Introduction
The incidence of gastric cancer in the digestive
tract is 13.9% with a continuous increasing trend (1). The complex pathogenesis of cancer
involves oncogene activation, mutation of tumor suppressor genes,
and proliferation of malignant cells. In addition, apoptosis is
inhibited in the malignant cells (2).
The deregulation of proliferation and/or apoptosis induces the
appearance of malignant tumors (3).
MicroRNAs (miRs) are non-coding RNAs that control gene expression.
A recent study confirmed that the development of malignant tumors
is highly related to the activation and expression of specific miRs
(4). Abnormal expression of miRs can
contribute to the dysregulation of oncogenes or tumor suppressor
genes, exerting a tremendous influence on the development of
malignant tumors and the progression of cancers (5). Investigating miRs can contribute to
understanding the mechanism of proliferation, apoptosis, invasion,
and metastasis of gastric cancer cells, and have the potential to
become alternative therapeutic targets. Previous findings verified
the abnormal expression of miRs in gastric cancer tissues (5,6). Thus,
specific miRs can be used as markers to distinguish normal and
malignant tissues, with the potential to become promising targets
for testing, diagnosing, and treating gastric cancer (7,8).
Bcl-2 is an anti-apoptotic gene located on human
chromosome 18q21 (9) highly expressed
in stem cells of human tissues, including skin basal collagen cells
and small intestinal crypt bottom cells. By inhibiting cell
apoptosis, Bcl-2 ensures that the cells have sufficient time to
complete their transformation from stem cells into highly
differentiated cells (10). Previous
results have verified that Bcl-2 is closely related to the
occurrence and development of lymphoma, colorectal, breast,
cervical, and thyroid cancer (11–13). Other
studies demonstrate that Bcl-2 is also linked to the prognosis of
certain tumors (14). In addition, a
high expression of Bcl-2 is closely associated with the invasion
and metastasis of malignant tumor cells and recent studies showed
that the expression of Bcl-2 in tumor cells can be inhibited by
artificial methods, leading to the increase of cancer cell
sensitivity to chemotherapeutic drugs (15). In addition, we downregulation of Bcl-2
induces and speeds apoptosis in primary tumor cells.
We further investigated the role of miR-21 in the
occurrence and development of gastric cancers by analyzing gastric
cancer pathology and adjacent normal tissues. We examined the
potential mechanisms of miR-21 to provide a novel biomarker for
early diagnosis and provide a rationale for new treatments of
gastric cancer.
Materials and methods
Tissue processing
Gastric carcinoma and the corresponding normal
gastric tissue were stored at −80°C. The samples were ground and
the powder was placed in a pre-processed tube, adding 500 µl
TRIzol, and mixed for 15 sec; 170 µl chloroform was added with 15
sec mixing and centrifugation at 10,000 × g for 10 min. Supernatant
(400 µl) was added to a new tube, 500 µl isopropanol was added with
15 sec mixing with the vortex, then centrifuge at 10,000 × g for 10
min at 4°C. Supernatant was discard, the white sediment at the
bottom of the tube was air dried. Total RNA was dissolved in 50 µl
DEPC water.
Western blot analysis
Two hundred milligrams of tissue was sheared and 1
ml of lysate was added; homogenated and centrifuged at 6,200 × g
for 10 min, the supernatant was transferred to a new tube;
centrifuge at 10,000 × g for 60 min, and the supernatant was
transferred to a new tube. The protein content was determined with
the protein kit BCA™.
Flow cytometry
The MGC803 human gastric cancer cell line was
transfected with miR-21, after which the cells were starved for 48
h after transfection. Then, we trypsinized the cells (0.25%
trypsin) into a single cell suspension and used Annexin V-FITC/PI
to detect apoptosis by cytometer. Primer sequences used were:
miR-21 forward, 5′-TCCGAAGTTGTAGTCAGACT-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′.
MTT assay
MGC803 cells transfected with miR-21 were
trypsinized and re-suspended in Dulbecco's modified Eagle's medium
(DMEM) culture medium containing 10% fetal bovine serum (FBS). The
cells were seeded in 96-well culture plates with a volume of 200 µl
per well. The 96-well culture plates were placed in 5%
CO2 cell culture incubator with saturated humidity and
37°C for 3–5 days. MTT solution (20 µl) was then added to each
well, and the cells were incubated in the incubator for 4 h. The
culture medium was discarded, 150 µl DMSO was added per well, and
shaken for 10 min. Absorbance was measured at 92 nm in the cell
culture medium using enzyme-linked immunosorbent assay, and the
cell growth curve was drawn with the time as the horizontal
coordinate. The DMEM (FBS-free) was used to dilute the Matrigel.
MGC803 cells were cultured with serum-free DMEM for 24 h. The
supernatant was used as the chemotaxis solution with 0.05–0.2% BSA.
MGC803 cells were washed with PBS 2–3 times. Cell culture medium
(DMEM-free) was added and the cells were placed in the incubator
for 24 h. The supernatant was discarded and digested with 0.25%
trypsin. The supernatant was discarded, and DMEM with 5% BSA was
used to re-suspend cells; 300 µl DMEM (FBS-free) was added to the
upper chamber; 200 µl chemotaxis solution was added to the lower
chamber, and the Matrigel was covered; 400 µl cell suspension was
added to the upper chamber and incubated for 48 h; 95% ethanol was
used for 30 min and then stained with H&E. Five fields of view
were selected to count and take the average value of the
transmembrane cells. The above experiments were repeated three
times.
Results
Levels of miR-21 and Bcl-2 mRNA in
gastric cancer and adjacent normal tissue
To examine the role of miR-21 and Bcl-2 in gastric
cancer, we extracted total RNA from gastric cancer samples and
adjacent normal tissue to measure mRNA levels. We measured mRNA
levels of miR-21 and Bcl-2 by fluorescent
quantitative PCR in 50 pairs of gastric cancer tissues and the
adjacent normal tissues (Tables I and
II). The ΔCT values for
miR-21 in the gastric cancer group were significantly higher
than those in the control group (Table
I). In addition, the ΔCT values for Bcl-2 mRNA in the
gastric cancer group was also significantly higher that in the
control group (Table II).
 | Table I.Expression of miR-21 mRNA (mean ± SD,
n=50). |
Table I.
Expression of miR-21 mRNA (mean ± SD,
n=50).
| Groups | ΔCq | ΔΔCq |
2−ΔΔCq |
|---|
| Para-gastric
cancer | 14.78±0.15 | 6.69±0.32 | 1.06±0.13 |
| Gastric cancer
tissues | 8.63±0.26 | 0.54±0.12 |
8.12±0.21a |
 | Table II.Expression of Bcl-2 mRNA (mean ± SD,
n=50). |
Table II.
Expression of Bcl-2 mRNA (mean ± SD,
n=50).
| Groups | ΔCq | ΔΔCq |
2−ΔΔCq |
|---|
| Gastric cancer | 12.18±0.15 | 7.81±0.19 | 1.13±0.55 |
| Para-gastric
cancer | 5.63±0.26 | 0.43±0.27 |
9.26±0.37a |
Expression of Bcl-2 in gastric
cancer
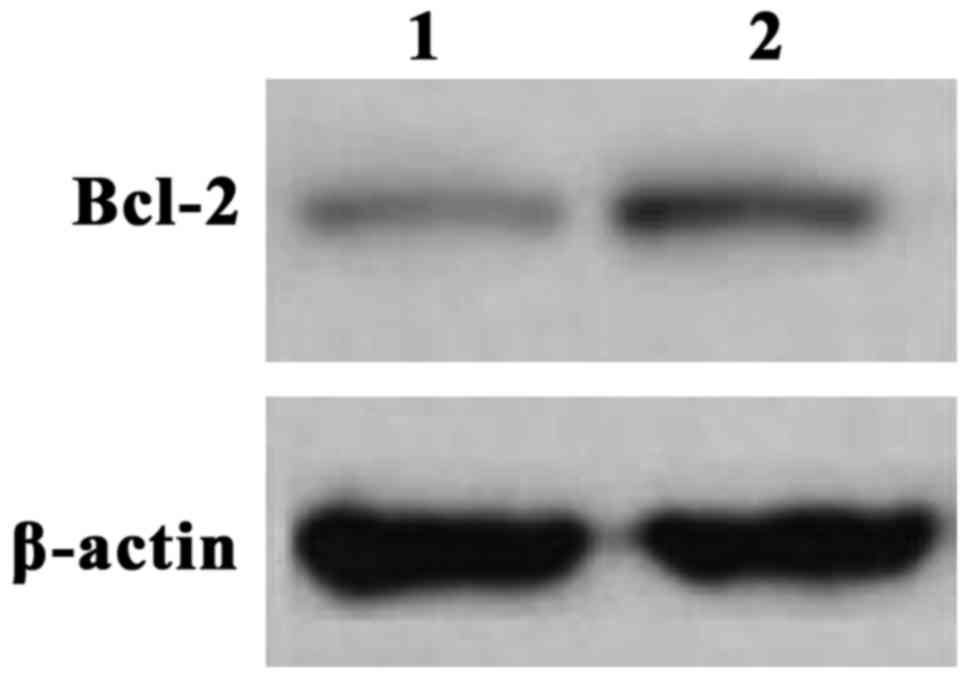
We next validated the elevated Bcl-2 mRNA
expression by analyzing the protein levels. The expression of Bcl-2
in the gastric cancer and control group were measured and tested by
western blot analysis. Compared to the control group, the level of
the Bcl-2 protein in the gastric cancer group was significantly
higher (Fig. 1), supporting the
results with mRNA levels.
Correlation of Bcl-2 expression and
clinical features
We investigated the correlation between Bcl-2
protein expression in gastric adenocarcinoma and clinical features
(Table III). Expression of Bcl-2
protein was not associated to age and locations of the tumor.
However, we found a strong association with the tumor clinical
stage, tumor cell invasion of lymph nodes, and the tumor metastasis
degree (Table III). We also
compared the relative expression of miR-21 and Blc-2 mRNA by
Spearman test. We found a strong correlation of the expression of
miR-21 and Blc-2 mRNA.
 | Table III.Bcl-2 protein expression and the
clinical features. |
Table III.
Bcl-2 protein expression and the
clinical features.
| Group | Cases (n) | High Bcl-2 (n) | Low Bcl-2 (n) | P-value |
|---|
| Age (years) |
|
|
| 0.72 |
| ≤65 | 14 | 8 | 6 |
|
|
>65 | 36 | 18 | 18 |
|
| Clinical stages |
|
|
| 0.024 |
| T1 | 14 | 3 | 11 |
|
| T2 | 20 | 8 | 12 |
|
| T3 | 10 | 8 | 2 |
|
| T4 | 6 | 5 | 1 |
|
| Lymph nodes |
|
|
| 0.025 |
| Yes | 9 | 8 | 1 |
|
| No | 41 | 13 | 28 |
|
| Metastasis
degree |
|
|
| 0.041 |
|
High | 21 | 18 | 3 |
|
|
Middle | 16 | 10 | 6 |
|
|
Low | 11 | 4 | 7 |
|
| Tumor location |
|
|
| 0.701 |
| Before
cardiac stomach | 15 | 7 | 8 |
|
| After
cardiac stomach | 4 | 1 | 3 |
|
| On
cardiac stomach | 31 | 17 | 14 |
|
Inhibition of miR-21 in MGC803
cells
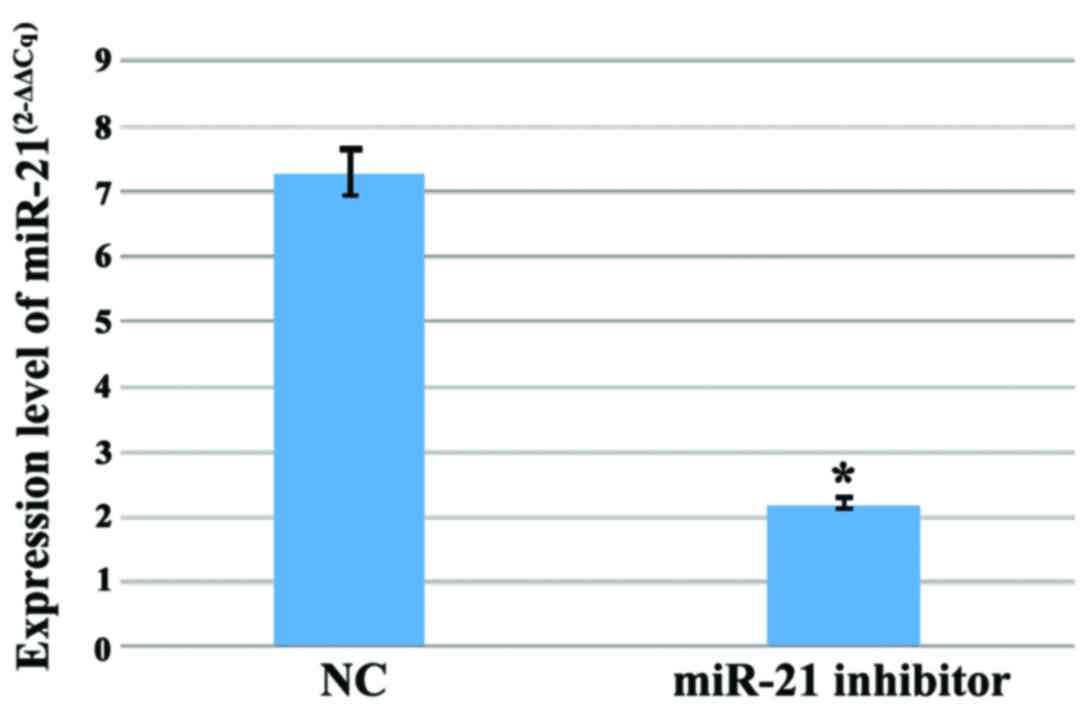
To investigate the functional relevance of miR-21
expression in gastric adenocarcinoma, we introduced the miR-21
inhibitor into MGC803 cells and normal cells. We measured the
expression levels of miR-21 after 48 h. Compared to normal cells,
the expression of miR-21 in MGC803 cells was significantly reduced
(Fig. 2).
The Protein expression of Bcl-2 in
MGC803 lineage and normal cells
We examined Bcl-2 expression, cell proliferation,
apoptosis and invasion on MGC803 cells treated with miR-21. We
found that miR-21 inhibition resulted in significantly lower levels
of Bcl-2 protein (Table IV).
 | Table IV.The protein expression of Bcl-2 in
MGC803 lineage and normal cells. |
Table IV.
The protein expression of Bcl-2 in
MGC803 lineage and normal cells.
|
|
| Bcl-2 |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Groups | n | + | − | Positive rate
(%) | χ2 | P-value |
|---|
| Gastric cancer | 96 | 35 | 61 | 36.5 | 63.548 | <0.001 |
| Control | 96 | 88 | 8 | 91.7 |
|
|
Proliferation of MGC803 after miR-21
inhibition
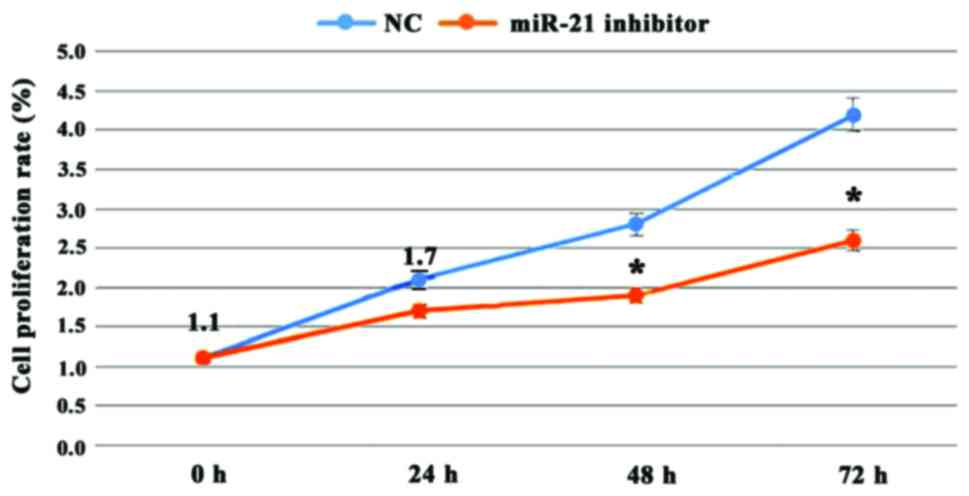
The proliferation of the MGC803 cells using the MMT
assay was determined. We incubated normal and MGC803 cells for 24,
48 and 72 h with miR-21 inhibitor found at each time-point the
proliferation of the MGC803 cells was decreased compared to the
control group (Fig. 3). At each
time-point the differences were statistically significance
(P<0.01).
Apoptosis in MGC803 cells after miR-21
inhibition
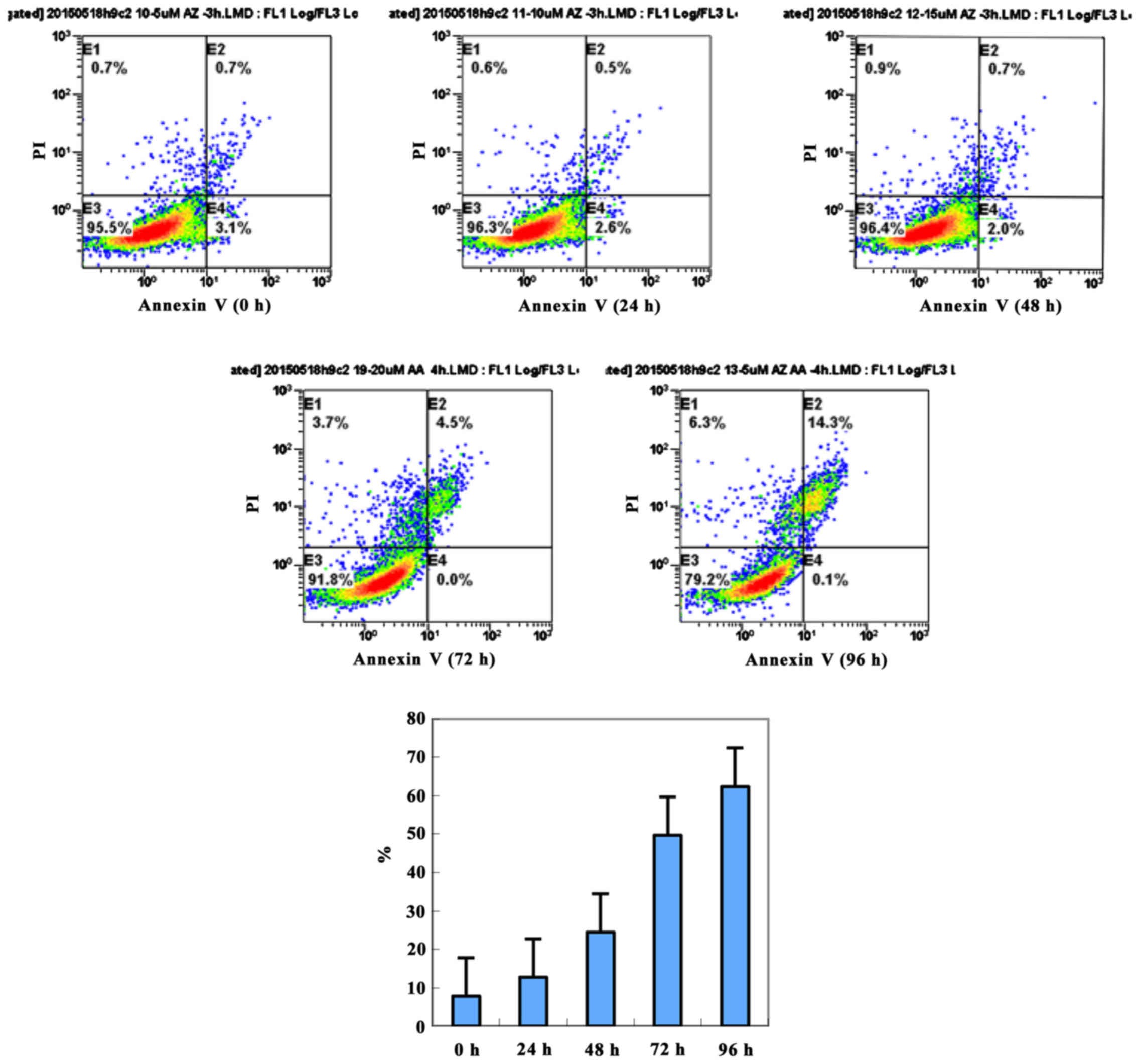
To further investigate the effects of miR-21 in
MGC803 apoptosis, we used Annexin V-FITC/PI double stain. We
evaluated MGC803 apoptosis at different time-points (0, 24, 48, 72
and 96 h) after miR-21 inhibition. As time progressed, MGC803
apoptosis showed acceleration (Fig.
4). At each time-point, the apoptosis of the MGC803 cells was
higher than that in normal cells (Fig.
4).
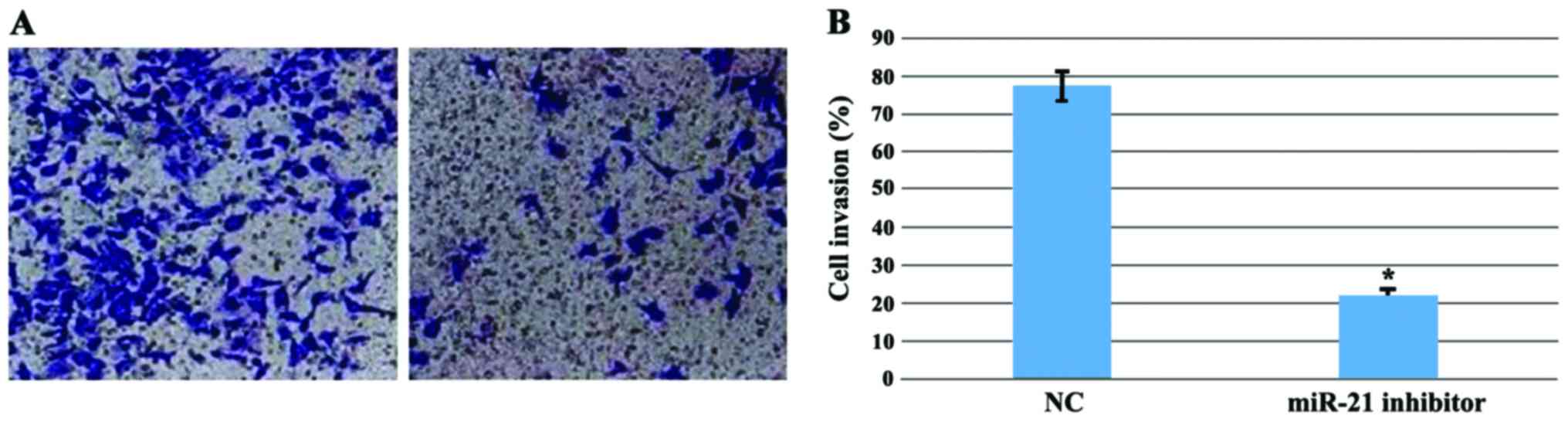
Invasion of MGC803 cells after miR-21
inhibition
The cell invasion ability is one of the key features
of tumors, which can represent the malignancy degree of the tumor.
To investigate gastric cancer cell invasion, the Transwell assay
was used to evaluate the ability of MGC803 to invade after miR-21
inhibition. Compared with the control group, the invasion ability
of the MGC803 was significantly decreased (P<0.01) (Fig. 5).
Discussion
The rapid development of molecular biology and
modern cancer medicine has revealed the correlation between miR
expression and the development of malignant tumors. miRs can
regulate the expression of one third of genes in the human genome
and have been shown to exert many physiological functions in cell
development, proliferation, differentiation, apoptosis, and
metastasis. The expression levels of miRs are altered changed
(increased or decreased) in most malignant tumor tissues compared
to normal tissues, suggesting strong connection between miRs and
the development of tumors (16–20). The
malignant tumor growth, proliferation, invasion, and metastasis,
and apoptosis are closely correlated to abnormal expression of miRs
and its aberrant regulatory activities.
Our experiments show that miR-21 mRNA and
Bcl-2 mRNAs were significantly elevated in gastric
adenocarcinoma and miR-21 inhibition reduced the proliferation and
increase the apoptosis of MGC803 cells. Antisense inhibition of
miR-21 can activate malignant cells apoptosis (21). The study of antisense oligonucleotides
decreased the expression of miR-21 in glioma cells and cell number,
while the activity of caspase-3 and −7 were significantly
increased. Our experiments show that miR-21 inhibition had similar
effects on gastric adenocarcinoma. The inhibitory effect of Bcl-2
on apoptosis mainly form channel protein in three steps (22–25): i)
Increase cell membrane permeability to inhibit the release of
mitochondrial apoptotic proteins, and ultimately inhibit apoptosis;
ii) improve cellular antioxidant, scavenging oxygen free radicals
to suppress apoptosis; and iii) block calcium ion transmembrane
flow through intracellular calcium ion concentration regulation to
inhibit apoptosis.
Using artificial approaches to inhibit the miR-21 in
cholangiocarcinoma cells showed that miR-21 promoted the
effectiveness of the chemotherapy drug gemcitabine by inducing
apoptosis (26). This suggests that
miR-21 activates the PI3K signaling pathway. Lund et al
(27) showed that miR-21 can reduce
the expression of PDCD4 in breast cancer cells, thus promoting the
transformation of tumor cells. In colon cancer samples and cell
lines, miR-21 can regulate the target gene of PDCD4, thus,
suggesting a key role in growth and invasion.
Our study also found that Bcl-2 was associated with
clinical staging, lymph node metastasis, and tumor differentiation.
The relative expression of miR-21 and Bcl-2 mRNA were
strongly correlated with gastric cancer. Other research found that
the content of miR-21 in breast cancer tissue was significantly
higher than in normal tissue (28,29).
miR-21 was also associated with tumor clinical stage, vascular
invasion, and tumor cell proliferation, suggesting a similar role
for miR-21 in breast and gastric cancer. A study found that the
apoptosis induced factor of PDCD4 inhibits the expression of miR-21
in MCF-7 cells, and miR-21 can play an antagonistic role to p53
apoptosis pathway by inhibition of the tumor suppressor protein p53
(30). Finally, we found that miR-21
promotes the expression of Bcl-2 protein, and miR-21 inhibition
decreased cell proliferation. The mechanisms regulating miR-21 high
expression in gastric adenocarcinoma tissues are still unclear. One
possibility is that miR-21 promotes the proliferation and invasion
of human MGC803 cells and the inhibition of apoptosis.
In conclusion, our study reports higher levels of
miR-21 in gastric adenocarcinoma, and we discuss the possible role
of miR-21 in regulating MGC803 cell apoptosis. Our study supports
the potential for miR-21 as a marker for early diagnosis and target
treatment for gastric adenocarcinoma.
References
|
1
|
Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK,
Jeon TJ, Kim JM, Kim YI, Ryu KW, Kong SH, et al: Clinical practice
guidelines for gastric cancer in Korea: An evidence-based approach.
J Gastric Cancer. 14:87–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Long ZW, Yu HM, Wang YN, Liu D, Chen YZ,
Zhao YX and Bai L: Association of IL-17 polymorphisms with gastric
cancer risk in Asian populations. World J Gastroenterol.
21:5707–5718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen XZ, Chen H, Castro FA, Hu JK and
Brenner H: Epstein-Barr virus infection and gastric cancer: a
systematic review. Medicine (Baltimore). 94:e7922015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanaan Z, Rai SN, Eichenberger MR, Roberts
H, Keskey B, Pan J and Galandiuk S: Plasma miR-21: a potential
diagnostic marker of colorectal cancer. Ann Surg. 256:544–551.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li T, Li RS, Li YH, Zhong S, Chen YY,
Zhang CM, Hu MM and Shen ZJ: miR-21 as an independent biochemical
recurrence predictor and potential therapeutic target for prostate
cancer. J Urol. 187:1466–1472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu XG, Zhu WY, Huang YY, Ma LN, Zhou SQ,
Wang YK, Zeng F, Zhou JH and Zhang YK: High expression of serum
miR-21 and tumor miR-200c associated with poor prognosis in
patients with lung cancer. Med Oncol. 29:618–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han M, Wang Y, Liu M, Bi X, Bao J, Zeng N,
Zhu Z, Mo Z, Wu C and Chen X: MiR-21 regulates
epithelial-mesenchymal transition phenotype and hypoxia-inducible
factor-1α expression in third-sphere forming breast cancer stem
cell-like cells. Cancer Sci. 103:1058–1064. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lakomy R, Sana J, Hankeova S, Fadrus P,
Kren L, Lzicarova E, Svoboda M, Dolezelova H, Smrcka M, Vyzula R,
et al: MiR-195, miR-196b, miR-181c, miR-21 expression levels and
O-6-methylguanine-DNA methyltransferase methylation status are
associated with clinical outcome in glioblastoma patients. Cancer
Sci. 102:2186–2190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu QL, Abel P, Foster CS and Lalani EN:
bcl-2: role in epithelial differentiation and oncogenesis. Hum
Pathol. 27:102–110. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luanpitpong S, Chanvorachote P, Stehlik C,
Tse W, Callery PS, Wang L and Rojanasakul Y: Regulation of
apoptosis by Bcl-2 cysteine oxidation in human lung epithelial
cells. Mol Biol Cell. 24:858–869. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Flangea C, Potencz E, Mihăescu R, Gîju S
and Anghel A: Bcl-2 expression in Hodgkin's lymphoma progression.
Rom J Morphol Embryol. 49:357–363. 2008.PubMed/NCBI
|
|
12
|
Manne U, Weiss HL and Grizzle WE: Bcl-2
expression is associated with improved prognosis in patients with
distal colorectal adenocarcinomas. Int J Cancer. 89:423–430. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou XL and Wang M: Expression levels of
survivin, Bcl-2, and KAI1 proteins in cervical cancer and their
correlation with metastasis. Genet Mol Res. 14:17059–17067. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hajnóczky G, Csordás G, Das S,
Garcia-Perez C, Saotome M, Sinha Roy S and Yi M: Mitochondrial
calcium signalling and cell death: approaches for assessing the
role of mitochondrial Ca2+ uptake in apoptosis. Cell
Calcium. 40:553–560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimamoto S, Tsuchiya M, Yamaguchi F,
Kubota Y, Tokumitsu H and Kobayashi R: Ca2+/S100
proteins inhibit the interaction of FKBP38 with Bcl-2 and Hsp90.
Biochem J. 458:141–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Yimamu M, Wang X, Zhang X, Mao M, Fu
L, Aisimitula A, Nie Y and Huang Q: Addition of rituximab to a CEOP
regimen improved the outcome in the treatment of non-germinal
center immunophenotype diffuse large B cell lymphoma cells with
high Bcl-2 expression. Int J Hematol. 99:79–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simsek EN and Uysal T: In vitro
investigation of cytotoxic and apoptotic effects of Cynara L.
species in colorectal cancer cells. Asian Pac J Cancer Prev.
14:6791–6795. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo KW, Ko CH, Yue GGL, Lee JK, Li KK, Lee
M, Li G, Fung KP, Leung PC and Lau CB: Green tea (Camellia
sinensis) extract inhibits both the metastasis and osteolytic
components of mammary cancer 4T1 lesions in mice. J Nutr Biochem.
25:395–403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu CJ, Zhou L and Cai Y:
Dihydroartemisinin induces apoptosis of cervical cancer cells via
upregulation of RKIP and downregulation of bcl-2. Cancer Biol Ther.
15:279–288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pushkarev VM, Kovzun OI, Pushkarev VV and
Tronko M: Biochemical effects of combined action of γ-irradiation
and paclitaxel on anaplastic thyroid cancer cells. Ukr Biokhim Zh
(1999). 85:51–61. 2013.PubMed/NCBI
|
|
21
|
Banzhaf-Strathmann J and Edbauer D: Good
guy or bad guy: the opposing roles of microRNA 125b in cancer. Cell
Commun Signal. 12:302014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lim L, Balakrishnan A, Huskey N, Jones KD,
Jodari M, Ng R, Song G, Riordan J, Anderton B, Cheung ST, et al:
MicroRNA-494 within an oncogenic microRNA megacluster regulates
G1/S transition in liver tumorigenesis through suppression of
mutated in colorectal cancer. Hepatology. 59:202–215. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Melo SA, Sugimoto H, O'Connell JT, Kato N,
Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, et
al: Cancer exosomes perform cell-independent microRNA biogenesis
and promote tumorigenesis. Cancer Cell. 26:707–721. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kasinski AL, Kelnar K, Stahlhut C,
Orellana E, Zhao J, Shimer E, Dysart S, Chen X, Bader AG and Slack
FJ: A combinatorial microRNA therapeutics approach to suppressing
non-small cell lung cancer. Oncogene. 34:3547–3555. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chan JA, Krichevsky AM and Kosik KS:
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
Cancer Res. 65:6029–6033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meng F, Henson R, Lang M, Wehbe H,
Maheshwari S, Mendell JT, Jiang J, Schmittgen TD and Patel T:
Involvement of human micro-RNA in growth and response to
chemotherapy in human cholangiocarcinoma cell lines.
Gastroenterology. 130:2113–2129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lund E, Güttinger S, Calado A, Dahlberg JE
and Kutay U: Nuclear export of microRNA precursors. Science.
303:95–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Asangani IA, Rasheed SAK, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Frankel LB, Christoffersen NR, Jacobsen A,
Lindow M, Krogh A and Lund AH: Programmed cell death 4 (PDCD4) is
an important functional target of the microRNA miR-21 in breast
cancer cells. J Biol Chem. 283:1026–1033. 2008. View Article : Google Scholar : PubMed/NCBI
|