Introduction
Cancer, a prevalent health concern, is a complex
disease that affects the quality of life and life expectancy of
patients globally (1). The growth of
transformed cells is regulated by a number of genes that also
regulate normal cell proliferation (2). At present, chemotherapy is one of the
most important modalities for cancer treatments; however, it is not
completely effective (3). Drug
discovery has identified a number of natural products that may be
able to treat cancer (4). The skin of
amphibians fulfills a wide range of functions necessary for their
survival and previous studies have demonstrated that amphibians,
including frogs and toads, possess a number of bioactive agents in
their skin (5,6). This has led to a growing interest in
whether these agents may exhibit anticancer effects.
The extracts of amphibian skin have been used in
Chinese medicine to treat different types of diseases (7,8). A number
of natural products have been identified as major pharmacological
constituents in toad skin, including hydrosoluble indole alkaloids
(bufotenine, bufotenidine, and cinobufotenine) and liposoluble
steroidal cardiac glycosides, including bufadienolides (9,10).
Bufadienolides, including cinobufagin, bufotalin and bufalin, have
been demonstrated to inhibit Na+K+-ATPase and
exhibit wide-spectrum cytotoxic effects on a number of human cancer
cells (11). Wen et al
(12) demonstrated the
anti-inflammatory and anti-nociceptive activities of bufalin in
rodents. In addition, a previous study demonstrated that
cinobufacini effectively induced the apoptosis of lens epithelial
cells by suppressing the expression of B-cell lymphoma 2 (Bcl-2)
family proteins (13). Due to its low
toxicity, the medication prevented and treated posterior capsule
opacification (14). In addition,
bufalin induced cancer cell apoptosis by repressing the expression
of microRNA-181a to decrease the gene expression of Bcl-2 (15). Wang et al (16) demonstrated that bufalin and
cinobufagin induced HepG2 cell apoptosis through the extrinsic Fas-
and intrinsic mitochondria-mediated signaling pathways, where the
Fas-mediated caspase-10-dependent pathway may serve a more
important function (16). Bufalin
inhibited SK-Hep1 cell migration and invasion by inhibiting the
nuclear factor-κ-light-chain-enhancer of activated B cells and
matrix metalloproteinase-2/−9- pathways (17). Cinobufacini induced the apoptosis of
T-47D cells in a caspase-3-dependent manner (18). In pancreatic cancer cells, bufalin
exhibited its anticancer action through induction of cell cycle
arrest and cell apoptosis (19).
Arenobufagin has been demonstrated to induce human hepatocellular
carcinoma cell apoptosis and autophagy by inhibiting the
phosphoinositide 3-kinase/RAC-α serine/threonine-protein
kinase/mammalian target of rapamycin signaling pathway (20). These results have demonstrated the
application potential of bufadienolides for the treatment of
cancer; however, the effects and the underlying molecular
mechanisms of the effect of bufadienolides on esophageal squamous
cell carcinoma (ESCC) cells remain elusive.
In the present study, the anticancer activities of
two bufadienolides, bufotalin and bufalin, were investigated in
vitro and in vivo. The results suggested that bufotalin
and bufalin effectively impeded the viability of a panel of five
ESCC cell lines by inducing cell apoptosis through activation of
the tumor protein p53 (p53) signaling pathway. Furthermore,
bufotalin exhibited in vivo anticancer efficacy in a nude
mouse model, where bufotalin inhibited the growth of tumors through
activation of the p53 signaling pathway. Overall, these findings
indicated that bufadienolides exert their effects against ESCC
through the regulation of the p53 signaling pathway.
Materials and methods
Reagents and materials
Bufotalin (10102631) and bufalin (11070631)
standards were provided by Shanghai Tauto Biotech Co., Ltd.
(Shanghai, China). All other reagents were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Cell culture
Human ESCC cell lines (Eca-109, EC9706, TE5, Hec2
and TE11) were purchased from the American Type Culture Collection
(Manassas, VA, USA) and the non-malignant human esophageal squamous
cell line Het-1A was purchased from GuangZhou Jennio Biotech Co.,
Ltd. (Guangzhou, China). Eca-109, EC9706, TE5, Hec2 and TE11 cells
were cultured and maintained in RPMI-1640 (Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), penicillin (100 U/ml) and streptomycin (50 µg/ml) at 37°C in
a humidified incubator with 5% CO2. Het-1A cells were
cultured and maintained in Dulbecco's modified Eagle's medium
(DMEM; Hyclone; GE Healthcare Life Sciences) supplemented with 10%
FBS, penicillin (100 U/ml) and streptomycin (50 µg/ml) at 37°C in a
humidified incubator with 5% CO2.
Examination of cell viability by MTT
assay
The effects of bufotalin on cell viability were
determined using an MTT assay as previously described (10). Briefly, Eca-109 (2×104
cells/ml), EC9706 (2×104 cells/ml), TE5
(2×104 cells/ml), Hec2 (2×104 cells/ml), TE11
(2×104 cells/ml) and Het-1A cells (2×104
cells/ml) were cultured in DMEM supplemented with 10% FBS, 100 U/ml
penicillin and 50 µg/ml streptomycin at 37°C in a humidified
incubator under 5% CO2. The cell viability
(2×104 cells/ml) following treatment with 2 and 4 µM
bufalin or bufotalin at 37°C for 72 h was determined by the MTT
assay. Eca-109, EC9706, TE5, Hec2, TE11 and Het-1A cells without
drug treatment functioned as control groups. The color intensity
was measured at 575 nm on a microplate spectrophotometer (VersaMax;
Molecular Devices, LLC, Sunnyvale, CA, USA) (21).
Flow cytometry analysis
Following exposure to different treatments as
aforementioned in the MTT assay section, Eca-109, EC9706, TE5,
Hec2, TE11 and Het-1A cells (2×104 cells/ml) were washed
with PBS twice and then fixed with 70% ethanol at −20°C in the dark
overnight. The fixed cells were stained with propidium iodide (50
µg/ml) for 2 h at 37°C in the dark and then flow cytometry was
performed using a Coulter® Epics®
XL™ flow cytometer (Beckman Coulter, Inc., Brea, CA,
USA). Apoptotic cells were quantified using the sub-G1 peak in the
cell cycle distribution histogram as previously described (22).
Examination of apoptotic DNA
fragmentation by terminal deoxynucleotidyl transferase dUTP nick
end labeling (TUNEL) and DAPI co-staining assay
Following treatment, cells (1×105
cells/ml) cultured in confocal dishes were fixed with formaldehyde
for 10 min at room temperature and permeabilized with 0.1% Triton
X-100 in PBS for 2 min at 4°C. The cells were then incubated with
100 µl/well TUNEL reaction mixture containing nucleotide mixture
and terminal deoxynucleotidyl transferase for 1 h at 37°C and then
stained with DAPI (1 µg/ml) for 20 min at 37°C. Following washing
with PBS, the cells were examined on a confocal fluorescence
microscope (Zeiss LSM 510 Meta confocal microscope; Zeiss AG,
Oberkochen, Germany) as described previously (10). Three fields of view were captured and
selected randomly.
Activation of caspases by
bufadienolides
The enzymatic activities of caspase-3, −8 and −9 in
cells treated with bufadienolides were determined with a
fluorometric method using specific caspase substrates (Ac-DEVD-AMC
for caspase-3, Ac-IETD-AMC for caspase-8 and Ac-LEHD-AMC for
caspase-9 respectively), as described previously (16).
Western blot analysis
Following treatment with 2 and 4 µM bufotalin for 24
h, cells (1×105 cells/ml) were harvested using a cell
scraper at 4°C and the cell pellets were collected by
centrifugation at a speed of 1,000 × g for 15 min at 4°C, and were
lysed in RIPA cell lysis buffer on ice for 1 h. The total proteins
were obtained from supernatant after centrifugation (16,099 × g) at
4°C for 30 min. The protein concentration of cell lysates was
determined using a BCA assay kit (Sigma-Aldrich; Merck KGaA) as
described previously (23). Equal
amounts of protein (20 µg/lane) were separated on a 12% SDS-PAGE
gel and transferred to polyvinylidene fluoride membranes. Following
blocking with 5% non-fat milk at 4°C for 2 h, the membranes were
incubated with primary antibodies p53 (cat. no. 9282; Cell
Signaling Technology, Inc., Danvers, MA, USA) and phosphorylated
p53 serine-15 (p-p53; cat. no. 9284, Cell Signaling Technology,
Inc.) both at a dilution of 1:1,000 at 4°C overnight. Following
washing with TBS solution, the membranes were incubated with
corresponding secondary antibodies for 2 h at 4°C and visualized by
Pierce enhanced chemiluminescence western blotting substrate
(Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
p53 small interfering (si)RNA
transfection
Cells were seeded at a density of 2×106
cells/ml in 6-well plates, which were allowed to grow to 60%
confluence after 24 h. The cells were then incubated with p53 siRNA
(100 nmol/l; cat. no. 6231; Cell Signaling Technology, Inc.) in
serum-free DMEM for 24 h, with Lipofectamine® 2000 (cat.
no. 11668027; Thermo Fisher Scientific, Inc.) used as a
transfection reagent according to the manufacturer's protocol.
Then, fresh serum-free DMEM medium with 10% fetal bovine serum was
added to each well for 24 h with or without 2 and 4 µM
bufadienolides at 4°C for 24 h. Control cells were transfected with
a fluorescein-labeled non-targeted scramble control siRNA (cat. no.
78193; Cell Signaling Technology, Inc.), which allowed for the
monitoring of transfection efficiency. Following siRNA
transfection, the cells were subjected to apoptotic analysis as
aforementioned.
Immunofluorescence analysis of protein
expression in cells
Levels of p53 (cat. no. 9282; Cell Signaling
Technology, Inc.) and phosphorylated p53 serine-15 (p-p53; cat. no.
9284; Cell Signaling Technology, Inc.), both at a dilution of
1:1,000 in the treated cells were analyzed by immunofluorescence.
Following treatment, 4×104 cells were seeded, washed
with PBS and fixed with 3.7% formaldehyde in PBS for 15 min at 4°C.
Following rinsing with PBS, the cells were permeabilized with 0.2%
Triton X-100 for 5 min at room temperature. The permeabilized cells
were then blocked for 30 min with 0.1% bovine serum albumin (Gibco;
Thermo Fisher Scientific, Inc.) at room temperature and incubated
with p-p53 primary antibodies at 4°C overnight. Cells were then
incubated with Alexa-488 labeled anti-rabbit immunoglobulin G (IgG)
antibody (cat. no. 4412; 1:250; Cell Signaling Technology, Inc.)
for 1 h at room temperature. The cells nuclei were stained with
Hoechst 33342 (1 µg/ml) for 20 min at 37°C. A total of 3 fields of
view of each slide were selected randomly and analyzed using a
confocal fluorescence microscope (Zeiss LSM 510 Meta confocal
microscope with LSM 510 software; Zeiss AG).
Tumor xenograft in nude mice
The female Balb/c nude mice (age, 3–4 weeks; weight,
14–15 g; n=40) were purchased from Beijing HuaFuKang Bioscience
Co., Ltd. (Beijing, China) and housed ad libitum at ~25°C.
The pressure difference was 25 Pa, relative humidity was 40–70%,
and the light/dark cycle was 12/12 h. The mice were acclimated to
the environment for 10 days prior to treatment. Eca-109 cells
(5×106) suspended in PBS were injected subcutaneously
into the right lower hind flank of each 6-week-old male nude mouse.
The mice were then randomly assigned into three groups with 10 mice
in each group. After 10 days, bufotalin dissolved in solution
(N.N-dimethylformamidev:Tween-80v:salinev=10:2:88)
was administered (intraperitoneal; 2 or 4 mg/kg body weight every
other day) for 20 days. Control mice received an equal volume of
the vehicle (saline). The body weights and tumor volumes were
monitored every two days. After 20 days, tumor size had grown to
~10 mm (the humane end-point) and tumor xenografts were collected
and weighed.
The tumor dimension was measured with calipers, and
the tumor volume was calculated with the following formula: Volume
= lx w2/2, where l is the maximal length and w is the
width. A portion of the tumors from control and treated groups were
fixed in 10% formalin at room temperature for 24 h, dehydrated with
graded ethanol (80, 90, 95 and 100%) and embedded in paraffin
(section thickness, 4 µm) for hematoxylin (0.5 mg/ml) staining for
15 min at room temperature and eosin (0.5 mg/ml) staining for 5 min
at room temperature and immunohistochemical analyses.
Immunofluorescence staining analysis (magnification, ×20) using a
confocal fluorescence microscope (Zeiss LSM 510 Meta confocal
microscope with LSM 510 software; Zeiss AG) was performed with
anti-p-p53 (dilution, 1:100; Ser15; cat. no. 9284; Cell
Signaling Technology, Inc.) at 4°C overnight and ki67 antibodies at
4°C overnight (dilution, 1:100; cat. no. 9129; Cell Signaling
Technology, Inc.) and Alexa Fluor 488-conjugated anti-rabbit IgG
(cat. no. 4340; Cell Signaling Technology, Inc.) antibodies (1:100)
at room temperature for 1 h. Blocking was performed with 5% bovine
serum albumin for 15 min at room temperature. All animal studies
were approved by the Animal Experimentation Ethics Committee of
Guangdong No. 2 Provincial People's Hospital (Guanzhou, China).
Statistical analysis
In the present study, all experiments were performed
using SPSS 10.0 (SPSS, Inc., Chicago, IL, USA) ≥3 times and the
data were presented as the mean ± standard error of the mean.
Differences between two groups were analyzed using a two-tailed
Student's t-test and differences among three or more groups was
analyzed by one-way analysis of variance and Fisher's least
significant difference post hoc test. P<0.05 was considered to
indicate a statistically significant difference. Quantitative
analysis was performed using Quantity-one 1D software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Results and Discussion
Bufotalin and bufalin effectively
inhibit ESCC cell viability
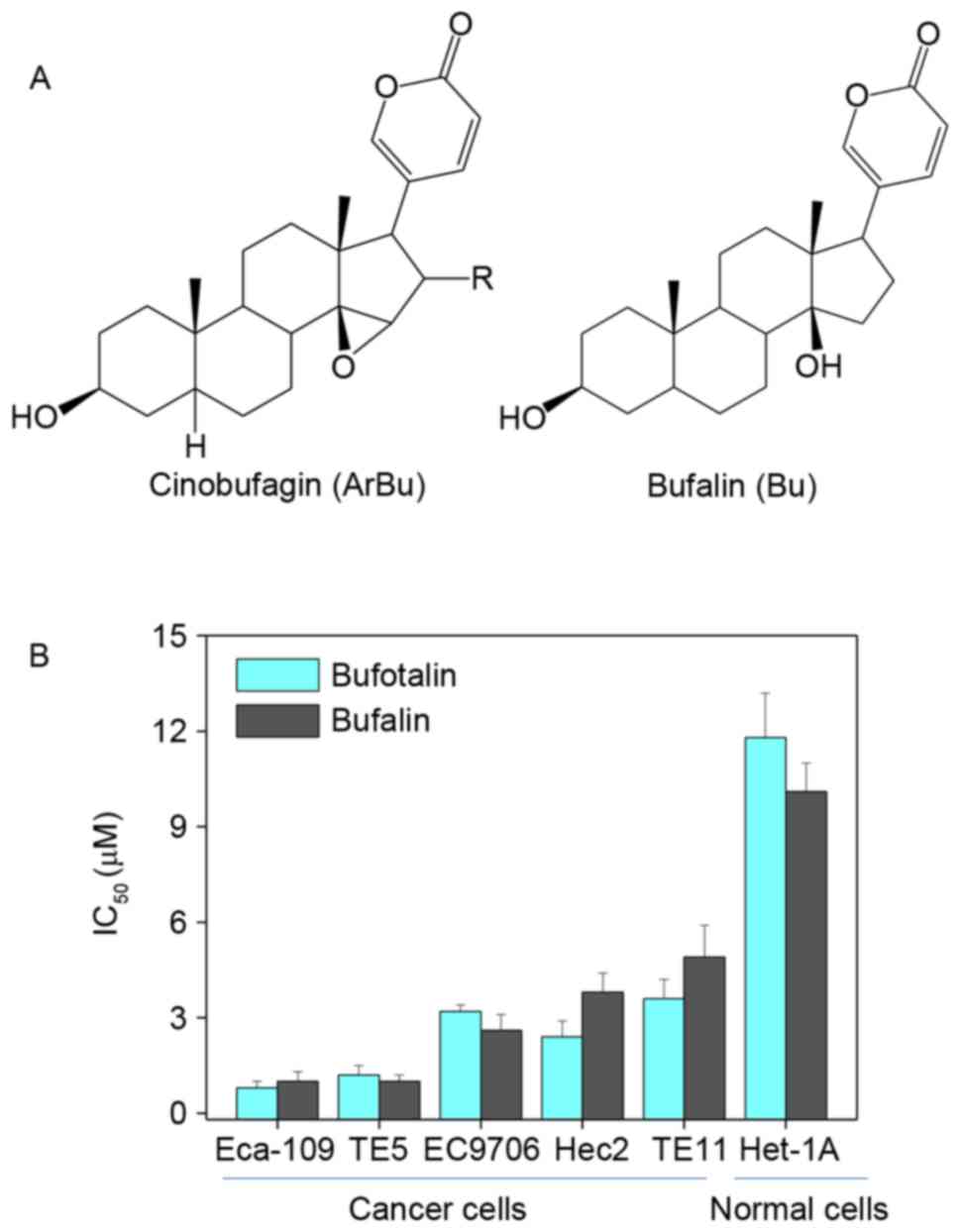
The in vitro anticancer effects of bufotalin
and bufalin (chemical structures are illustrated in Fig. 1A) were investigated in five human ESCC
cancer cell lines, including Eca-109, EC9706, TE5, Hec2 and TE11,
and a human esophageal squamous cell line Het-1A. Following
treatment with 2 µM bufotalin and 4 µM bufalin at 37°C for 72 h,
cell viability was detected using an MTT assay. Half-maximal
inhibitory concentration (IC50) values revealed that
bufotalin and bufalin effectively inhibited the cell viability of
ESCC cells, particularly bufotalin, which exhibited IC50
values of 0.8, 1.2, 3.2, 2.4 and 3.6 µM for Eca-109, TE5, EC9706,
Hec2 and TE11 cells, respectively (Fig.
1B). This improved effect of bufotalin compared with bufalin
was observed in the TE5, EC9706 and HET-1A cell lines. Notably,
bufotalin and bufalin exhibited markedly lower toxicity towards
Het-1A human esophageal squamous cells, indicating the high
selectivity of these two compounds towards cancer cells.
Collectively, these results indicated that bufotalin and bufalin
may have applications in the treatment of ESCC.
Activation of cell apoptosis by
bufotalin
Dysregulation of cell apoptosis (also known as
programmed cell death) has been demonstrated to be associated with
a number of human chronic diseases, including cancer (24). Induction of cancer cell apoptosis has
been identified as an effective way to treat cancer (25). The growth inhibitory activities of the
majority of anticancer drugs on cancer cells are achieved through
the induction of apoptosis (26).
Results from the MTT assay in the present study demonstrated that
bufotalin exhibited markedly increased anticancer efficacy compared
with bufalin. Therefore, further studies were performed to explore
the molecular mechanisms underlying the effects of bufotalin on
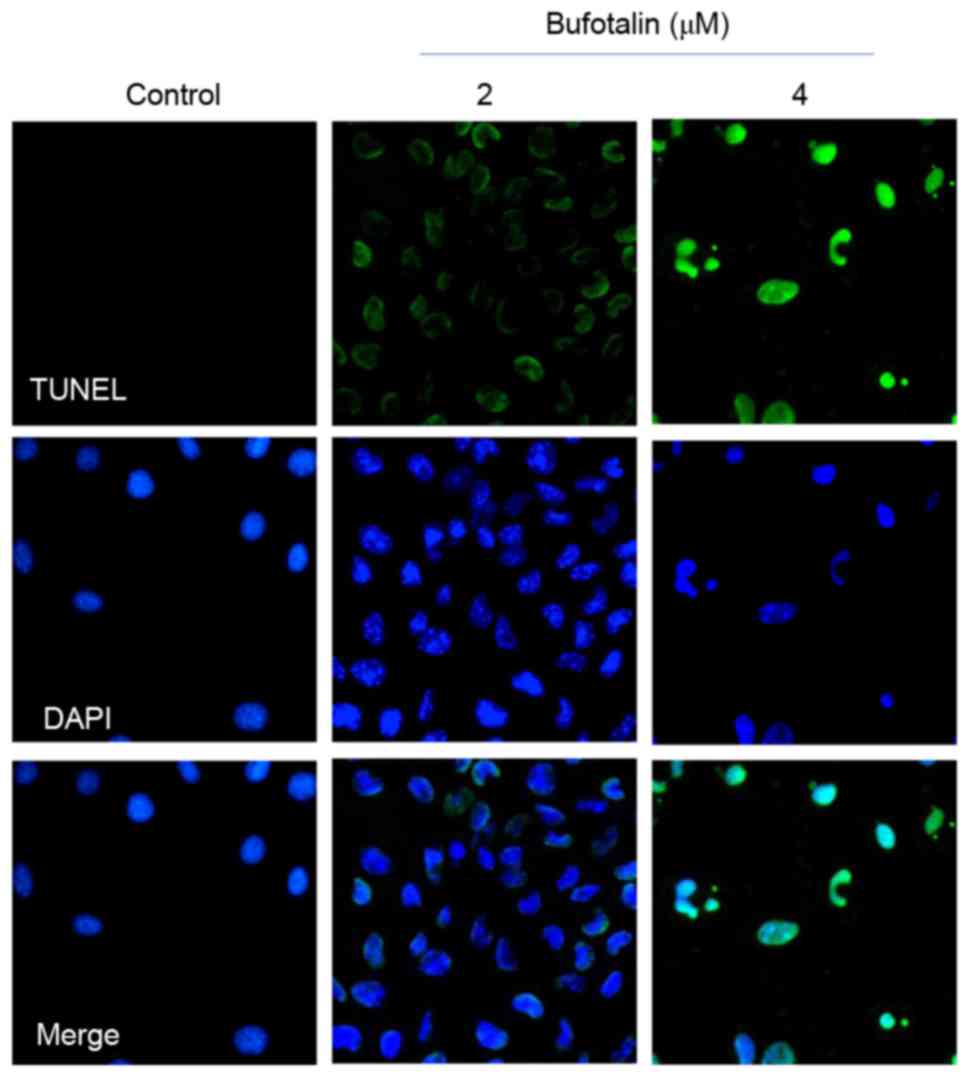
cancer cell death. As demonstrated by a TUNEL-DAPI co-staining
assay, Eca-109 cells exposed to 2 and 4 µM bufotalin for 24 h
exhibited marked and dose-dependent apoptosis compared with the
control (Fig. 2). In addition, DAPI
staining revealed that Eca-109 cells treated with bufotalin
exhibited DNA fragmentation and nuclear condensation. Quantitative
analysis (data not shown) by counting the apoptotic cells, revealed
that bufotalin induced dose-dependent increased apoptosis in the
treated cells compared with the control cells.
Caspase family proteins act as important regulators
in the induction of apoptosis through the enzymolysis of series of
substrates (27). Activated caspases
subsequently induce proteolytic cleavage of poly ADP ribose
polymerase and finally result in cell apoptosis (27). Cell apoptosis is initiated by two
central mechanisms; the death receptor-mediated extrinsic and the
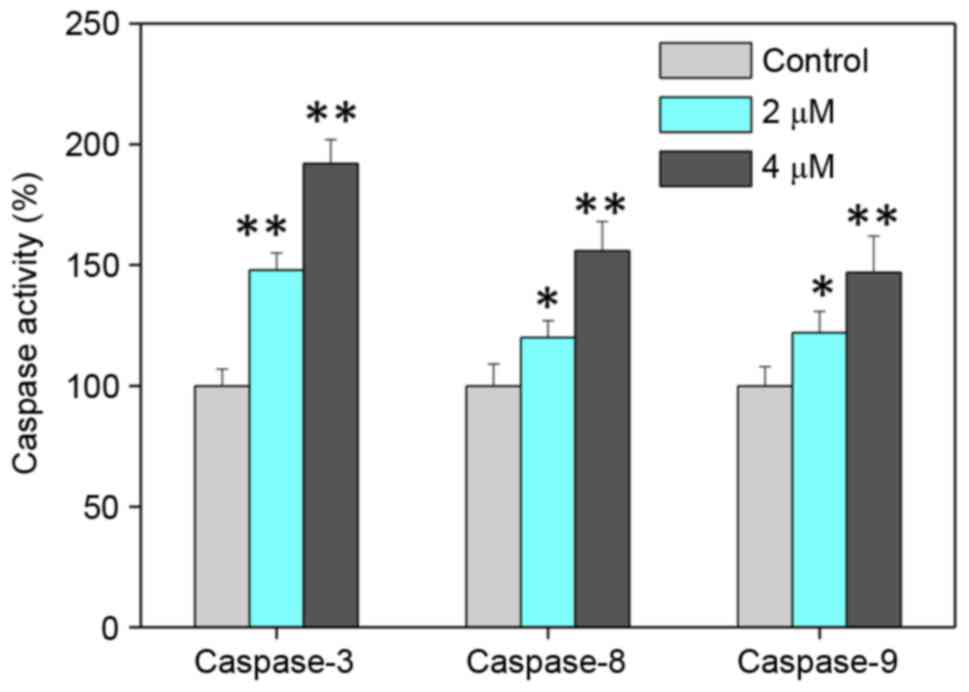
mitochondria-mediated intrinsic apoptotic pathways (28). In the present study, intracellular
caspase activities in cells exposed to bufotalin were measured in
order to examine the requirement of caspase proteins for
bufotalin-induced apoptosis. Exposure of Eca-109 cells to bufotalin
significantly induced the activation of caspase-3, −8 and −9
compared with the control (caspase-3, P<0.01 and P<0.01;
caspase-8, P<0.05 and P<0.01; caspase-9, P<0.05 and
P<0.01 for 2 and 4 µM bufotalin, respectively; Fig. 3). The activation of caspase-9
suggested the induction of the mitochondria-mediated apoptotic
pathway, while the activation of caspase-8 indicated the induction
of death receptor-mediated apoptosis (29). These results revealed that bufotalin
induces ESCC cell apoptosis through the activation of
caspase-mediated apoptosis, with the involvement of mitochondria
and death receptors.
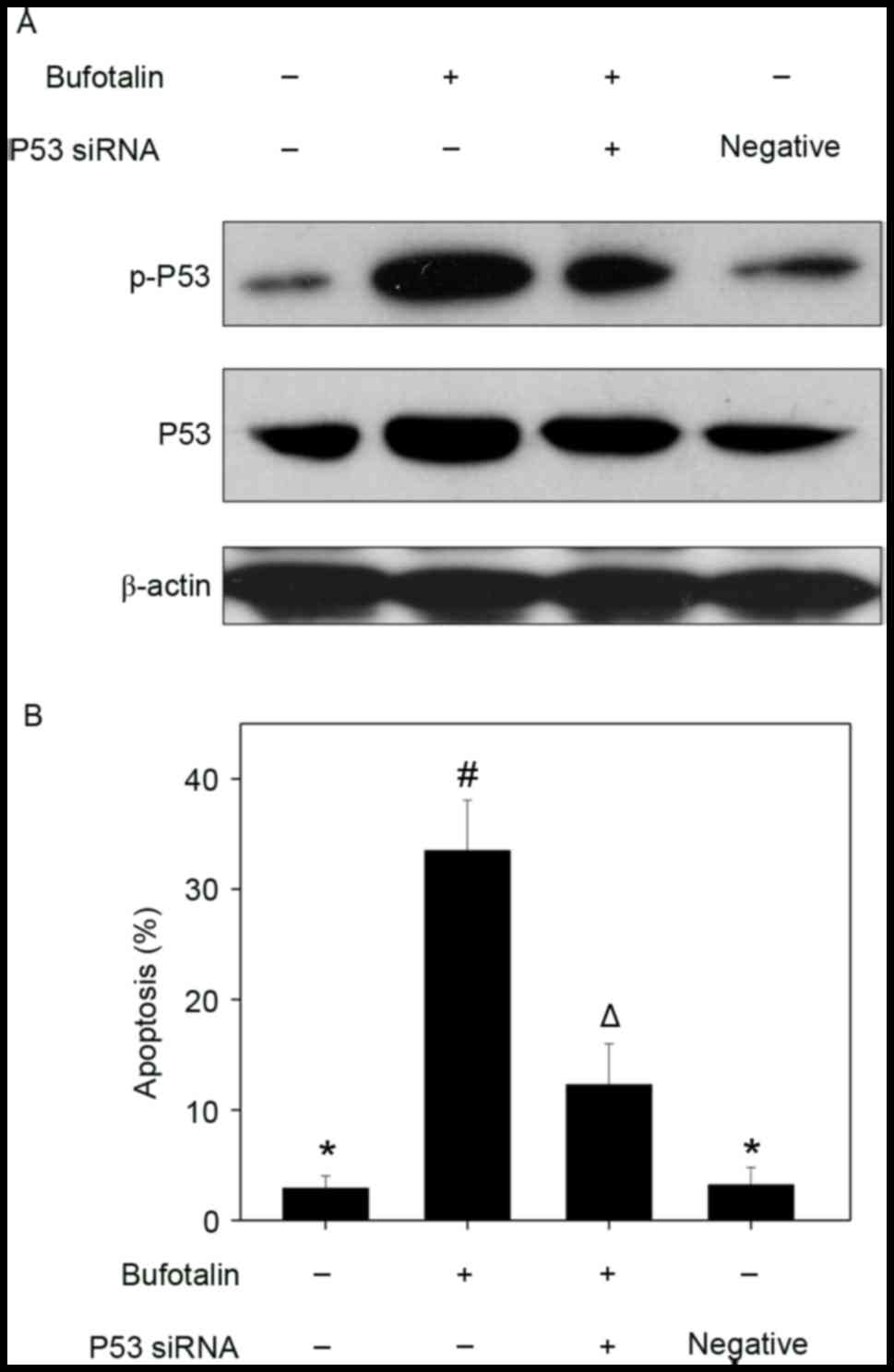
Involvement of p53 in
bufotalin-induced cell apoptosis
The p53 tumor suppressor integrates a number of
stress signals and signaling pathways (30). One of the most important functions of
p53 is the regulation of cell apoptosis by activation of the
expression of p53-inducible targets, including Bcl-2 associated X
protein, p53 upregulated modulator of apoptosis and
cyclin-dependent kinase inhibitor 1 in cancer cells, which may
promote tumor chemotherapy by enhancing the apoptosis-inducing
activities of anticancer drugs (25,31). In
addition, p53 regulates cell apoptosis through upregulation of the
expression of DNA damage-associated proteins and the inhibition of
DNA repair (32). Therefore, in the
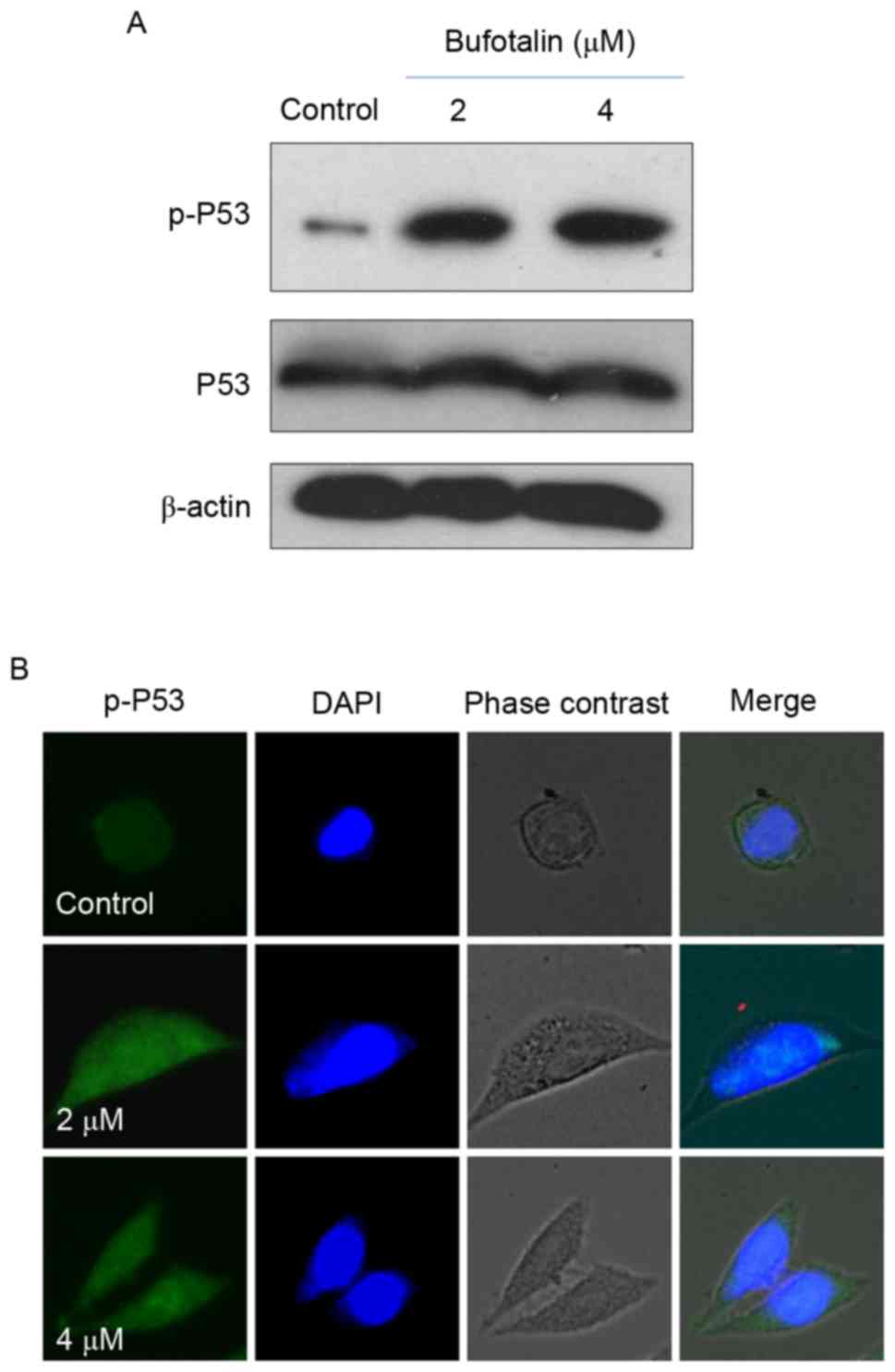
present study, the levels of total p53 and p-p53 were examined by
western blot analysis and fluorescence imaging in order to
elucidate the molecular mechanisms underlying the anticancer
effects of bufotalin. Bufotalin markedly increased the expression
of p53 total protein and p-p53 (Ser15) in Eca-109 cells compared
with the control (Fig. 4A).
Fluorescence imaging confirmed the increase in p-p53 levels
following treatment with bufotalin (Fig.
4B). The involvement of p53 in bufotalin-induced ESCC cell
apoptosis was further verified using RNA interference. Transfection
with p53 siRNA markedly inhibited bufotalin-induced p53
phosphorylation compared with bufotalin treatment alone (Fig. 5A) and significantly decreased
apoptotic cell death compared with bufotalin treatment alone
(P<0.05; Fig. 5B), whereas
transfection with the negative control siRNA exhibited no
significant effects (Fig. 5A and B).
These results suggested that bufotalin activates ESCC cell
apoptosis through activation of the p53-dependent signaling
pathway.
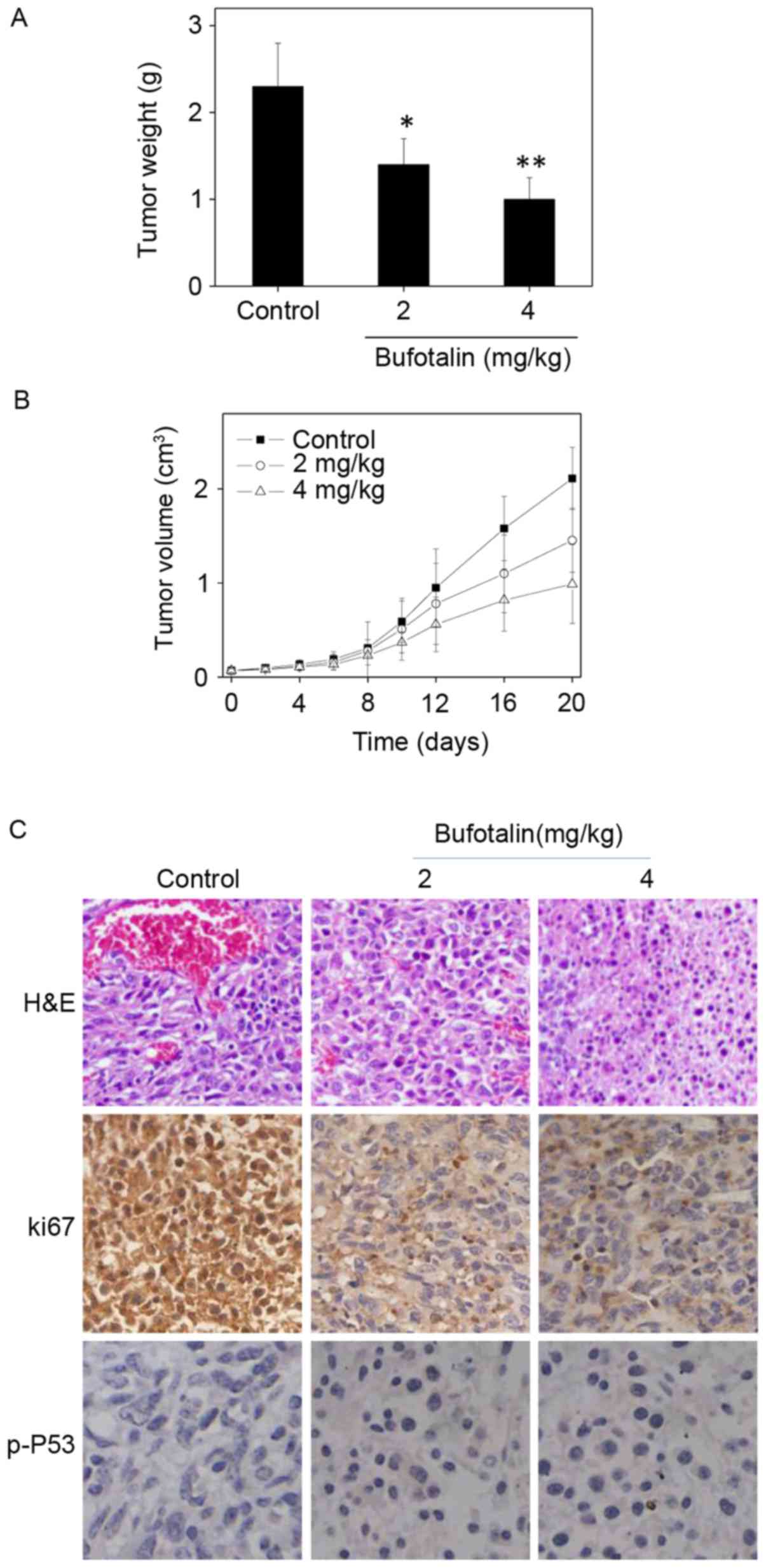
In vivo anticancer activities of
bufotalin with involvement of p53 phosphorylation
The in vivo anticancer activity of bufotalin
against ESCC was evaluated in Eca-109 xenografts in a nude mice
model. Following administration of 2 or 4 mg/kg body weight
bufotalin every other day for 20 days, the tumor weight was
significantly reduced from 2.3 to 1.4 and 1.0 g, respectively
(P<0.05 and P<0.01, respectively; Fig. 6A). In addition, the tumor volume was
markedly decreased to 68 and 46% of the control group (Fig. 6B). The mice that received bufotalin
treatment maintained normal body weight throughout the treatment
process. These results demonstrate the in vivo anticancer
efficacy of bufotalin against ESCC. In addition, immunostaining of
histological sections was performed in order to examine the in
vivo anticancer mechanisms. A marked decrease in blood vessel
and cancer cell density was observed in tumors treated with 2 and 4
mg/kg bufotalin compared with the control (Fig. 6C). The expression of Ki-67, a
biomarker of proliferation, was markedly inhibited by bufotalin. In
addition, the expression of phosphorylated p53 was markedly
enhanced following treatment. These in vivo data support the
hypothesis that bufotalin inhibits tumor growth through activation
of the p53 signaling pathway.
To conclude, in the present study, the in
vitro and in vivo anticancer efficacies of two
bufadienolides (bufotalin and bufalin) were examined. The results
demonstrated that bufotalin and bufalin effectively suppressed the
viability of ESCC cell lines through induction of cell apoptosis
occurring through activation of the p53 signaling pathway. In
addition, bufotalin demonstrated in vivo anticancer efficacy
in a nude mouse model, where bufotalin markedly suppressed tumor
growth through activation of the p53 signaling pathway.
Collectively, these results illustrated the therapeutic potential
of bufadienolides against ESCC by regulating the p53 signaling
pathway.
Acknowledgements
The present study was supported by the Construction
of Scientific Medicine Research Project of Guangdong Province
(grant no. 2013 20131105).
References
|
1
|
Song G, Cheng L, Chao Y, Yang K and Liu Z:
Emerging nanotechnology and advanced materials for cancer radiation
therapy. Adv Mater. 29:2017. View Article : Google Scholar
|
|
2
|
Humbert PO, Verona R, Trimarchi JM, Rogers
C, Dandapani S and Lees JA: E2f3 is critical for normal cellular
proliferation. Genes Dev. 14:690–703. 2000.PubMed/NCBI
|
|
3
|
Chang Y, He L, Li Z, Zeng L, Song Z, Li P,
Chan L, You Y, Yu XF, Chu PK and Chen T: Designing core-shell gold
and selenium nanocomposites for cancer radiochemotherapy. Acs Nano.
11:4848–4858. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh SK, Banerjee S, Acosta EP, Lillard
JW and Singh R: Resveratrol induces cell cycle arrest and apoptosis
with docetaxel in prostate cancer cells via a p53/p21(WAF1/CIP1)
and p27(KIP1) pathway. Oncotarget. 8:17216–17228. 2017.PubMed/NCBI
|
|
5
|
Liong M, Lu J, Kovochich M, Xia T, Ruehm
SG, Nel AE, Tamanoi F and Zink JI: Multifunctional inorganic
nanoparticles for imaging, targeting, and drug delivery. ACS Nano.
2:889–896. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oelkrug C, Hartke M and Schubert A: Mode
of action of anticancer peptides (ACPs) from amphibian origin.
Anticancer Res. 35:635–643. 2015.PubMed/NCBI
|
|
7
|
Chen T and Wong YS: Selenocystine induces
apoptosis of A375 human melanoma cells by activating ROS-mediated
mitochondrial pathway and p53 phosphorylation. Cell Mol Life Sci.
65:2763–2775. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen T and Wong YS: Selenocystine induces
caspase-independent apoptosis in MCF-7 human breast carcinoma cells
with involvement of p53 phosphorylation and reactive oxygen species
generation. Int J Biochem Cell Biol. 41:666–676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi FH, Li AY, Lv H, Zhao L, Li JJ, Gao B
and Tang W: Apoptosis-inducing effect of cinobufacini, Bufo bufo
gargarizans Cantor skin extract, on human hepatoma cell line
BEL-7402. Drug Discov Ther. 2:339–343. 2008.PubMed/NCBI
|
|
10
|
Chen Y, Qiao L, Yu B, Li G, Liu C, Ji L
and Chao H: Mitochondria-specific phosphorescent imaging and
tracking in living cells with an AIPE-active iridium(III) complex.
Chem Commun (Camb). 49:11095–11097. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Z, Wen J, Zhang J, Ye M and Guo D:
Simultaneous determination of four bufadienolides in human liver by
high-performance liquid chromatography. Biomed Chromatogr.
18:318–322. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wen L, Huang Y, Xie X, Huang W, Yin J, Lin
W, Jia Q and Zeng W: Anti-inflammatory and antinociceptive
activities of bufalin in rodents. Mediators Inflamm.
2014:1718392014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang DL, Qi FH, Xu HL, Inagaki Y, Orihara
Y, Sekimizu K, Kokudo N, Wang FS and Tang W: Apoptosis-inducing
activity of compounds screened and characterized from cinobufacini
by bioassay-guided isolation. Mol Med Rep. 3:717–22.
2010.PubMed/NCBI
|
|
14
|
Xu GX and Wang TT: Apoptosis of lens
epithelial cells induced by cinobufagin in vitro. Int J Ophthalmol.
3:128–131. 2010.PubMed/NCBI
|
|
15
|
Zhai XF, Fang FF, Liu Q, Meng YB, Guo YY
and Chen Z: MiR-181a contributes to bufalin-induced apoptosis in
PC-3 prostate cancer cells. BMC Complement Altern Med. 13:3252013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang F, Zhang D, Zhang Q, Chen Y, Zheng D,
Hao L, Duan C, Jia L, Liu G and Liu Y: Synergistic effect of
folate-mediated targeting and verapamil-mediated P-gp inhibition
with paclitaxel-polymer micelles to overcome multi-drug resistance.
Biomaterials. 32:9444–9456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YY, Lu HF, Hsu SC, Kuo CL, Chang SJ,
Lin JJ, Wu PP, Liu JY, Lee CH, Chung JG and Chang JB: Bufalin
inhibits migration and invasion in human hepatocellular carcinoma
SK-Hep1 cells through the inhibitions of NF-kB and matrix
metalloproteinase-2/−9-signaling pathways. Environ Toxicol.
30:74–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang W, Shi A and Fan Z: Apoptosis of
T-47D cells induced by cinobufacini via a caspase-3-dependent
manner. Chem Res Chinese Univer. 30:108–113. 2014. View Article : Google Scholar
|
|
19
|
Li M, Yu X, Guo H, Sun L, Wang A, Liu Q,
Wang X and Li J: Bufalin exerts antitumor effects by inducing cell
cycle arrest and triggering apoptosis in pancreatic cancer cells.
Tumour Biol. 35:2461–2471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang DM, Liu JS, Deng LJ, Chen MF, Yiu A,
Cao HH, Tian HY, Fung KP, Kurihara H, Pan JX and Ye WC:
Arenobufagin, a natural bufadienolide from toad venom, induces
apoptosis and autophagy in human hepatocellular carcinoma cells
through inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis.
34:1331–1342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo H, Wang F, Bai Y, Chen T and Zheng W:
Selenium nanoparticles inhibit the growth of HeLa and MDA-MB-231
cells through induction of S phase arrest. Colloids Surf B
Biointerfaces. 94:304–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang F, Tang Q, Zhong X, Bai Y, Chen T,
Zhang Y, Li Y and Zheng W: Surface decoration by Spirulina
polysaccharide enhances the cellular uptake and anticancer efficacy
of selenium nanoparticles. Int J Nanomedicine. 7:835–844.
2012.PubMed/NCBI
|
|
23
|
Zhang Y, Li X, Huang Z, Zheng W, Fan C and
Chen T: Enhancement of cell permeabilization apoptosis-inducing
activity of selenium nanoparticles by ATP surface decoration.
Nanomedicine. 9:74–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maiese K, Chong ZZ, Shang YC and Wang S:
Targeting disease through novel pathways of apoptosis and
autophagy. Expert Opin Ther Targets. 16:1203–1214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen T and Wong YS: Selenocystine induces
apoptosis of A375 human melanoma cells by activating ROS-mediated
mitochondrial pathway and p53 phosphorylation. Cell Mol Life Sci.
65:2763–2775. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen T and Wong YS: Selenocystine induces
caspase-independent apoptosis in MCF-7 human breast carcinoma cells
with involvement of p53 phosphorylation and reactive oxygen species
generation. Int J Biochem Cell Biol. 41:666–676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
He L, Lai H and Chen T: Dual-function
nanosystem for synergetic cancer chemo-/radiotherapy through
ROS-mediated signaling pathways. Biomaterials. 51:30–42. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li M, Lai L, Zhao Z and Chen T: Aquation
is a crucial activation step for anticancer action of ruthenium(II)
polypyridyl complexes to trigger cancer cell apoptosis. Chem Asian
J. 11:310–320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie Q, Lan G, Zhou Y, Huang J, Liang Y,
Zheng W, Fu X, Fan C and Chen T: Strategy to enhance the anticancer
efficacy of X-ray radiotherapy in melanoma cells by platinum
complexes, the role of ROS-mediated signaling pathways. Cancer
Lett. 354:58–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fridman JS and Lowe SW: Control of
apoptosis by p53. Oncogene. 22:9030–9040. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He L, Huang Y, Zhu H, Pang G, Zheng W,
Wong YS and Chen T: Cancer-targeted monodisperse mesoporous silica
nanoparticles as carrier of ruthenium polypyridyl complexes to
enhance theranostic effects. Adv Funct Mater. 24:2754–2763. 2014.
View Article : Google Scholar
|