of response to cell cycle checkpoint
arrest, retarded cell proliferation, and increased sensitivity to
DNA-damaging agents (17,18). Thus, understanding the status of the
pathways is crucial for effective targeted therapies against the
progression of BUC.
p53, one of the major tumor suppressors, similar to
CHK1, exerts several mechanisms underlying the anticancer function
and also plays a role in apoptosis, genomic stability, and
inhibition of angiogenesis (19). p53
occurs as wild-type and mutant isoforms. The antitumor activity of
wild-type p53 primarily enabling the DNA-damaged cells into G1/G0
arrest and DNA repair process before entering the S phase in
anti-cancer mechanisms is carried out by i) activating the DNA
repair proteins when DNA has sustained damage; ii) arresting the
cell growth by stalling the cell cycle at the G1/S checkpoint upon
recognition of DNA damage; iii) initiating apoptosis if DNA damage
proves to be irreparable (20,21). On
the other hand, the mutant p53 loses its antitumor function and
induces abnormal gene expression, thereby leading to tumor
progression (21,22). Although many reports described an
abnormal p53 in BUC, whether it could serve as a prognostic factor
in BUC is still unclear.
In the study, we examined CHK1 and p53 in BUC
specimens and peritumoral tissues, investigated their expression
and interaction in different histological grades, clinical
pathological staging, and 5-year survival rate. In addition, we
also assessed their value as potential therapeutic targets and for
prognosis in BUC.
Materials and methods
Specimens and clinical data
A total of 110 specimens of bladder cancer and 45
peritumoral bladder tissues (the adjacent normal tissues >2 cm
from the cancer tissue) were collected between 2009 and 2014 at the
Zhejiang Cancer Hospital (Zhejiang, China). The detailed
clinicopathological data including age, sex, TNM stage,
histological subtype and grade, tumor diameter, single/multiple
sites, and patients with incipience/recurrence were assimilated
(Table I). Tumor grades were
determined based on the 2016 World Health
Organization/International Society of Urologic Pathology
classification (23); the pathologic
stage was assigned according to the 2010 American Joint Committee
on Cancer 7th TNM staging system (24). The specimens were frozen at −80°C or
fixed in 10% formalin, embedded in paraffin, sliced continually,
and subjected to hematoxylin and eosin (H&E) and
immunohistochemistry (IHC) staining. This reterospective study has
been approved by the Ethics Committee of the Zhejiang Cancer
Hospital.
 | Table I.Clinicopathological characteristics of
BUC patients. |
Table I.
Clinicopathological characteristics of
BUC patients.
| Characteristic | N | CHK1-positive cases
(%) |
χ2-value | P-value | p53-positive cases
(%) |
χ2-value | P-value |
|---|
| Sex |
| Male | 94 | 70 (74.5) | 0.41 | 0.63 | 49 (43.6) | 0.002 | 0.96 |
|
Female | 16 | 11 (68.5) |
|
| 9 (50.0) |
|
|
| Age, years |
|
<63 | 48 | 35 (72.9) | 1.17 | 0.27 | 20 (41.7) | 2.40 | 0.12 |
| ≥63 | 62 | 46 (74.2) |
|
| 38 (61.3) |
|
|
| Histologic grade |
| High
grade | 47 | 31 (66.0) | 7.54 | 0.006 | 23 (48.9) | 4.45 | 0.016 |
| Low
grade | 63 | 50 (79.3) |
|
| 35 (55.6) |
|
|
| Pathologic T |
|
Ta-T1 | 50 | 32 (64.0) | 4.54 | 0.032 | 17 (34.0) | 5.46 | 0.019 |
|
T2-T4 | 60 | 45 (72.3) |
|
| 36 (60.0) |
|
|
| Lymphatic
metastasis |
|
Positive | 51 | 42 (82.4) | 5.07 | 0.024 | 36 (70.1) | 4.89 | 0.027 |
|
Negative | 59 | 39 (66.1) |
|
| 22 (37.3) |
|
|
| Tumor diameter,
cm |
|
<2 | 18 | 11 (61.1) | 0.002 | 0.96 | 7 (38.9) | 0.12 | 0.65 |
| ≥2 | 92 | 70 (76.1) |
|
| 51 (55.4) |
|
|
| Numbers of
tumor |
|
Single | 47 | 34 (72.3) | 0.003 | 0.82 | 21 (44.9) | 1.28 | 0.27 |
|
Multiple | 64 | 47 (73.4) |
|
| 37 (57.9) |
|
|
| Tumor
formation |
| No
recurrence | 78 | 63 (80.8) | 0.44 | 0.51 | 45 (57.8) | 0.27 | 0.61 |
|
Recurrence | 32 | 18 (56.3) |
|
| 13 (40.6) |
|
|
IHC
Mouse anti-human monoclonal antibodies against CHK1
and p53 were purchased from Santa Cruz Biotechnology (Santa Cruz,
CA, USA). IHC EnVision method was performed as follows: 4 µm
paraffin-embedded tissue sections were mounted on glass slides.
After deparaffinization in xylene, the slides were immersed in a
target retrieval buffer solution; subsequently, the slides were
treated with EDTA buffer (pH 9.0) bath. The endogenous peroxidase
was blocked by incubation in methanol containing 0.3%
H2O2 for 30 min. IHC staining was performed
using the EnVision System (EnVision+; Dako, Carpentaria,
CA, USA). The slides were incubated overnight at 4°C with primary
antibody against CHK1 and p53. followed by immersion in
diaminobenzidine (DAB) for signal visualization. The negative
control slides were probed with phosphate buffered saline (PBS)
instead of the primary antibody.
Western blot analysis
Total protein from BUC or normal tissues was
extracted using RIPA buffer containing the protease inhibitor PMSF
(Bocai Bio Company, Shanghai, China) and quantified by
bicinchoninic acid (BCA) method (Pierce, Rockford, IL, USA). The
protein samples were resolved on 8% SDS-polyacrylamide gel and
transferred to PVDF membrane (Amersham, Buckinghamshire, UK). The
membranes were blocked with 5% dry milk for 1 h in Tris-buffered
saline and 0.1% Tween-20 (TBS/Tween-20) (Dako). After washing in
TBS/Tween-20, the membranes were probed with rabbit polyclonal CHK1
(1:2,000), or mouse monoclonal p53 (1:2,000), or mouse monoclonal
GAPDH (1:5,000; all Abcam, Cambridge, UK) antibodies overnight at
4°C. After washing with TBS/Tween-20, the membranes were probed
with HRP-conjugated rabbit anti-mouse or goat anti-rabbit IgG
(1:5,000; Abcam) for 1 h at room temperature and washed with
TBS/Tween-20. The immunoreactive bands on the membrane were
detected using ECL Plus™ Western Blotting Detection Reagents
(Amersham). The signal of western blotting band was quantified
using gray-scale analysis software and data was normalized to
GAPDH.
Determination of results
CHK1 is expressed mainly in the cytoplasm, also
slightly in the nucleus, whereas p53 is primarily expressed in the
nucleus. Semi-quantitative results were determined according to the
percentage and intensity of the staining pattern of positive cells:
i) According to the percentage of positive cells in the total
counted cells, the scores were divided into 4 levels: 0, positive
cells <10%; 1, positive cells moderate 10–24%; 2, positive cells
moderate 25–49%; 3, positive cells moderate 50–74%; 4, positive
cells >75%. ii) According to the intensity of the staining
pattern, the scores were ascribed as follows: 0, colorless; 1,
yellow; 2, brown; 3, tan. The cell staining was comprehensively
judged based on the multiplication of scores of the positive cells
and the intensity of staining: 0–1, negative; ≥2, positive.
Statistical methods. SPSS 21.0 (IBM SPSS, Armonk,
NY, USA) was used to analyze the data and determine the
correlations among various parameters. The chi-square and Fisher's
exact tests were applied to determine the statistical significance,
and Spearman's rank correlation test was employed for determining
the relationship among CHK1 and p53 in BUC. Kaplan-Meier survival
analysis and log-rank test were used for determining the survival
curves. P<0.05 was considered to indicate a statistically
significant difference.
Results
CHK1 and p53 are expressed
significantly in BUC than in peritumoral tissue, showing a large
difference between Ta-T1 and T2-T4 groups
The positive rates of CHK1 in BUC and peritumoral
tissues were 73.6% (81/110) and 6.7% (3/45), respectively, and that
of p53 were 52.7% (58/110) and 2.2% (1/45), respectively. The
differences were statistically significant (P<0.05) (Table II). IHC demonstrated that CHK1 and
p53 were expressed significantly in both Ta-T1 and T2-T4 groups
than the normal group. In addition, CHK1 and p53 were stained
deeply in T2-T4 than in Ta-T1 group, indicating significant
differences. A total of 110 cases were checked and the
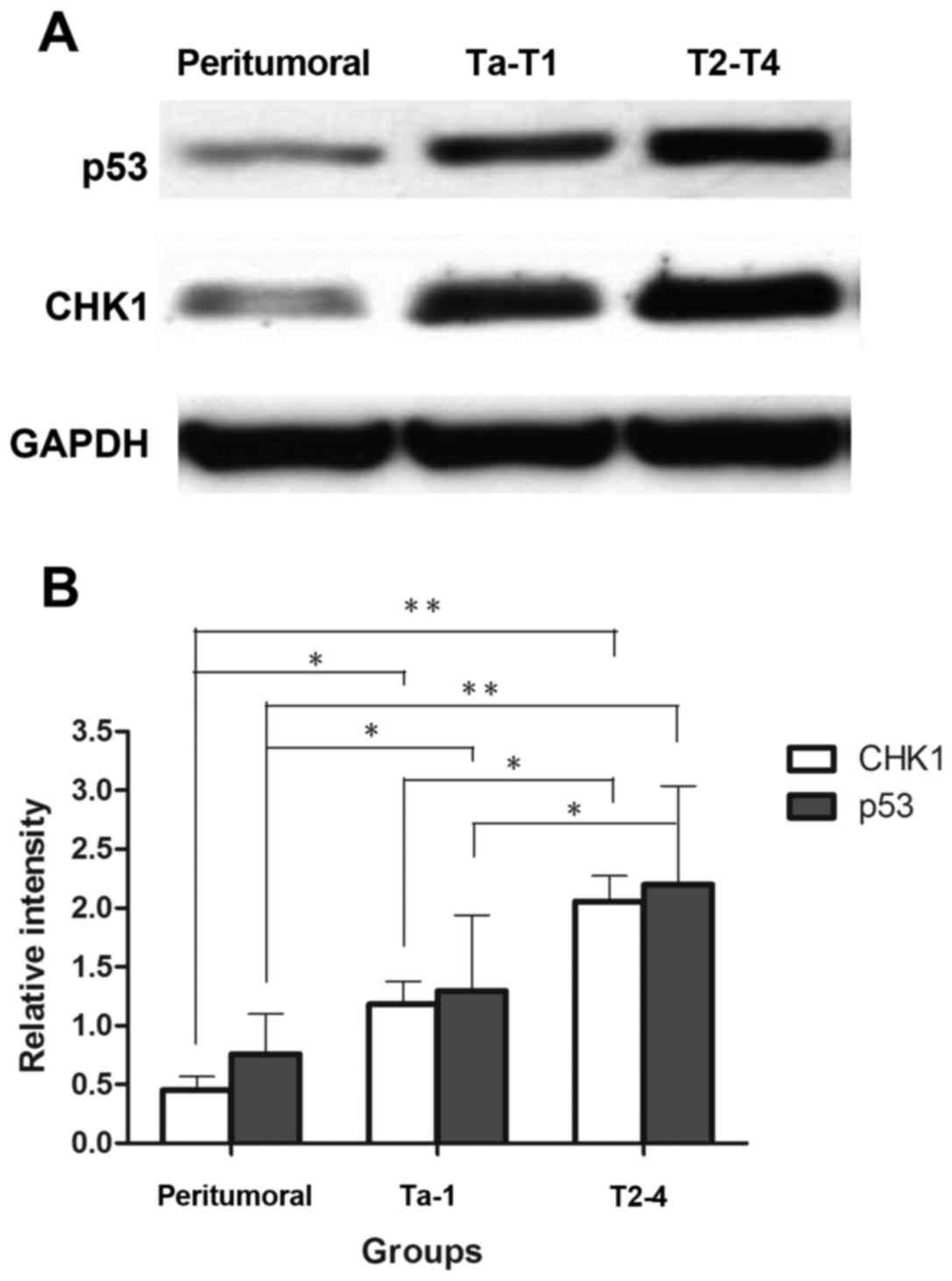
representative pictures were shown in (Fig. 1). For further assessment, we examined
protein expression of four cases in each group by western blot
analysis, the representative bands were selected to be
demonstrated; and the intensity of the immunoreactive bands
markedly supported the previous results (Fig. 2).
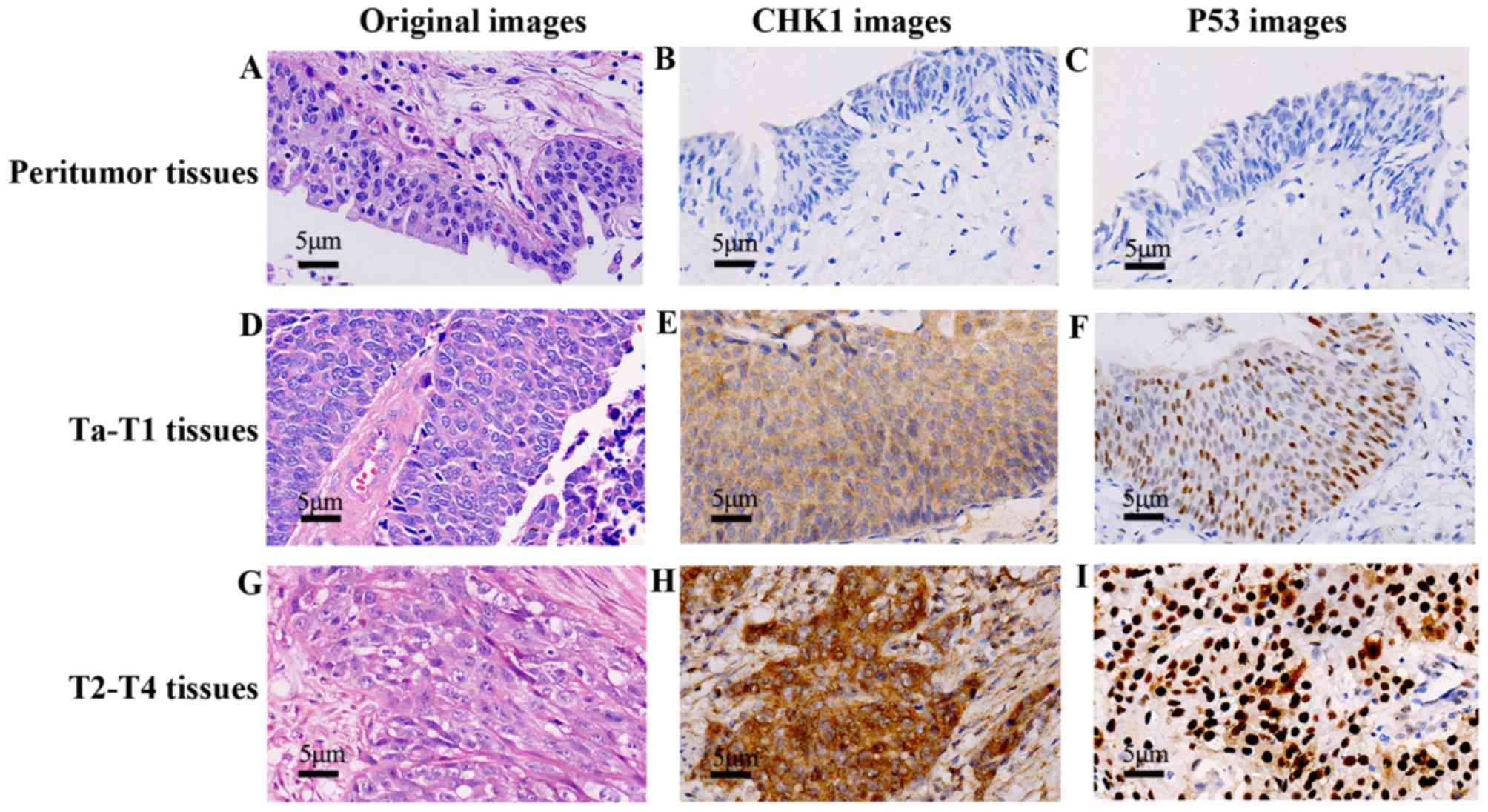 | Figure 1.CHK1 and p53 expression profiles in
peritumoral, Ta-T1, and T2-T4 BUC tissues, respectively. A total of
110 cases were checked in immunohistochemistry EnVision method
(×400), and the representative pictures were shown. In the
peritumoral group, we checked the H&E staining (A) IHC staining
with CHK1 (B) and p53 (C) respectively, both the CHK1 and p53
displayed a negative expression. In Ta-T1 group, H&E staining
showed atypical tumor cells (e.g., large, irregular and partial
nucleus) (D) IHC staining with CHK1 (E) and p53 (F) were positive,
the location and depth of color were preponderant than the
peritumoral group (B and C). In T2-T4 group, H&E staining
displayed cancer nests (G) CHK1 (H) and p53 (I) in IHC were stained
intensely than the Ta-T1 group (E and F). |
 | Table II.Expression of CHK1 and p53 proteins
in the normal or BUC tissues. |
Table II.
Expression of CHK1 and p53 proteins
in the normal or BUC tissues.
| Group | Total number | CHK1-positive cases
(%) |
χ2-value | P-value | p53-positive cases
(%) |
χ2-value | P-value |
|---|
| Normal | s45 | 3 (6.7) | 6.53 | 0.011 | 1 (2.2) | 7.03 | 0.008 |
| BUC | 110 | 81 (73.6) |
|
| 58 (52.7) |
|
|
|
Ta-T1 | 50 | 32 (64.0) | 4.54 | 0.032 | 17 (34.0) | 5.46 | 0.019 |
|
T2-T4 | 60 | 49 (81.7) |
|
| 41 (68.3) |
|
|
Expressions of CHK1 and p53 proteins are related
with clinical pathological stage, histological grade, and lymphatic
metastasis in BUC. We demonstrated that the positive rate of CHIK1
in T2-T4 (81.7%) was higher than in Ta-T1 period (64.0%) and that
of p53 was 68.3% in T2-T4 and 34.0% in Ta-T1 BUC. In the
histological grade, CHK1 occupied 79.3 and 66% in the low and high
grade, respectively, whereas p53 was 55.6% in low grade and 48.9%
in high. Furthermore, in 51 cases with lymphatic metastasis, CHK1
showed a positive rate of 82.4%, whereas p53 was 70.3%. All the
data were statistically significant (P<0.05). However, the
protein expression did not correlate with sex, age, tumor diameter,
single/multiple sites, and patients with incipience/recurrence
(Tables I, II).
Positive correlation of CHK1 and p53
expressions in BUC
The positive correlation between CHK1 and p53
(r=0.480, P<0.05) demonstrated a synergistic influence in BUC
development (Table III).
 | Table III.Correlation between CHK1 and p53 in
BUC. |
Table III.
Correlation between CHK1 and p53 in
BUC.
|
| CHK1 |
|
|
|---|
|
|
|
|
|
|---|
| p53 | + | − |
χ2-value | P-value |
|---|
| + | 48 | 10 | 5.259 | 0.022 |
| − | 33 | 19 |
|
|
CHK1 and p53 as potential prognostic
factors for BUC
In the cohort of 110 BUC cases, the shortest
survival time was 7 months and the longest was 82 months, with an
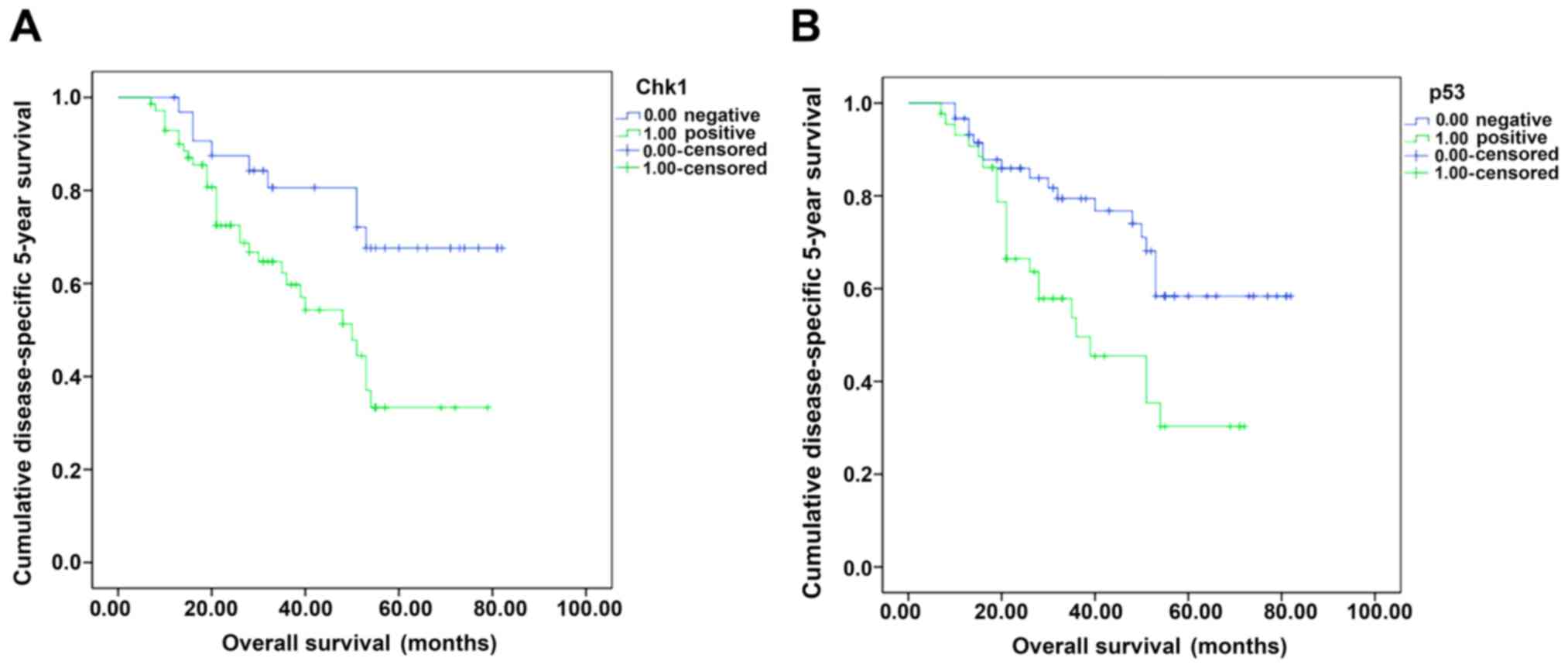
average of 55.16 months and median 53 months. Kaplan-Meier and the
log-rank test analyses showed that the overall 5-year survival rate
was 62.7% (67/110). The 5-year survival rate of CHK1-positive and
negative cases was 56.8 and 75.0%, respectively, with a significant
difference (χ2=6.98, P=0.008) (Fig. 3). With respect to p53, the 5-year
survival rate of positive and negative cases was 47.7 and 72.7%,
respectively (χ2=7.63, P=0.006) (Fig. 3B). In further, we divided the cohort
into NMIBC (Ta-1 in pathologic, 50 cases) and MIBC (T2-4 in
pathologic, 60 cases) groups, and further in sub-groups according
the markers: CHK1 positive and p53 positive (NMIBC, 8 cases; MIBC,
27 cases); CHK1 positive and p53 negative (NMIBC, 24 cases; MIBC,
18 cases); CHK1 negative and p53 positive (NMIBC, 9 cases; MIBC,9
cases); CHK1 negative and p53 negative (NMIBC, 9 cases; MIBC, 6
cases). The data in NMIBC group indicated that CHK1 negative and
p53 negative sub-group had obvious advantage in the 5-year survival
rate than other sub-groups (χ2=18.97, P<0.001)
(Fig. 4A). However, in MIBC group,
the 5-year survival rate did not had significant difference among
the sub-groups (χ2=0.34, P=0.952) (Fig. 4B). Furthermore, we checked the
multivariate Cox analyses to elucidate the prognostic effects of
the biological marker, the data revealed that only metastasis was
an independent prognostic risk factor (P<0.001) (Table IV).
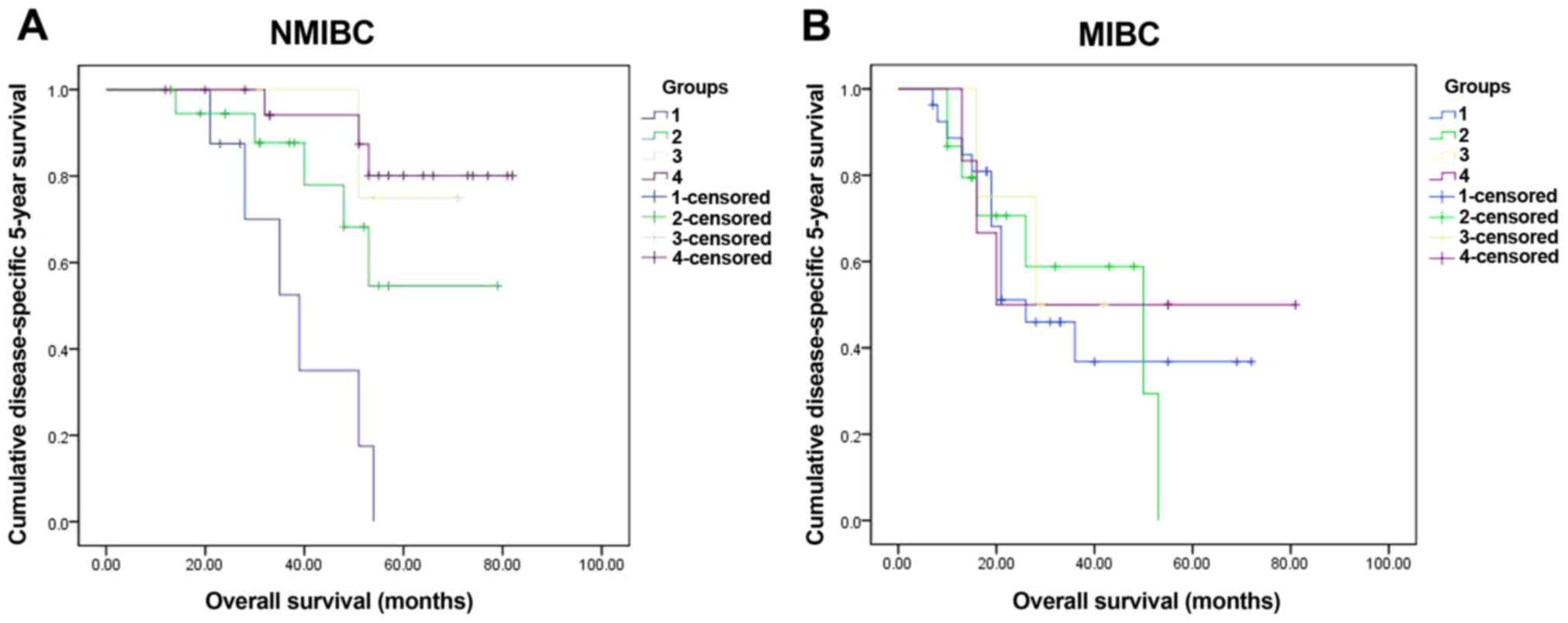 | Figure 4.The 5-year survival rate of sub-groups
in NMIBC (50 cases) and MIBC (60 cases). Four sub-groups were
divided according to the markers: group 1, CHK1 positive and p53
positive (NMIBC, 8 cases; MIBC, 27 cases); group 2, CHK1 positive
and p53 negative (NMIBC, 24 cases; MIBC, 18 cases); group 3, CHK1
negative and p53 positive (NMIBC, 9 cases; MIBC, 9 cases); group 4,
both CHK1 and p53 were negative (NMIBC, 9 cases; MIBC, 6 cases).
Significant differences were checked in NMIBC (χ2=18.97,
P<0.001) (A) yet in MIBC no significant differences
(χ2=0.34, P=0.952) (B). CHK1, checkpoint kinase 1;
NMIBC, non-muscle-invasive bladder cancer; MIBC, muscle-invasive
bladder cancer. |
 | Table IV.Univariate and multivariate Cox
regression analyses estimating the associations of CHK1 and p53
expression with patient survival. |
Table IV.
Univariate and multivariate Cox
regression analyses estimating the associations of CHK1 and p53
expression with patient survival.
| Characteristic | Crude HR | 95% CI | P-value | Adjust HR | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
<63 | 1 |
|
|
|
|
|
|
≥63 | 2.45 | 1.28–4.67 | 0.006 |
|
|
|
| Pathologic T |
|
|
|
|
|
|
|
Ta-T1 | 1 |
|
|
|
|
|
|
T2-T4 | 2.40 | 1.30–4.43 | 0.005 |
|
|
|
| Lymphatic
metastasis |
|
|
|
|
|
|
|
Negative | 1 |
|
| 1 |
|
|
|
Positive | 5.45 | 2.80–10.61 | <0.001 | 4.75 | 2.39–9.44 | <0.001 |
| p53 |
|
|
|
|
|
|
|
Negative | 1 |
|
|
|
|
|
|
Positive | 2.07 | 1.13–3.79 | 0.019 |
|
|
|
| CHK1 |
|
|
|
|
|
|
|
Negative | 1 |
|
|
|
|
|
|
Positive | 2.76 | 1.31–5.81 | 0.007 |
|
|
|
Discussion
DNA damage response is a complex signaling network,
which includes cell cycle checkpoints and DNA damage and repair
pathways. DNA damage induces G1/S arrest or G2/M arrest to prevent
the cells carrying damaged chromosomes from progressing into
mitosis. The G2/M arrest is principally mediated by the activation
of serine/threonine kinase CHK1, whereas the G1/S checkpoint is
primarily mediated through the tumor suppressor p53 (25). p53 is known to enhance the
CHK1-mediated G2/M checkpoint activation induced by
chemotherapeutics. The downregulation of the molecule can be
prevented by inhibitors against JAK2, BCR/ABL, or the PI3K/Akt
pathway (26).
The current study indicated the expression of CHK1
between BUC and peritumoral tissues differed significantly
(P<0.05). CHK1 exhibited an increasing deterioration of the
pathological grading and clinical staging. Survival analysis
indicated a negative prognosis in CHK1 overexpression cases. On the
other hand, the evaluation of p53 in BUC specimens retrieved
results similar to that of CHK1, demonstrating a remarkable
difference between BUC and peritumoral tissues (P<0.05), and
growing trend with an elevated degree of BUC malignancy. To further
confirm the results, we assessed the correlation between CHK1 and
p53 in BUC; the close correlation of CHK1 and p53 indicated
synergistic interaction in BUC progression. These data confirmed
the evidence that both CHK1 and p53 were associated with BUC and
could serve as putative targets for treating BUC. This conclusion
was in agreement with previous studies with respect to CHK1 and p53
in cancer research (14,25,27).
However, the precise underlying mechanism is not yet
elucidated.
The ATM/CHK signaling requires p53 to mediate the
physiological function (28). In
ATM-CHK-p53 axis, the enhanced proliferative pressure and genomic
instability of both precancerous lesions and cancers generate a
considerable amount of spontaneous DNA damage. This accumulated DNA
damage induces cell cycle arrest, senescence, and apoptosis via p53
activation. Followed by full activation of the replication stress
response, the activated ATM/ATR phosphorylates CHK1 resulting in
the activation of downstream effector molecules, including p53. In
another pathway, p53 putatively regulates CHK1 in a positive
feedback mechanism. For example, Bernard et al reported that
phosphorylation of CHK1 on Ser317 was regulated by p53; thus, p53
may act as a molecular ‘on/off’ switch for the phosphorylation of
CHK1 on Ser317 (29).
To confirm the deduction above, we checked the
5-year survival rate of sub-groups in NMIBC and MIBC groups
(Fig. 4A and B). In NMIBC group, the
survival images indicated CHK1 positive and p53 positive sub-groups
had the worst prognosis, CHK1 negative and p53 negative sub-groups
had the best prognosis, and the other sub-groups were in middle.
The results confirmed CHK1 and p53 could be prognostic factors in
patients suffering from BUC, CHK1 and p53 probably had synergistic
interaction in CHK1 positive and p53 positive cases, which probably
indicated poor prognosis. However, in MIBC group, there was no
significant difference in the sub-groups. This result might be due
to the limited sample and a high mortality in MIBC. In the
multivariate Cox analyses, we found metastasis was an independent
prognostic risk factor, other factors including sex, age, tumor
diameter, single/multiple sites, histological grade, clinical
pathological staging, CHK1, p53, patients with incipient/recurrence
were not independent prognostic risk factors. The results indicated
that an effective biomarker to predict tumor metastasis of BUC.
Our study had several limitations. Firstly, as there
was small sample size in NMIBC and MIBC, we did not do EORTC table
to confirm the markers' validation in further; then, the small
cases in each sub-group in NMIBC or MIBC group, probably increased
the error in 5-year survival rate analysis. So, we expected to
collect more samples and finish the analysis in further
research.
In summary, those results indicated CHK1 and p53 in
BUC, especially in NMIBC, could be regarded as the potential
chemotherapeutic target. And further research about the underlying
mechanism remains needed.
As delineated above, the present study, for the
first time, assessed the association of CHK1 and p53 in BUC,
suggesting potential prognosis or therapeutic target in the
progression of BUC.
Acknowledgements
The authors would like to thank Jan-Guo Feng for
their technical assistance.
References
|
1
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prasad SM, Decastro GJ and Steinberg GD:
Medscape: URothelial carcinoma of the bladder: Definition,
treatment and future efforts. Nat Rev Urol. 8:631–642. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abdollah F, Gandaglia G, Thuret R,
Schmitges J, Tian Z, Jeldres C, Passoni NM, Briganti A, Shariat SF,
Perrotte P, et al: Incidence, survival and mortality rates of
stage-specific bladder cancer in united states: A trend analysis.
Cancer Epidemiol. 37:219–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bishr M, Lattouf JB, Latour M and Saad F:
Tumour stage on re-staging transurethral resection predicts
recurrence anad progression-free survival of patients with
high-risk non-muscle invasive bladder cancer. Can Urol Assoc J.
8:E306–E310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yafi FA, Aprikian AG, Chin JL, Fradet Y,
Izawa J, Estey E, Fairey A, Rendon R, Cagiannos I, Lacombe L, et
al: Contemporary outcomes of 2287 patients with bladder cancer who
were treated with radical cystectomy: A canadian multicentre
experience. BJU Int. 108:539–545. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soloway MS: Bladder cancer: Lack of
progress in bladder cancer-what are the obstacles? Nat Rev Urol.
10:5–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nedjadi T, Al-Maghrabi J, Assidi M, Dallol
A, Al-Kattabi H, Chaudhary A, Al-Sayyad A, Al-Ammari A, Abuzenadah
A, Buhmeida A and Al-Qahtani M: Prognostic value of HER2 status in
bladder transitional cell carcinoma revealed by both IHC and BDISH
techniques. BMC Cancer. 16:6532016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang ZY, Zhang W, Yang JJ, Song DK, Wei JX
and Gao S: Association of thymosin β4 expression with
clinicopathological parameters and clinical outcomes of bladder
cancer patients. Neoplasma. 63:991–998. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou J, Dong D, Cheng R, Wang Y, Jiang S,
Zhu Y, Fan L, Mao X, Gui Y, Li Z, et al: Aberrant expression of
KPNA2 is associated with a poor prognosis and contributes to OCT4
nuclear transportation in bladder cancer. Oncotarget.
7:72767–72776. 2016.PubMed/NCBI
|
|
10
|
van der Heijden AG, Mengual L, Lozano JJ,
Ingelmo-Torres M, Ribal MJ, Fernandez PL, Oosterwijk E, Schalken
JA, Alcaraz JA, Alcaraz A and Witjes JA: A five-gene expression
signature to predict progression in T1G3 bladder cancer. Eur J
Cancer. 64:127–136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pignot G, Cizeron-Clairac G, Vacher S,
Susini A, Tozlu S, Vieillefond A, Zerbib M, Lidereau R, Debre B,
Amsellem-Ouazana D and Bieche I: microRNA expression profile in a
large series of bladder tumors: identification of a 3-miRNA
signature associated with aggressiveness of muscle-invasive bladder
cancer. Int J Cancer. 132:2479–2491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Segersten U, Spector Y, Goren Y, Tabak S
and Malmström PU: The role of microRNA profiling in prognosticating
progression in Ta and T1 urinary bladder cancer. Urol Oncol.
32:613–618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goto H, Kasahara K and Inagaki M: Novel
insights into Chk1 regulation by phosphorylation. Cell Struct
Funct. 40:43–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Toledo LI, Murga M and Fernandez-Capetillo
O: Targeting ATR and Chk1 kinases for cancer treatment: A new model
for new (and old) drugs. Mol Oncol. 5:368–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Petermann E, Maya-Mendoza A, Zachos G,
Gillespie DA, Jackson DA and Caldecott KW: Chk1 requirement for
high global rates of replication fork progression during normal
vertebrate S phase. Mol Cell Biol. 26:3319–3326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Petermann E, Woodcock M and Helleday T:
Chk1 promotes replication fork progression by controlling
replication initiation. Proc Natl Acad Sci USA. 107:pp.
16090–16095. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Herman-Antosiewicz A, Stan SD, Hahm ER,
Xiao D and Singh SV: Activation of a novel ataxia-telangiectasia
mutated and Rad3 related/checkpoint kinase 1-dependent prometaphase
checkpoint in cancer cells by diallyl trisulfide, a promising
cancer chemopreventive constituent of processed garlic. Mol Cancer
Ther. 6:1249–1261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Myers K, Gagou ME, Zuazua-Villar P,
Rodriguez R and Meuth M: ATR and Chk1 suppress a
caspase-3-dependent apoptotic response following DNA replication
stress. PLoS Genet. 5:e10003242009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wasylishen AR and Lozano G: Attenuating
the p53 pathway in human cancers: many means to the same end. Cold
Spring Harb Perspect Med. 6:a0262112016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vazquez A, Bond EE, Levine AJ and Bond GL:
The genetics of the p53 pathway, apoptosis and cancer therapy. Nat
Rev Drug Discov. 7:979–987. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Williams AB and Schumacher B: p53 in the
DNA-Damage-Repair Process. Cold Spring Harb Perspect Med. 6:2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Freed-Pastor WA and Prives C: Mutant p53:
One name, many proteins. Genes Dev. 26:1268–1286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Humphrey PA, Moch H, Cubilla AL, Ulbright
TM and Reuter VE: The 2016 WHO classification of tumours of the
urinary system and male genital organs-part B: Prostate and bladder
tumours. Eur Urol. 70:106–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rath S, Connors JM, Dolman PJ, Rootman J,
Rootman DB and White VA: Comparison of American Joint Committee on
Cancer TNM-based staging system (7th edition) and Ann Arbor
Classification for predicting outcome in ocular adnexal lymphoma.
Orbit. 33:23–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Drayton RM and Catto JW: Molecular
mechanisms of cisplatin resistance in bladder cancer. Expert Rev
Anticancer Ther. 12:271–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li CC, Yang JC, Lu MC, Lee CL, Peng CY,
Hsu WY, Dai YH, Chang FR, Zhang DY, Wu WJ and Wu YC: ATR-Chk1
signaling inhibition as a therapeutic strategy to enhance cisplatin
chemosensitivity in urothelial bladder cancer. Oncotarget.
7:1947–1959. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Umezawa Y, Kurosu T, Akiyama H, Wu N,
Nogami A, Nagao T and Miura O: Down regulation of Chk1 by p53 plays
a role in synergistic induction of apoptosis by chemotherapeutics
and inhibitors for Jak2 or BCR/ABL in hematopoietic cells.
Oncotarget. 7:44448–44461. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Manic G, Obrist F, Sistigu A and Vitale I:
Trial Watch: Targeting ATM-CHK2 and ATR-CHK1 pathways for
anticancer therapy. Mol Cell Oncol. 2:e10129762015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bernard JJ, Lou YR, Peng QY, Li T, Conney
AH and Lu YP: Inverse relationship between p53 and phospho-Chk1
(Ser317) protein expression in UVB-induced skin tumors in SKH-1
mice. Exp Mol Pathol. 96:126–131. 2014. View Article : Google Scholar : PubMed/NCBI
|