Gastric cancer (GC) is one of the four most common
types of cancer in China, and is associated with a high mortality
rate. Between 33 and 50% of worldwide GC diagnoses occur in China
(1). GC exhibits significant
heterogeneity regarding its biological behavior and results in
differing prognoses, independent of clinical stage (2). Despite cancer cells being extensively
studied, advances in cancer research have highlighted that cancer
progression is primarily determined by individual biological
behaviors that are modulated via the cross-talk between cancer
cells and the tumor microenvironment (TME) (3). Numerous studies have demonstrated the
pivotal function of TME in GC progression (4–6). TMEs are
heterogeneous in nature, containing a surrounding extracellular
matrix (ECM) and several different types of cell including
fibroblasts, endothelial cells, immune cells, local and bone
marrow-derived stromal stem and progenitor cells (7). In the present review, the current
knowledge of cancer-associated fibroblasts (CAFs), which are
important components in the TME, are summarized in order to
elucidate the exact function(s) of CAFs in the regulation of
different biological behaviors which occur in GC progression
(8–11).
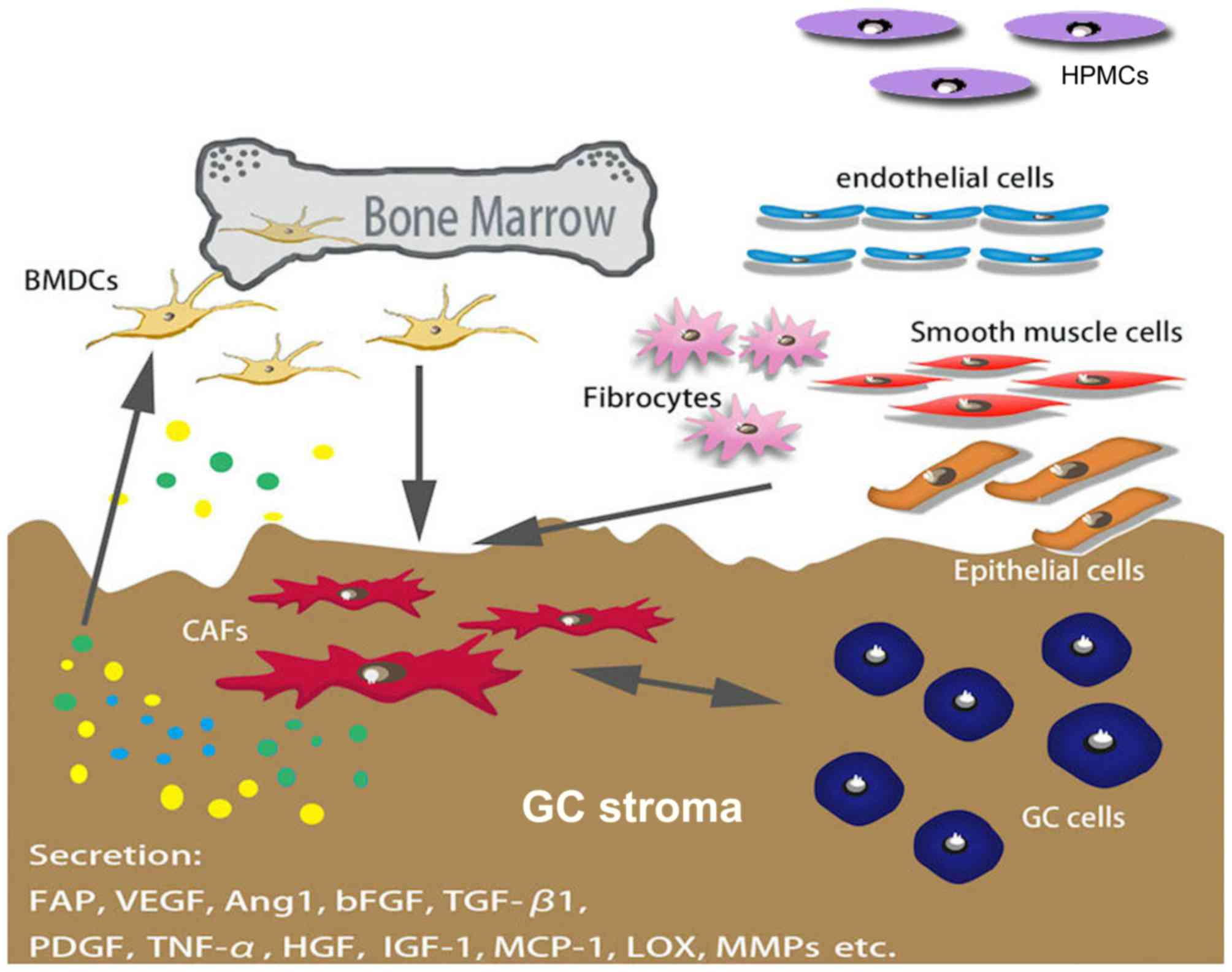
CAFs are spindle-shaped blast-like cells with an
unclear origin; however, a previous study demonstrated that bone
marrow-derived stromal cells are a major source of CAFs, as well as
mesenchymal stem cells (MSCs) (12).
Several factors mediate the differentiation of CAFs, and certain
markers, including α-smooth muscle actin (α-SMA), fibroblast
activation protein (FAP) and platelet-derived growth factor (PDGF)
receptor α/β, have been used to distinguish CAF from other types of
fibroblast (Fig. 1) (10,13–15).
The interaction between cancer cells and adjacent
stroma may motivate specific TMEs to promote GC tumor progression
(16,17). Accumulating evidence has suggested
that CAFs may increase the proliferation rate of GC cells through a
variety of mechanisms, for example by targeting PTEN via
microRNA-106b in CAFs or by targeting the TGF-β/Smad pathway
(18–21). It has been demonstrated previously
that bone marrow-derived fibrocytes may migrate into the GC TME
using the stromal cell-derived factor 1 (SDF-1)/CXC chemokine
receptor type 4 (CXCR4) system, and may increase cancer cell
proliferation and the rate of fibrosis, in a similar manner to CAFs
(18). In addition, Han et al
(22) demonstrated that neuregulin 1,
secreted by GC stem cells (GCSCs), regulated the activation of the
nuclear factor κB (NF-κB) signaling pathway, and modulated the
proliferation and invasion of GC cells by culturing GCSCs and CAFs
directly from patients with GC. Kikuchi et al (23) demonstrated that periostin (POSTN) was
overexpressed due to CAF, and POSTN may regulate the primary tumor
niche by supporting cancer cell proliferation through the
extracellular-signal-related kinase (ERK) signaling pathway in GC
when testified in the mouse fibroblast cell line NIH3T3 C57BL/6
POSTN−/− and human diffuse-type GC cell lines OCUM-2MLN
and OCUM-12.
CAFs directly and indirectly improve the ability of
invasion and metastasis, fundamental behaviors in cancer cells
(24,25). CAFs are able to induce an aggressive
phenotype and cause functional changes in GC cells in order to
enhance the ability of cells to invade directly. This biological
behavior is termed the epithelial-mesenchymal transition (EMT)
(12). It has been reported
previously that HSC-39 cells modulate EMT by communicating with
CAFs during the process of cancer metastasis (26). Tsukada et al (27) demonstrated, using a GC mouse xenograft
model, that human peritoneal mesothelial cells may be an origin of
CAFs, and are activated by transforming growth factor β (TGFβ)-1
signaling, leading to the acquirement of the ability to invade
basement membranes in GC.
In addition to the direct effects of CAFs on GC
cells, accumulating evidence focused primarily on the invasion
ability of GC cells has demonstrated that CAFs are able to
indirectly improve the ability of GC cells to invade and
metastasize by secreting numerous functional molecules (24,25,27). Yang
et al (19) used conditioned
media from CAFs and normal fibroblasts (NFs) to stimulate GC cells,
and demonstrated that GC cell invasion rates were significantly
increased in the CAF group compared with the NF group. Furthermore,
by utilizing a co-culturing system containing chromatic assembly
factor 1 and atypical glandular cells (gastric cell line) as an
in vitro model for an invasion study, Fukui et al
(28) demonstrated that interleukin
(IL)-22 is produced by CAFs and promotes GC cell invasion via
signal transducer and activator of transcription 3 and ERK
signaling pathways. Similarly, He et al (29) co-cultured GC cells with CAFs that were
transfected with galectin (Gal)-1 small interfering RNA, and
demonstrated that CAFs increased the capability for GC cells to
migrate into and invade the stroma through the overexpression of
Gal-1 protein. Sun et al (30)
demonstrated that glia-activating factor 9 secreted from CAFs may
upregulate the expression of matrix metalloproteinase (MMPs)
dose-dependently, and resulted in an increase in the number of
invasive cells. Results from a previous study suggest that the
proportion of CAFs in scirrhous GC is increased and results in a
poor clinical prognosis as cancer cells are able to invade the
submucosa, which contains an abundance of stromal cells (21). Additionally, Sung et al
(31) demonstrated that the
expression of Twist-related protein 1 was observed more frequently
in GC CAFs compared with other cells, and also led to a significant
increase in the invasive ability of GC cells in vitro.
It is well-known that cancer cells generate a
supportive microenvironment in order to activate fibroblasts and
facilitate the secretion of growth factors and proteases at the
peritoneal dissemination site through numerous stroma-modulating
growth factors, including fibroblast growth factor (FGF) family
members, PDGF, vascular endothelial growth factor (VEGF) family
members, epidermal growth factor ligands, ILs and TGF-β1 (32–34).
Comparatively, the potential invasive and metastatic ability of
cancer cells may be enhanced by the transdifferentiation process
via EMT. In this process, MSCs promote tumor growth by
differentiating into CAFs and remodeling the TME, and facilitate
invasion and metastasis observed in GC (20,35).
Karnoub et al (36) compared
growth kinetics between MSC-containing tumors [breast cancer cells
(BCCs) and MSCs]. BCCs were injected into a xenograft model of
immunocompromised mice, and results demonstrated that chemokine
ligand 5-chemokine receptor 5 paracrine interactions serve a
pivotal function in the process of enabling MSCs to induce
metastasis. Furthermore, a previous study suggested that MSCs
acquired a CAF phenotype when exposed to GC-derived exosomes, and
the differentiation of MSCs to CAFs was associated with the
activation of the TGF-β/Smad signaling pathway (20). Additionally, this study demonstrated
that tumor exosomes are able to promote the migration of human
umbilical cord MSCs in vitro. Xu et al (37) demonstrated that MSC-like cells are
able to be isolated from human GC tissues (hGC-MSCs) and adjacent
non-cancerous tissues (hGCN-MSCs) from the same patient, and
results demonstrated several characteristic discrepancies between
the cell surface markers, the pluripotency and the
proliferation-associated gene expression in these two cell types.
Notably, another study used a Transwell migration assay to confirm
the difference in the migration abilities of hGCN-MSCs and
hGC-MSCs, which may partially result from the difference in the
cluster of differentiation (CD) 44 expression level, as CD44 is one
of the most important adhesion molecules and serves a crucial
function in cell migration and invasion processes (38). Tsukada et al (27) demonstrated that TGF-β, derived from
cancer cells in the peritoneal TME was able to activate human
peritoneal mesothelial cells (HPMCs) and lead to the progression
and fibrosis of GC. However, it was suggested that HPMCs are one of
the origins of CAFs and contribute to the EMT mechanism (26). Yu et al (39) demonstrated that CAFs promoted GC cell
migration and invasion by upregulating transgelin (TAGLN) levels
and TAGLN-induced MMP-2 production in human GC stroma. Furthermore,
it was also demonstrated that TAGLN promoted tumor metastasis by
upregulating MMP-2 enzymes that are capable of degrading the
basement membrane.
Cancer is a highly complex process, involving
numerous cancer cells and the surrounding stroma, which is
constructed of various different types of mesenchymal cell and the
ECM (40). The ECM is a complex
ecosystem scaffold populated by different types of stromal cell,
including fibroblast-like cells, endothelial cells and immune
cells, and is morphologically defined by desmoplasia, angiogenesis,
inflammation and the immune response (41,42).
Histopathological and genetic evidence suggests that
tumor-associated stromal proportions or signatures may refine the
prognostic assessment of tumors, therefore CAF-induced desmoplasia
may serve a pivotal function in cancer progression (43–45).
Within a tumor, the tissue structure becomes disordered and the ECM
is remodeled by mesenchymal cells, including CAFs (46). CAFs serve fundamental functions in ECM
remodeling, metabolic and immune reprogramming of the TME, and have
a marked effect on adaptive resistance to chemotherapy. Numerous
ECM and basement membrane constituents are produced by activated
fibroblasts or myofibroblasts (47).
Furthermore, myofibroblasts are a major source of ECM-degrading
proteases, including MMPs, and serve a vital function in the
contribution of ECM desmoplasia by expressing α-SMA, an important
marker for myofibroblasts, and serves as a prognostic marker in
multiple types of cancer (48,49). CAFs
maintain the mesenchymal phenotype in breast cancer cells by
producing linear bundles in the ECM, which is a radial alignment of
type I collagen fibers relative to tumors associated with local
invasion and poor disease-free survival (DFS) (50). Furthermore, the ECM may be modified by
interstitial flow and enzymes including lipoxygenase, which is
secreted by CAFs. It has been demonstrated previously that CAFs are
able to express a wide range of factors including cytokines, growth
factors and chemokines, all of which are critical to induce the
degradation of the ECM, promote angiogenesis and EMT, regulate
metabolic reprogramming, and increase proliferation rates and
chemotherapy resistance (51). In GC,
invading the surrounding tissue and the ECM via enzymatic
degradation is the first step of migration away from the primary
tumor (52).
Cancer is associated with fibroblasts throughout all
stages of disease progression, including metastasis, and CAFs are a
key component of the general host response to tissue damage caused
by cancer cells (12,53). CAFs are activated and respond to
cross-talk with cancer cells during carcinogenesis, and create a
suitable niche for tumor growth and metastasis (54). A previous study has demonstrated that
the quantity of CAFs in tumor stroma is associated with the stage
of the tumor and may provide prognostic information (55). Shan et al (56) demonstrated the association between
quantitative levels of FAP in GC stromal and clinicopathological
characteristics. FAPs are secreted by CAFs and act as a regulator
of GC cell invasion and migration, and are highly expressed in
advanced-stage disease (stages III–IV). FAP expression is markedly
increased in patients with lymph node involvement and metastases
compared with patients without metastases. Furthermore, the study
also demonstrated that stromal fibroblasts from the GC invasion
front (the interface zone fibroblasts) had a marked positive FAP
expression compared with NFs and CAFs (56).
It has been demonstrated previously that the
predominant cell type in desmoplastic tumor is CAFs (57). De Monte et al (58) demonstrated the association between the
quantity of T helper cell (Th) 2 and Th1 tumor immune infiltrate
present in the tumor stroma, and determined a poor prognosis in
patients with pancreatic cancer who had R0 or R1 resection at stage
IB-III. In addition, it was demonstrated that the CAFs served a
significant function in Th2 immune deviation, which led to the
secretion of thymic stromal lymphopoietin (TSLP) and activated
myeloid dendritic cells (DCs) with features of TSLP-conditioned DCs
with Th2-polarizing capability. Berdiel-Acer et al (59) observed the fibroblast migratory
potential between normal colonic fibroblasts (NCFs) obtained from
normal colonic mucosa between 5 and 10 cm from the surgical margin,
and CAFs from primary tumors and hepatic metastasis (CAF-LM)
obtained from fresh liver metastases. Results demonstrated that the
transcriptomic signature of fibroblasts, which were defined in the
study, was able to function as an independent predictor of patient
outcome and facilitate the selection of patients at risk of disease
recurrence, particularly high-risk patients. Furthermore, genetic
analysis demonstrated that the ZEB1, SNAI1, SLUG1, E-cadherin and
N-cadherin genes exhibited a gradual increase in expression during
cancer progression from ECM to liver metastasis, which may regulate
CAF-LM to induce EMT phenotypes in epithelial cells more
efficiently compared with other types of myofibroblasts.
Previous studies have demonstrated that the
inflammatory status and the immune microenvironment promote the
progression of cancer (76–78). Numerous studies have demonstrated that
inflammatory cells, mediators (including chemokines and
TNF-α/IL-1β) and key transcription factors are present in the
cancer TME in experimental animal models and human tissues
(79–81). Notably, simultaneous acute recruitment
of immune cell infiltration and fibrosis has been reported
previously, which may demonstrate the association between CAF and
the immune microenvironment (82,83).
The coevolution between cancer cells and stromal
cells increases the number of inflammatory mediators and leads to
the formation of a cancer-associated immune microenvironment
(79,84,85). There
have been previous attempts to classify the tumor stroma into three
groups, Collagen-dominant, fibroblast-dominant or
lymphocyte-dominant, on the basis of the stromal status. Notably,
the dominant stromal type may serve as an independent predictor of
DFS, particularly in patients with high-grade tumors. Furthermore,
lymphocyte-dominant types predicted the longest DFS compared with
the two other types; this suggested that lymphocytic infiltration
is associated with a favorable prognosis (83,86–88).
Notably, a previous study (89) has
provided evidence that CAFs produce pro-inflammatory factors
including IL-6, cyclooxygenase 2 (COX-2) and CXC chemokine ligand 1
that drive leukocyte infiltration. Thus, CAFs may promote tumor
progression by facilitating immune cell infiltration. Macrophages
are derived from CD34+ bone marrow progenitors which
continually proliferate and differentiate into specific types of
resident tissue macrophages, and are prominent components in the
stroma accounting for almost all types of malignancy (90). Additionally, macrophages at the tumor
periphery are able to foster local invasion by supplying
matrix-degrading enzymes, including MMPs and cysteine cathepsin
proteases (91). It has been
demonstrated previously that tumor-associated macrophage (TAM)
infiltration is associated with poor prognostic features, higher
tumor grades and decreased DFS in patients with cancer (91–93).
Herrera et al (94)
demonstrated that the combination of CAFs and M2 macrophages were
associated with poor disease-free survival and overall survival
rates in advanced-stage patients, and also provided evidence of the
prognostic potential of combining these two cells types of cell.
The mechanism of action described previously indicated that
histidine-rich glycoprotein suppressed placental growth
factor-dependent polarization of the tumor immune environment, and
regulated the suppression of macrophages from a pro-tumor (M2) to
an anti-tumor (M1) phenotype (95).
VEGF, secreted by CAFs, served an immunosuppressive function by
affecting T-cell progenitors and leading to an increase in the
infiltration of regulatory T cells and myeloid-derived suppressor
cells, triggering immunosuppression (96). When investigating the function of
CAF-rich desmoplastic stroma in pancreatic ductal adenocarcinoma,
results demonstrated that there was an increase in the number of
inflammatory markers including MCP-1, also termed chemokine ligand
(CCL) 2 (CCL20, TGFβ, indoleamine-pyrrole 2,3-dioxygenase, IL-6 and
COX-2 were also identified) (97).
MCP-1 is a well-characterized chemokine involved in attracting
macrophages into the TME, and inducing the differentiation of
macrophages into an immunosuppressive M2 type (98). CAFs that are isolated from
pre-neoplastic skin lesions expressed a pro-inflammatory gene
signature and promoted macrophage recruitment in vivo in an
NF-κB-dependent manner (99). It has
been previously demonstrated that CCL2 and CXCL14 (secretions from
CAFs) are able to increase the recruitment of macrophages and
promote their intravasive ability (100,101). A
previous study also demonstrated that TGF-β (a product of TAMs and
MDSCs including CAFs) possesses the ability to improve the
phagocytic ability of TAMs and limit the ability of DC to
internalize, present the antigen and transport the antigen to the
draining lymphatic system (102).
Additionally, TGF-β attenuated interferon-γ secretion by natural
killer (NK) cells, resulting in the impairment of Th1
differentiation, and inhibited the expression of NK cell-activating
receptors including NK group 2, member D, NKp6, NKp44 and NKp30
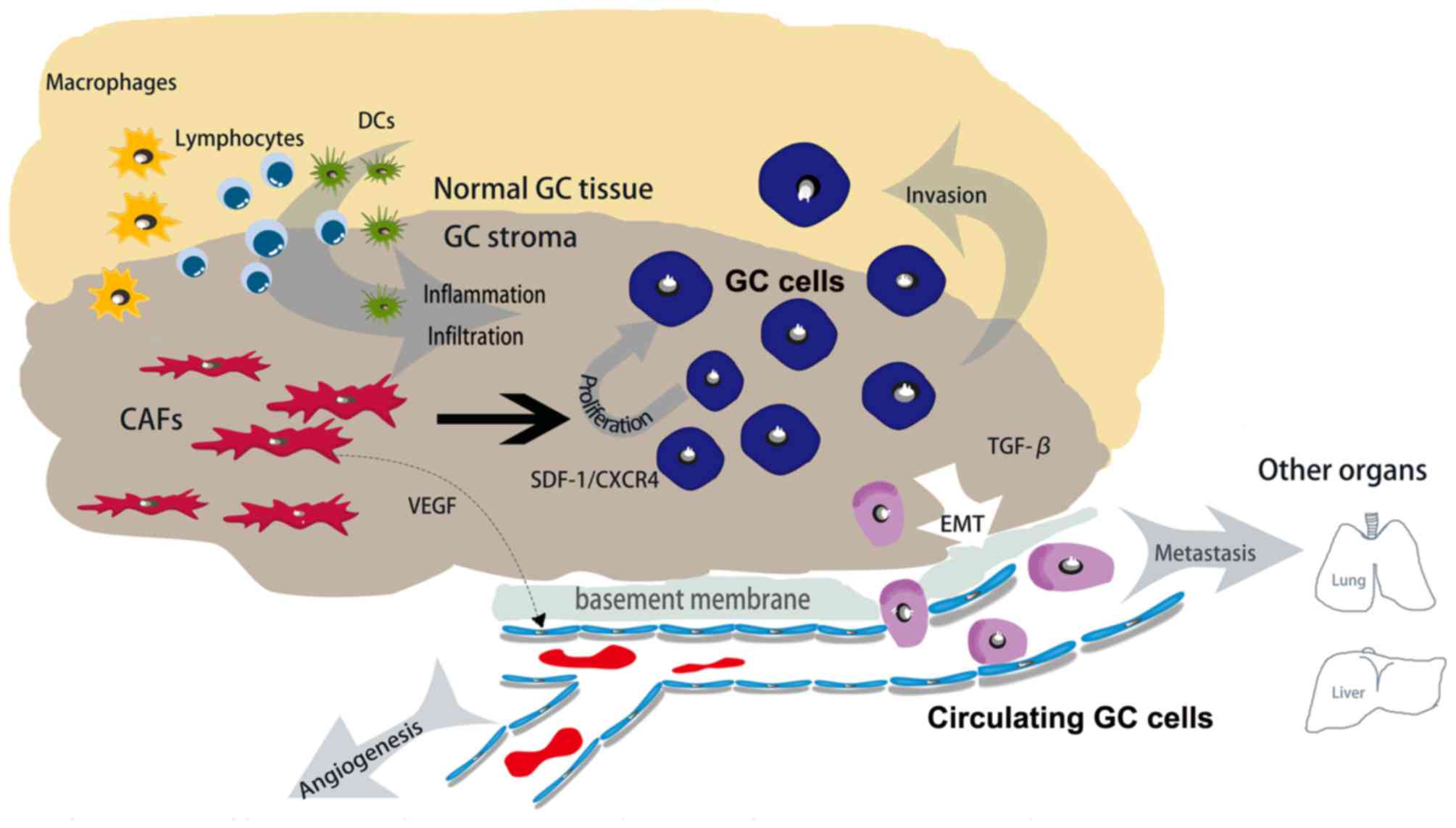
(103,104). Essentially, the direct action of
CAFs may be able to mediate the infiltration of immune cells for a
sustained anticancer immune response (Fig. 2).
CAFs are an important component in the TME in GC,
the research of which is becoming increasingly important. In the
present review, the potential origin of CAFs in GC, their
distinctive secretions that may be used to identify CAFs and how
CAFs are able to influence GC progression have been discussed. The
studies discussed in the present review demonstrate that CAFs may
modulate several aspects of tumor biological behavior in GC
including the ability to proliferate, metastasize and invade.
Additionally, CAFs increase the infiltration of immune cells into
GC stroma and increase the rate of angiogenesis by secreting VEGF.
However, further investigation is required in order to determine
the precise origin of CAFs in GC and the mechanisms underlying the
role of CAFs in regulating the evolution of cancer cells in GC.
The present review was supported by the Science Fund
of the National Natural Science Foundation of China (grant nos.
81401515 and 81230031), the Fundamental Research Funds for the
Central Universities of Ministry of Education of China (grant no.
2042014kf0096) and the 351 Talent Project (Luojia Young Scholars)
of Wuhan University.
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wong H and Yau T: Targeted therapy in the
management of advanced gastric cancer: Are we making progress in
the era of personalized medicine? Oncologist. 17:346–358. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki HI, Katsura A, Matsuyama H and
Miyazono K: MicroRNA regulons in tumor microenvironment. Oncogene.
34:3085–3094. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peng CW, Tian Q, Yang GF, Fang M, Zhang
ZL, Peng J, Li Y and Pang DW: Quantum-dots based simultaneous
detection of multiple biomarkers of tumor stromal features to
predict clinical outcomes in gastric cancer. Biomaterials.
33:5742–5752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee K, Hwang H and Nam KT: Immune response
and the tumor microenvironment: How they communicate to regulate
gastric cancer. Gut Liver. 8:131–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JW, Nam KH, Ahn SH, Park DJ, Kim HH,
Kim SH, Chang H, Lee JO, Kim YJ, Lee HS, et al: Prognostic
implications of immunosuppressive protein expression in tumors as
well as immune cell infiltration within the tumor microenvironment
in gastric cancer. Gastric Cancer. 19:42–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim MG, Shon Y, Kim J and Oh YK: Selective
activation of anticancer chemotherapy by cancer-associated
fibroblasts in the tumor microenvironment. J Natl Cancer Inst.
109:pii: djw1862017. View Article : Google Scholar
|
|
9
|
Kanemaru A, Yamamoto K, Kawaguchi M,
Fukushima T, Lin CY, Johnson MD, Camerer E and Kataoka H:
Deregulated matriptase activity in oral squamous cell carcinoma
promotes the infiltration of cancer-associated fibroblasts by
paracrine activation of protease-activated receptor 2. Int J
Cancer. 140:130–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ouyang L, Chang W, Fang B, Qin J, Qu X and
Cheng F: Estrogen-induced SDF-1α production promotes the
progression of ER-negative breast cancer via the accumulation of
MDSCs in the tumor microenvironment. Sci Rep. 6:395412016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamangar F: Gastric cancer: Descriptive
epidemiology, risk factors, screening and prevention. Cancer
Epidemiol Biomarkers Prev. 23:700–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Quante M, Tu SP, Tomita H, Gonda T, Wang
SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, et al: Bone
marrow-derived myofibroblasts contribute to the mesenchymal stem
cell niche and promote tumor growth. Cancer cell. 19:257–272. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Räsänen K and Vaheri A: Activation of
fibroblasts in cancer stroma. Exp Cell Res. 316:2713–2722. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang J, Lu Y, Lin YY, Zheng ZY, Fang JH,
He S and Zhuang SM: Vascular mimicry formation is promoted by
paracrine TGF-β and SDF1 of cancer-associated fibroblasts and
inhibited by miR-101 in hepatocellular carcinoma. Cancer Lett.
383:18–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia Q, Zhang FF, Geng F, Liu CL, Wang YQ,
Xu P, Lu ZZ, Xie Y, Wu H, Chen Y, et al: Improvement of anti-tumor
immunity of fibroblast activation protein α based vaccines by
combination with cyclophosphamide in a murine model of breast
cancer. Cell Immunol. 310:89–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Polanska UM and Orimo A:
Carcinoma-associated fibroblasts: Non-neoplastic tumour-promoting
mesenchymal cells. J Cell Physiol. 228:1651–1657. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maeda K, Chung YS, Ogawa Y, Takatsuka S,
Kang SM, Ogawa M, Sawada T and Sowa M: Prognostic value of vascular
endothelial growth factor expression in gastric carcinoma. Cancer.
77:858–863. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Terai S, Fushida S, Tsukada T, Kinoshita
J, Oyama K, Okamoto K, Makino I, Tajima H, Ninomiya I, Fujimura T,
et al: Bone marrow derived ‘fibrocytes’ contribute to tumor
proliferation and fibrosis in gastric cancer. Gastric Cancer.
18:306–313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang TS, Yang XH, Chen X, Wang XD, Hua J,
Zhou DL, Zhou B and Song ZS: MicroRNA-106b in cancer-associated
fibroblasts from gastric cancer promotes cell migration and
invasion by targeting PTEN. FEBS Lett. 588:2162–2169. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu J, Qian H, Shen L, Zhang X, Zhu W,
Huang L, Yan Y, Mao F, Zhao C, Shi Y and Xu W: Gastric cancer
exosomes trigger differentiation of umbilical cord derived
mesenchymal stem cells to carcinoma-associated fibroblasts through
TGF-β/Smad pathway. PloS one. 7:e524652012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fuyuhiro Y, Yashiro M, Noda S, Kashiwagi
S, Matsuoka J, Doi Y, Kato Y, Hasegawa T, Sawada T and Hirakawa K:
Upregulation of cancer-associated myofibroblasts by TGF-β from
scirrhous gastric carcinoma cells. Brit J Cancer. 105:996–1001.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han ME, Kim HJ, Shin DH, Hwang SH, Kang CD
and Oh SO: Overexpression of NRG1 promotes progression of gastric
cancer by regulating the self-renewal of cancer stem cells. J
Gastroenterol. 50:645–656. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kikuchi Y, Kunita A, Iwata C, Komura D,
Nishiyama T, Shimazu K, Takeshita K, Shibahara J, Kii I, Morishita
Y, et al: The niche component periostin is produced by
cancer-associated fibroblasts, supporting growth of gastric cancer
through ERK activation. Am J Pathol. 184:859–870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goetz JG, Minguet S, Navarro-Lérida I,
Lazcano JJ, Samaniego R, Calvo E, Tello M, Osteso-Ibáñez T,
Pellinen T, Echarri A, et al: Biomechanical remodeling of the
microenvironment by stromal caveolin-1 favors tumor invasion and
metastasis. Cell. 146:148–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Erez N, Truitt M, Olson P, Arron ST and
Hanahan D: Cancer-associated fibroblasts are activated in incipient
neoplasia to orchestrate tumor-promoting inflammation in an
NF-kappaB-dependent manner. Cancer cell. 17:135–147. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Semba S, Kodama Y, Ohnuma K, Mizuuchi E,
Masuda R, Yashiro M, Hirakawa K and Yokozaki H: Direct
cancer-stromal interaction increases fibroblast proliferation and
enhances invasive properties of scirrhous-type gastric carcinoma
cells. Brit J Cancer. 101:1365–1373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsukada T, Fushida S, Harada S, Yagi Y,
Kinoshita J, Oyama K, Tajima H, Fujita H, Ninomiya I, Fujimura T
and Ohta T: The role of human peritoneal mesothelial cells in the
fibrosis and progression of gastric cancer. Int J Oncol.
41:476–482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fukui H, Zhang X, Sun C, Hara K, Kikuchi
S, Yamasaki T, Kondo T, Tomita T, Oshima T, Watari J, et al: IL-22
produced by cancer-associated fibroblasts promotes gastric cancer
cell invasion via STAT3 and ERK signaling. Br J Cancer.
111:763–771. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He XJ, Tao HQ, Hu ZM, Ma YY, Xu J, Wang
HJ, Xia YJ, Li L, Fei BY, Li YQ and Chen JZ: Expression of
galectin-1 in carcinoma-associated fibroblasts promotes gastric
cancer cell invasion through upregulation of integrin β1. Cancer
Sci. 105:1402–1410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun C, Fukui H, Hara K, Zhang X, Kitayama
Y, Eda H, Tomita T, Oshima T, Kikuchi S, Watari J, et al: FGF9 from
cancer-associated fibroblasts is a possible mediator of invasion
and anti-apoptosis of gastric cancer cells. BMC Cancer. 15:3332015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sung CO, Lee KW, Han S and Kim SH: Twist1
is up-regulated in gastric cancer-associated fibroblasts with poor
clinical outcomes. Am J Pathol. 179:1827–1838. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song YH, Zhu YT, Ding J, Zhou FY, Xue JX,
Jung JH, Li ZJ and Gao WY: Distribution of fibroblast growth
factors and their roles in skin fibroblast cell migration. Mol Med
Rep. 14:3336–3342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang X, Ibrahimi OA, Olsen SK, Umemori H,
Mohammadi M and Ornitz DM: Receptor specificity of the fibroblast
growth factor family. The complete mammalian FGF family. J Biol
Chem. 281:15694–15700. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Basilico C and Moscatelli D: The FGF
family of growth factors and oncogenes. Adv Cancer Res. 59:115–165.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cao H, Xu W, Qian H, Zhu W, Yan Y, Zhou H,
Zhang X and Xu X, Li J, Chen Z and Xu X: Mesenchymal stem cell-like
cells derived from human gastric cancer tissues. Cancer Lett.
274:61–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg
RA: Mesenchymal stem cells within tumour stroma promote breast
cancer metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu X, Zhang X, Wang S, Qian H, Zhu W, Cao
H, Wang M, Chen Y and Xu W: Isolation and comparison of mesenchymal
stem-like cells from human gastric cancer and adjacent
non-cancerous tissues. J Cancer Res Clin Oncol. 137:495–504. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ponta H, Sherman L and Herrlich PA: CD44:
From adhesion molecules to signalling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu B, Chen X, Li J, Qu Y, Su L, Peng Y,
Huang J, Yan J, Yu Y, Gu Q, et al: Stromal fibroblasts in the
microenvironment of gastric carcinomas promote tumor metastasis via
upregulating TAGLN expression. BMC Cell Biol. 14:172013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shimoda M, Mellody KT and Orimo A:
Carcinoma-associated fibroblasts are a rate-limiting determinant
for tumour progression. Semin Cell Dev Biol. 21:19–25. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bourget JM, Gauvin R, Larouche D, Lavoie
A, Labbé R, Auger FA and Germain L: Human fibroblast-derived ECM as
a scaffold for vascular tissue engineering. Biomaterials.
33:9205–9213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Venning FA, Wullkopf L and Erler JT:
Targeting ECM disrupts cancer progression. Front Oncol. 5:2242015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pickup MW, Mouw JK and Weaver VM: The
extracellular matrix modulates the hallmarks of cancer. EMBO Rep.
15:1243–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bonnans C, Chou J and Werb Z: Remodelling
the extracellular matrix in development and disease. Nat Rev Mol
Cell Biol. 15:786–801. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Carmeliet P: Angiogenesis in life, disease
and medicine. Nature. 438:932–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kalluri R: The biology and function of
fibroblasts in cancer. Nat Rev Cancer. 16:582–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Simian M, Hirai Y, Navre M, Werb Z,
Lochter A and Bissell MJ: The interplay of matrix
metalloproteinases, morphogens and growth factors is necessary for
branching of mammary epithelial cells. Development. 128:3117–3131.
2001.PubMed/NCBI
|
|
49
|
Tsujino T, Seshimo I, Yamamoto H, Ngan CY,
Ezumi K, Takemasa I, Ikeda M, Sekimoto M, Matsuura N and Monden M:
Stromal myofibroblasts predict disease recurrence for colorectal
cancer. Clin Cancer Res. 13:2082–2090. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sugimoto H, Mundel TM, Kieran MW and
Kalluri R: Identification of fibroblast heterogeneity in the tumor
microenvironment. Cancer Biol Ther. 5:1640–1646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Erkan M, Michalski CW, Rieder S,
Reiser-Erkan C, Abiatari I, Kolb A, Giese NA, Esposito I, Friess H
and Kleeff J: The activated stroma index is a novel and independent
prognostic marker in pancreatic ductal adenocarcinoma. Clin
Gastroenterol Hepatol. 6:1155–1161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shieh AC, Rozansky HA, Hinz B and Swartz
MA: Tumor cell invasion is promoted by interstitial flow-induced
matrix priming by stromal fibroblasts. Cancer Res. 71:790–800.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Crawford Y, Kasman I, Yu L, Zhong C, Wu X,
Modrusan Z, Kaminker J and Ferrara N: PDGF-C mediates the
angiogenic and tumorigenic properties of fibroblasts associated
with tumors refractory to anti-VEGF treatment. Cancer Cell.
15:21–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lazennec G and Richmond A: Chemokines and
chemokine receptors: New insights into cancer-related inflammation.
Trends Mol Med. 16:133–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Valcz G, Sipos F, Tulassay Z, Molnar B and
Yagi Y: Importance of carcinoma-associated fibroblast-derived
proteins in clinical oncology. J Clin Pathol. 67:1026–1031. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shan LH, Sun WG, Han W, Qi L, Yang C, Chai
CC, Yao K, Zhou QF, Wu HM, Wang LF and Liu JR: Roles of fibroblasts
from the interface zone in invasion, migration, proliferation and
apoptosis of gastric adenocarcinoma. J Clin Pathol. 65:888–895.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Elenbaas B and Weinberg RA: Heterotypic
signaling between epithelial tumor cells and fibroblasts in
carcinoma formation. Exp Cell Res. 264:169–184. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
De Monte L, Reni M, Tassi E, Clavenna D,
Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C and Protti MP:
Intratumor T helper type 2 cell infiltrate correlates with
cancer-associated fibroblast thymic stromal lymphopoietin
production and reduced survival in pancreatic cancer. J Exp Med.
208:469–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Berdiel-Acer M, Bohem ME, López-Doriga A,
Vidal A, Salazar R, Martínez-Iniesta M, Santos C, Sanjuan X,
Villanueva A and Molleví DG: Hepatic carcinoma-associated
fibroblasts promote an adaptative response in colorectal cancer
cells that inhibit proliferation and apoptosis: Nonresistant cells
die by nonapoptotic cell death. Neoplasia. 13:931–946. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Poon RT, Fan ST and Wong J: Clinical
implications of circulating angiogenic factors in cancer patients.
J Clin Oncol. 19:1207–1225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Weidner N, Carroll PR, Flax J, Blumenfeld
W and Folkman J: Tumor angiogenesis correlates with metastasis in
invasive prostate carcinoma. Am J Pathol. 143:401–409.
1993.PubMed/NCBI
|
|
64
|
Weidner N, Folkman J, Pozza F, Bevilacqua
P, Allred EN, Moore DH, Meli S and Gasparini G: Tumor angiogenesis:
A new significant and independent prognostic indicator in
early-stage breast carcinoma. J Natl Cancer Inst. 84:1875–1887.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hayashi Y, Tsujii M, Akasaka T, Kato M,
Inoue T, Tsujii Y, Maekawa A, Shinzaki S, Nishida T, Watabe K, et
al: Carcinoma-associated fibroblasts educated by P53-Incompetent
cancer cells contribute tumor growth through angiogenesis.
Gastroenterol. 146 Suppl:S488–S489. 2014. View Article : Google Scholar
|
|
66
|
Orimo A and Weinberg RA: Stromal
fibroblasts in cancer: A novel tumor-promoting cell type. Cell
Cycle. 5:1597–1601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
De Francesco EM, Lappano R, Santolla MF,
Marsico S, Caruso A and Maggiolini M: HIF-1α/GPER signaling
mediates the expression of VEGF induced by hypoxia in breast cancer
associated fibroblasts (CAFs). Breast Cancer Res. 15:R642013.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li H, Adachi Y, Yamamoto H, Min Y, Ohashi
H, Ii M, Arimura Y, Endo T, Lee CT, Carbone DP, et al: Insulin-like
growth factor-I receptor blockade reduces tumor angiogenesis and
enhances the effects of bevacizumab for a human gastric cancer cell
line, MKN45. Cancer. 117:3135–3147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Pinedo HM, Verheul HM, D'Amato RJ and
Folkman J: Involvement of platelets in tumour angiogenesis? Lancet.
352:1775–1777. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Bilen MA, Zurita AJ, Ilias-Khan NA, Chen
HC, Wang X, Kearney AY, Hodges S, Jonasch E, Huang S, Khakoo AY and
Tannir NM: Hypertension and circulating cytokines and angiogenic
factors in patients with advanced non-clear cell renal cell
carcinoma treated with sunitinib: results from a phase II trial.
Oncologist. 20:1140–1148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Taddei ML, Giannoni E, Comito G and
Chiarugi P: Microenvironment and tumor cell plasticity: An easy way
out. Cancer Lett. 341:80–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hellevik T, Pettersen I, Berg V, Bruun J,
Bartnes K, Busund LT, Chalmers A, Bremnes R and Martinez-Zubiaurre
I: Changes in the secretory profile of NSCLC-associated fibroblasts
after ablative radiotherapy: Potential impact on angiogenesis and
tumor growth. Transl Oncol. 6:66–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tang D, Gao J, Wang S, Ye N, Chong Y,
Huang Y, Wang J, Li B, Yin W and Wang D: Cancer-associated
fibroblasts promote angiogenesis in gastric cancer through
galectin-1 expression. Tumour Biol. 37:1889–1899. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hara M, Nagasaki T, Shiga K and Takeyama
H: Suppression of cancer-associated fibroblasts and endothelial
Cells by itraconazole in bevacizumab-resistant gastrointestinal
cancer. Anticancer Res. 36:169–177. 2016.PubMed/NCBI
|
|
75
|
Bai YP, Shang K, Chen H, Ding F, Wang Z,
Liang C, Xu Y, Sun MH and Li YY: FGF-1/−3/FGFR4 signaling in
cancer-associated fibroblasts promotes tumor progression in colon
cancer through Erk and MMP-7. Cancer Sci. 106:1278–1287. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Uso M, Jantus-Lewintre E, Bremnes RM,
Calabuig S, Blasco A, Pastor E, Borreda I, Molina-Pinelo S,
Paz-Ares L and Guijarro R: Analysis of the immune microenvironment
in resected non-small cell lung cancer: The prognostic value of
different T lymphocyte markers. Oncotarget. 7:52849–52861. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
He J, Hu Y, Hu M and Li B: Development of
PD-1/PD-L1 Pathway in tumor immune microenvironment and treatment
for non-small cell lung cancer. Sci Rep. 5:131102015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Whiteside TL: Apoptosis of immune cells in
the tumor microenvironment and peripheral circulation of patients
with cancer: Implications for immunotherapy. Vaccine. 20 Suppl
4:A46–A51. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Flossmann E and Rothwell PM; British
Doctors Aspirin Trial and the UK-TIA Aspirin Trial, : Effect of
aspirin on long-term risk of colorectal cancer: Consistent evidence
from randomised and observational studies. Lancet. 369:1603–1613.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chan AT, Ogino S and Fuchs CS: Aspirin and
the risk of colorectal cancer in relation to the expression of
COX-2. N Engl J Med. 356:2131–2142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Haviv I, Polyak K, Qiu W, Hu M and
Campbell I: Origin of carcinoma associated fibroblasts. Cell Cycle.
8:589–595. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sangai T, Ishii G, Kodama K, Miyamoto S,
Aoyagi Y, Ito T, Magae J, Sasaki H, Nagashima T, Miyazaki M and
Ochiai A: Effect of differences in cancer cells and tumor growth
sites on recruiting bone marrow-derived endothelial cells and
myofibroblasts in cancer-induced stroma. Int J Cancer. 115:885–892.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
De Falco V, Guarino V, Avilla E,
Castellone MD, Salerno P, Salvatore G, Faviana P, Basolo F, Santoro
M and Melillo RM: Biological role and potential therapeutic
targeting of the chemokine receptor CXCR4 in undifferentiated
thyroid cancer. Cancer Res. 67:11821–11829. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Borrello MG, Alberti L, Fischer A,
Degl'innocenti D, Ferrario C, Gariboldi M, Marchesi F, Allavena P,
Greco A, Collini P, et al: Induction of a proinflammatory program
in normal human thyrocytes by the RET/PTC1 oncogene. Pro Natl Acad
Scie USA. 102:14825–14830. 2005. View Article : Google Scholar
|
|
86
|
Ahn S, Cho J, Sung J, Lee JE, Nam SJ, Kim
KM and Cho EY: The prognostic significance of tumor-associated
stroma in invasive breast carcinoma. Tumour Biol. 33:1573–1580.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Martinet L, Garrido I, Filleron T, Le
Guellec S, Bellard E, Fournie JJ, Rochaix P and Girard JP: Human
solid tumors contain high endothelial venules: Association with T-
and B-lymphocyte infiltration and favorable prognosis in breast
cancer. Cancer Res. 71:5678–5687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Rosenwald A, Wright G, Chan WC, Connors
JM, Campo E, Fisher RI, Gascoyne RD, Muller-Hermelink HK, Smeland
EB, Giltnane JM, et al: The use of molecular profiling to predict
survival after chemotherapy for diffuse large-B-cell lymphoma. N
Engl J Med. 346:1937–1947. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Erez N, Glanz S, Raz Y, Avivi C and
Barshack I: Cancer associated fibroblasts express pro-inflammatory
factors in human breast and ovarian tumors. Biochem Biophys Res
Commun. 437:397–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lewis CE and Pollard JW: Distinct role of
macrophages in different tumor microenvironments. Cancer Res.
66:605–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Laoui D, Movahedi K, Van Overmeire E, van
den Bossche J, Schouppe E, Mommer C, Nikolaou A, Morias Y, De
Baetselier P and Van Ginderachter JA: Tumor-associated macrophages
in breast cancer: Distinct subsets, distinct functions. Int J Dev
Biol. 55:861–867. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Campbell MJ, Tonlaar NY, Garwood ER, Huo
D, Moore DH, Khramtsov AI, Au A, Baehner F, Chen Y, Malaka DO, et
al: Proliferating macrophages associated with high grade, hormone
receptor negative breast cancer and poor clinical outcome. Breast
Cancer Res Treat. 128:703–711. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lee AH, Happerfield LC, Bobrow LG and
Millis RR: Angiogenesis and inflammation in invasive carcinoma of
the breast. J Clin Pathol. 50:669–673. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Herrera M, Herrera A, Dominguez G,
Domínguez G, Silva J, García V, García JM, Gómez I, Soldevilla B,
Muñoz C, Provencio M, et al: Cancer-associated fibroblast and M2
macrophage markers together predict outcome in colorectal cancer
patients. Cancer Sci. 104:437–444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Rolny C, Mazzone M, Tugues S, Laoui D,
Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, et
al: HRG inhibits tumor growth and metastasis by inducing macrophage
polarization and vessel normalization through downregulation of
PlGF. Cancer Cell. 19:31–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wada J, Suzuki H, Fuchino R, Yamasaki A,
Nagai S, Yanai K, Koga K, Nakamura M, Tanaka M, Morisaki T and
Katano M: The contribution of vascular endothelial growth factor to
the induction of regulatory T-cells in malignant effusions.
Anticancer Res. 29:881–888. 2009.PubMed/NCBI
|
|
97
|
Tjomsland V, Spångeus A, Välilä J,
Sandström P, Borch K, Druid H, Falkmer S, Falkmer U, Messmer D and
Larsson M: Interleukin 1alpha sustains the expression of
inflammatory factors in human pancreatic cancer microenvironment by
targeting cancer-associated fibroblasts. Neoplasia. 13:664–675.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Roca H, Varsos ZS, Sud S, Craig MJ, Ying C
and Pienta KJ: CCL2 and interleukin-6 promote survival of human
CD11b+ peripheral blood mononuclear cells and induce M2-type
macrophage polarization. J Biol Chem. 284:34342–34354. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Mantovani A: La mala educacion of
tumor-associated macrophages: Diverse pathways and new players.
Cancer cell. 17:111–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Tsuyada A, Chow A, Wu J, Somlo G, Chu P,
Loera S, Luu T, Li AX, Wu X, Ye W, et al: CCL2 mediates cross-talk
between cancer cells and stromal fibroblasts that regulates breast
cancer stem cells. Cancer Res. 72:2768–2779. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Augsten M, Hägglöf C, Olsson E, Stolz C,
Tsagozis P, Levchenko T, Frederick MJ, Borg A, Micke P, Egevad L
and Ostman A: CXCL14 is an autocrine growth factor for fibroblasts
and acts as a multi-modal stimulator of prostate tumor growth. Proc
Natl Acad Sci USA. 106:pp. 3414–3419. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Byrne SN, Knox MC and Halliday GM: TGFbeta
is responsible for skin tumour infiltration by macrophages enabling
the tumours to escape immune destruction. Immunol Cell Biol.
86:92–97. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Bekeredjian-Ding I, Schäfer M, Hartmann E,
Pries R, Parcina M, Schneider P, Giese T, Endres S, Wollenberg B
and Hartmann G: Tumour-derived prostaglandin E and transforming
growth factor-beta synergize to inhibit plasmacytoid dendritic
cell-derived interferon-alpha. Immunology. 128:439–450. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Weber F, Byrne SN, Le S, Brown DA, Breit
SN, Scolyer RA and Halliday GM: Transforming growth factor-beta1
immobilises dendritic cells within skin tumours and facilitates
tumour escape from the immune system. Cancer Immunol Immunotherap.
54:898–906. 2005. View Article : Google Scholar
|