Introduction
Brain tumors are the most common solid neoplasms in
children (1). Pilocytic astrocytomas
(PAs), as WHO grade I neoplasias, represent up to 20% of brain
tumors in children and adolescents (2). They are found in the hypothalamus,
periventricular region of the third ventricle, and cerebellum
(3). They generally have a relatively
benign clinical course, with a 10-year survival rate of 95%
(4). This good prognosis is primarily
because PAs are usually sharply circumscribed, and thus, they can
often be completely resected. Hence, surgery is the gold standard
and represents the preferred therapy (5,6). In
addition, these tumors show only a slight tendency to infiltrate
healthy tissues (7). Nonetheless,
some PAs show a more malignant course, particularly in adult
patients (8,9).
The pathognomonic molecular characteristic of PAs in
pediatric patients is a KIAA1549-BRAF fusion transcript, resulting
from a somatic duplication of 7q34. Mutations of the proto-oncogene
B-Raf (BRAF V600E mutation) are found in less than 10% of tumors
(10,11). However, additional genetic alterations
can be present in the relatively uncommon case of PAs in adult
patients. The main genetic alterations in PAs in adult patients is
a KIAA1549-BRAF fusion transcript, found in 20–32% of cases;
FGFR1 mutation; and the absence of BRAF V600E mutation
(12–17). Moreover, IDH1 R132H mutation
might play a more important role in adult PAs (18,19). In
case of NF1 mutation, PAs may involve the optic pathways,
optic nerve, and chiasm (12,14). A review of the literature on adult PAs
has shown that most cases remain genetically uncharacterized.
Therefore, the question remains whether additional molecular
markers can be found at an epigenetic level to help predict the
clinical course of the disease. The best studied epigenetic
modification is DNA methylation. In this process, methyl groups are
covalently attached to CpG islands in the promoter regions of genes
by DNA methyltransferase, resulting in the suppression of
transcription. These CpG islands exist in approximately 40% of the
promoter regions found in humans. However, not all CP dinucleotides
are CpG islands that can be methylated. The methylation status of
P15, P16, RB1, and MGMT has been shown
to be important in the oncogenesis of WHO grade II–IV gliomas.
P15, P16, and RB1 play a crucial role in the
cell cycle as tumor suppressors and influence progression and
prognosis in glial tumors (20). P15
and p16 can bind and therefore inhibit CDK4 and CDK6. Inactive CDK4
and CDK6 are responsible for the hypophosphorylated status of RB1,
resulting in cell arrest (21).
Therefore, p15 and p16 act as tumor suppressors in the late G1
phase (22). Mutations of and
deletions in RB1, P15, and P16 are among the
most frequently observed genetic alterations in glial tumors and
can result in a more aggressive biological behavior of the tumor
(23–26).
MGMT is a DNA repair protein that removes alkyl
groups and adducts at the O6 position of guanine. It protects
healthy cells against mutagenic effects, and loss of expression due
to MGMT promoter hypermethylation has been proposed as a
predisposing factor for the acquisition of TP53 transition
mutations in oncogenesis (27).
MGMT hypermethylation is associated with a significantly
shorter progression-free survival (PFS) in patients with breast
cancer and low-grade astrocytomas (28–31). MGMT
can also protect cells with high-grade astrocytomas against the
cytotoxic effects of alkylating chemotherapeutic agents (32). The question arises whether specific
methylation patterns of these genes also correlate with the
clinical course of PAs as WHO grade I neoplasias. We hypothesize
that in PAs, promoter methylation of P15, P16,
RB1, and MGMT results in a higher frequency of
relapses with a reduced PFS and overall survival (OS). Furthermore,
we expect to find different specific methylation patterns in adult
and pediatric PAs.
Materials and methods
Patients
In this retrospective study, tumor tissues from
patients who underwent surgery at the Saarland University Medical
Center in Homburg between 1999 and 2014 and who had clinical data
available from January 1999 to December 2016 were used. Individual
follow-up periods ranged from 4 months to 14.7 years. Inclusion
criteria were a neuropathological diagnosis of PAs (WHO grade I)
and a sufficient amount of tumor tissue for DNA isolation. NF1
mutation was not detected in any tumor specimen. No included
patient had a tumor at the optic nerve. This study was approved by
the local Ethical Review Board, and written informed consent was
obtained from all patients or their representatives (Ärztekammer
des Saarlandes, Ethikkommission, No. 93/16). All procedures
performed in this study were in accordance with the ethical
standards of the 1964 Helsinki declaration. Genomic DNA was
extracted from the resected tumor tissue. All tissue samples were
stored at −80°C.
Methylation analysis
DNA isolation was performed using a DNA isolation
kit (Qiagen, QIAamp DNA Mini kit 50). The methylation status of
promoter regions of P15, P16, RB1, and
MGMT was determined by methylation-specific polymerase chain
reaction. Therefore, 500 ng DNA of each tumor specimen as well as
appropriate control samples were treated with bisulfite (Zymo
Research, EZ DNA Methylation-Gold kit 200) (33). In summary, unmethylated cytosine was
converted to uracil, whereas methylated cytosine remained
unchanged. The modified DNA was recovered by ethanol precipitation
and suspended in polymerase chain reaction (PCR) grade water. For
analyzing the methylation status, the primer sequences listed in
Table I were used (34–36). PCR
was performed using a 25-µl reaction volume and 38 PCR cycles. All
PCR products were electrophoretically separated on a 2% agarose
gel. As a positive control, a chemically globally methylated DNA
was used (Zymo Research, bisulfite-converted Human DNA). Genomic
DNA isolated from a non-neoplastic dura mater tissue served as a
negative control. In addition, each PCR included a control without
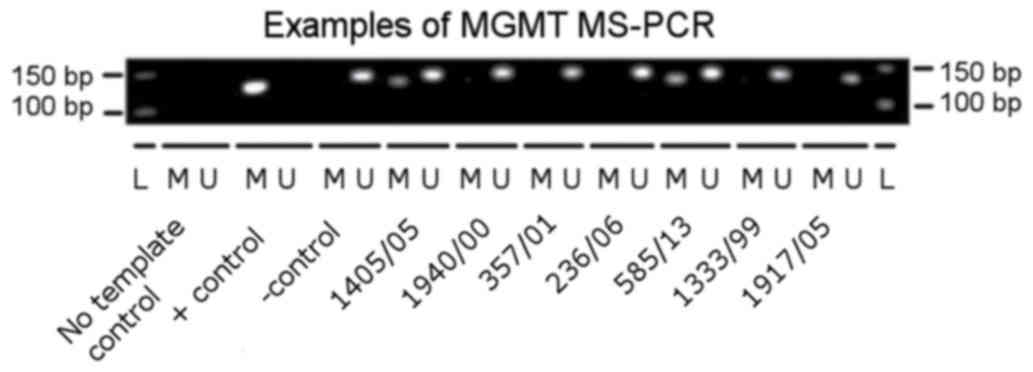
any DNA template. An example of PCR results is presented in
Fig. 1.
 | Table I.Primer sequences for methylation
specific-polymerase chain reaction. |
Table I.
Primer sequences for methylation
specific-polymerase chain reaction.
| Author, year | Gene name | Forward
(5′-3′) | Reverse
(5′-3′) | Methylation | Length, (bp) | Temperature
(°C) | (Refs.) |
|---|
| Felsberg, 2009 | MGMT |
GTTTTTAGAACGTTTTGCGTTTCGAC |
CACCGTCCCGAAAAAAAACTCCG | Methylated | 122 | 54 | (34) |
|
|
|
TGTGTTTTTAGAATGTTTTGTGTTTTGAT |
CTACCACCATCCCAAAAAAAAACTCCA | Unmethylated | 129 | 56 |
|
| Wong, 2000 | p15 |
GCGTTCGTATTTTGCGGTT |
CGTACAATAACCGAACGACCGA | Methylated | 148 | 60 | (35) |
|
|
|
TGTGATGTGTTTGTATTTTGTGGTT |
CCATACAATAACCAAACAACCAA | Unmethylated | 154 | 60 |
|
| Herman, 1996 | p16 |
TTATTAGAGGGTGGGGCGGATCGC |
GACCCCGAACCGCGACCGTAA | Methylated | 150 | 65 | (36) |
|
|
|
TTATTAGAGGGTGGGGTGGATTGT |
CAACCCCAAACCACAACCATAA | Unmethylated | 151 | 65 |
|
| Simpson, 2000 | RB1 |
GGGAGTTTCGCGGACGTGAC |
ACGTCGAAACACGCCCCG | Methylated | 172 | 55 | (37) |
|
|
|
GGGAGTTTTGTGGATGTGAT |
ACATCAAAACACACCCCA | Unmethylated | 172 | 55 |
|
IDH1-R123H staining
Immunohistochemistry was conducted on 4-µm-thick
formalin-fixed, paraffin-embedded tissue sections mounted on
StarFrost Advanced Adhesive slides (Engelbrecht, Kassel, Germany).
This was followed by drying at 80°C for 15 min.
Immunohistochemistry was performed on a BenchMark Ultra
immunostainer (Ventana Medical Systems, Tucson, AZ, USA). Sections
were stained with anti-IDH1-R132H antibody H09 (Dianova, Hamburg,
Germany) as previously described (37).
Statistical analysis
All samples were scrutinized comparing the
methylation status of P15, P16, RB1, and
MGMT for the determining the PFS, OS, and occurrence of
relapse. In addition, other clinical data such as age at onset,
gender, tumor location, and treatment modality were collected. The
Kaplan-Meier and log-rank test were used to calculate the PFS and
OS in relation to promoter methylation. For statistical evaluation
of the age at onset t-test for independent samples was applied. For
the analysis of gender, tumor location, and treatment modality, a
chi-square test was used. The significance level used in all tests
was P<0.05. SPSS v. 21 was used as the statistical program.
Results
A total of 18 patients (12 males and 6 females) met
the inclusion criteria. The most frequent localizations were the
cerebellum (12 patients), medulla oblongata and cervical spine (3
patients), and cerebrum (2 patient). In one patient, the tumor was
localized in the brainstem. The mean age at diagnosis was 17.9±15.8
years, ranging from 3.1 to 61.1 years. The mean follow-up duration
was 4.9±4.2 years, with a range from 4 months to 14.7 years. There
were six patients with an age at onset between 25.2 and 61.1 years;
there were categorized as adult patients. The other 12 patients had
disease onset between 3.1 and 18.4 years; they were categorized as
pediatric patients (38,39). Table II
shows an overview of collected data. Primary therapy after
diagnosis was tumor resection in all patients. Gross total
resection (GTR) was possible in nine patients. In the other nine
patients, only subtotal resection (STR) was possible because of
localization or infiltration of the tumor in eloquent areas of the
brain. The extent of resection was determined by magnetic resonance
imaging within 48 h postoperatively. Disease relapse occurred in
six patients. These patients underwent a second surgery, with
additional radiotherapy in two patients.
 | Table II.Clinical characteristics of the
patients. |
Table II.
Clinical characteristics of the
patients.
| Case | Sex | Age (years) | PFS (years), then
relapse | Therapy | Localisation | Methylation status
of RB1 | p15 | p16 | MGMT |
|---|
| 1646/05 | M | 25.2 | 0.9 | STR+STR | Cerebellum | 0 | 0 | 0 | 1 |
| 357/01 | M | 10.7 | No rel | GTR | Cerebellum | 0 | 0 | 0 | 0 |
| 04/14 | M | 13.8 | No rel | GTR | Cerebellum | 0 | 0 | 0 | 0 |
| 236/06 | M | 3.3 | No rel | GTR | Cerebellum | 0 | 0 | 0 | 0 |
| 56/04 | F | 34.9 | 0.3 | STR+(STR with
C) | Cervical spine | 0 | 1 | 0 | 1 |
| 1176/00 | F | 3.1 | No rel | GTR | Cerebellum | 0 | 0 | 0 | 1 |
| 1333/99 | M | 61.1 | 7.1 | STR+(STR with
RT) | Medulla
oblongata | 0 | 0 | 0 | 0 |
| 236/07 | F | 11.5 | No rel | GTR | Cerebellum | 0 | 0 | 0 | 0 |
| 2184/13 | F | 49 | 2.0 | STR+(STR with
RT) | Brainstem | 0 | 0 | 0 | 1 |
| 1940/00 | M | 4.1 | No rel | GTR | Cerebellum | 0 | 0 | 0 | 0 |
| 740/02 | M | 11.1 | No rel | GTR | Cerebellum | 0 | 0 | 0 | 1 |
| 1917/05 | M | 7.5 | No rel | GTR | Cerebellum | 0 | 0 | 0 | 0 |
| 1594/99 | F | 26.8 | No rel | STR | Right lat.
ventricle | 0 | 0 | 0 | 0 |
| 119/04 | M | 4.6 | No rel | GTR | Cerebellum | 0 | 0 | 0 | 0 |
| 203/09 | F | 7.4 | No rel | STR | Cerebellum | 0 | 0 | 0 | 0 |
| 1850/05 | M | 13.3 | 1.3 | STR+STR | Cervical spine | 0 | 0 | 0 | 1 |
| 585/13 | M | 46.1 | 0.3 | STR+STR+STR | Right lat.
ventricle | 0 | 0 | 0 | 1 |
| 1405/05 | M | 18.4 | No rel | STR | Cerebellum | 0 | 0 | 0 | 1 |
The PAs of all 18 patients were analyzed for
promoter methylation of P15, P16, RB1, and
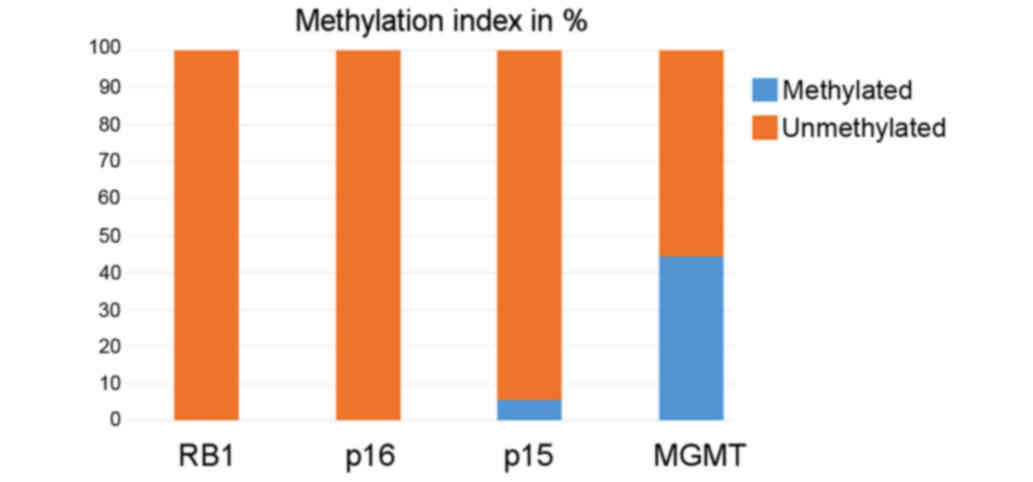
MGMT. The methylation index (MI) of P15, P16,
RB1, and MGMT was 0.0, 0.0, 5.6% (one patient, case
56/04), and 44.5% (8/18) (Fig. 2).
Because no methylated promoter of P16 and RB1 was
found, no further statistical analysis regarding these two genes
was conducted. Promotor methylation of P15 was found in one
patient; however, statistical analysis did not seem useful as only
one such patient was observed. However, this patient with
methylation of P15 had the only fatal clinical course in the
present cohort. The patient (case 56/04) showed relapse with local
metastasis 4 months after the first surgery. A second tumor
resection with subsequent chemotherapy (carboplatin + VCR) was
unsuccessful, and the patient died 6 months after the first
diagnosis.
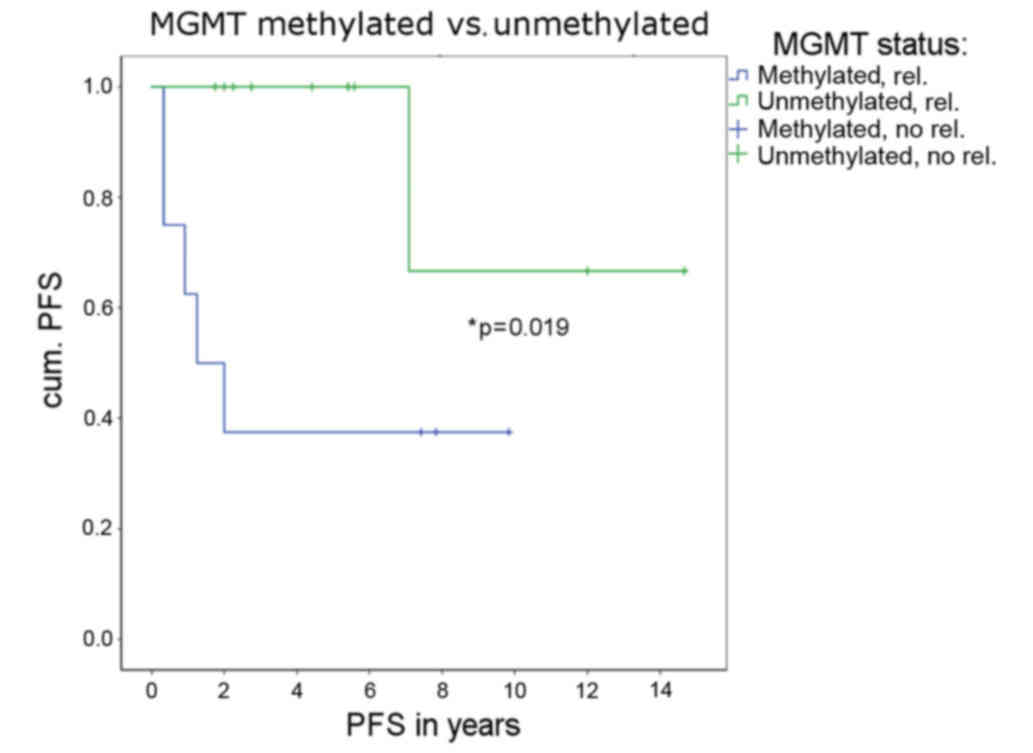
If the MGMT promoter was methylated, relapse
and second subsequent therapy occurred significantly more often
(P=0.019; Fig. 3). If the methylation
status of MGMT was used as a predictor for second therapy
due to relapse, 77.8% of all patients could be correctly classified
(binary logistic regression, P=0.016). When more closely examining
the six patients with relapse, a huge difference in PFS between the
patients with and those without methylation was found. One patient
with relapse (case 1333/99) showed an unmethylated MGMT
promoter. The PFS of that patient was 85.2 months. The other five
patients showing relapse with a methylated MGMT promoter had
an average PFS of 11.5 months.
There was no significant association between the age
of patients and a specific pattern of methylation. Adult patients
displayed a significant correlation with the non-cerebellar
location of PAs. Patients with a non-cerebellar tumor localization
were significantly older (38.5±17.08 years) at disease onset than
those with a cerebellar localization (11.41±7 years; P=0.01).
There was a significant correlation between the
extent of resection and occurrence of relapse (chi-square test,
P=0.005). If only STR was achieved, relapse was more likely. In
adult patients, STR was significantly more common (P=0.003). Adult
patients showed significantly more relapses after the first tumor
resection than pediatric patients (P=0.001). There was also a trend
that methylation status of MGMT correlated with the
frequency of STR (P=0.058).
However, a direct relation between age at disease
onset and methylation status of MGMT could not be found.
Age, gender, and localization of the tumor were not associated with
the methylation status of MGMT. The PAs of all 18 patients
had wild-type IDH1. An IDH1-R123H mutant could not be
demonstrated in any tumor.
Discussion
PAs represent up to 20% of brain tumors in children
and adolescents and are usually not malignant (2). However, some PAs show a more aggressive
clinical behavior, particularly in adult patients (8,9). This
trial aimed to identify new epigenetic markers to predict the
course of PAs. If these predictors are available, patients could be
stratified for an optimized follow-up. Because of their known
impacts on glial tumors, the analysis focused onP15,
P16, RB1, and MGMT in correlation with
patients' clinical courses.
RB1 and P16 showed no promoter
methylation. The promoter of P15 was methylated in one
patient. This is consistent with the results of Uhlmann et
al and Gonzales-Gomez et al who described PAs as not
commonly methylated (40,41). However, their trials described only
methylation profiles without the correlation of clinical parameters
or further stratification of patients for age. A remarkable case in
the present study was a patient with a PA at the cervical spine
(case 56/04) with promoter methylation of P15. Despite GTR,
local recurrence with meningeal metastases occurred. A second tumor
resection with subsequent chemotherapy was unsuccessful, and the
patient died 2 months later. Previous studies have shown that loss
of expression, resulting from deletion or methylation of
P15, is associated with a significantly worse prognosis for
survival in glioblastomas (20,42). It is
possible that promoter methylation of P15 in this patient
resulted in very aggressive tumor behavior and poor clinical
course.
The presented results regarding promoter methylation
of MGMT disproved the hypothesis that PAs are generally
unmethylated. The PAs of all 18 patients had an MGMT MI of
44.5%. This remarkably high frequency of methylation of MGMT
in PAs has not been reported in the literature thus far.
Nevertheless, loss of MGMT expression because of promoter
hypermethylation of the MGMT gene is a well-documented
phenomenon in high-grade brain tumors (43,44). In
the present study, patients showing tumors with promoter
methylation of MGMT showed a significantly higher risk of
relapse and necessity of secondary treatment. A closer look at the
six patients with relapse revealed that when MGMT was
methylated, the PFS was reduced. Studies on WHO grade II
astrocytomas demonstrated that methylation of MGMT can be
associated with a significantly shorter PFS (28). This supposes a higher malignancy in
PAs if the MGMT promoter is methylated. A higher malignancy
in patients having tumors with hypermethylation of MGMT vs.
a lower malignancy in patients having tumors with unmethylated
MGMT has also been demonstrated in breast cancer (28–31). In
glioblastoma multiforme, the hypermethylation of MGMT is a
well-known marker for better response characteristics than
alkylating chemotherapy, resulting in a better prognosis (32). This does not contradict the findings
in the present trial in PAs because none of the patients underwent
alkylating chemotherapy.
In PAs, different genetic characteristics between
adult and pediatric patients are known. Although a KIAA1549-BRAF
fusion transcript is dominant in pediatric patients, in adults,
FGFR1 mutation and the absence of BRAF V600E mutation can
also be found (12–17). In other recent investigations, an
IDH1 R132H mutation was described solely in adult patients
(18,19). Therefore, the hypothesis was that
methylation patterns are differently distributed between adult and
pediatric patients. This was not the case in the present trial.
RB1 and P16 were not methylated in adult or pediatric
patients. Because of the low number of promoter methylations of
P15, no reasonable conclusion can be drawn. The correlation
between methylation of MGMT and occurrence of relapse was
independent of age. However, adult patients displayed a significant
correlation with the non-cerebellar localization of PAs. Tumor
specimens of the included patients were scrutinized for analyzing
IDH1 R132H mutation. All patients showed wild-type
IDH1, suggesting that IDH1 R132H mutation in PAs is a
rare event in adult patients.
In this trial, tumor recurrence was significantly
more likely in cases of STR than in cases of GTR. This underlines
the huge importance of radical surgery for PAs. Alford et al
presented a similar correlation in a patient cohort with 51 PAs
(38).
The main limitation of this trial is the low number
of included patients. Hence, data in this trial should be
critically scrutinized. With only 18 patients included, we
acknowledge that the generalization of the results might be
limited. Nevertheless, the results show that even in benign tumors,
stratification based on molecular markers is becoming increasingly
important. In the present trial, methylation of MGMT was a
significant age-independent predictor of the necessity of a second
therapy. Consequently, a further evaluation of epigenetic markers
in larger cohorts of patients with PAs under the special aspect of
MGMT is recommendable. Though speculative, a further idea is
to assess methylation of MGMT in fluid probes obtained by
liquid biopsy (45). The proof of
principle has already been furnished in colorectal cancer (46). In cases of tumors of the central
nervous system, such as PAs, the cerebrospinal fluid next to blood
samples could be used. This could enable a prognosis even before
surgery.
Acknowledgements
The authors wish to thank Lisa Senger, Sonja
Hoffman, Petra Ludowicy, Juliane Riedl, and Sigrid Welsch for their
support in methylation analysis and statistics.
References
|
1
|
US Cancer Statistics Working Group: United
States Cancer Statistics: 1999–2009 incidence and mortality
web-based report. Atlanta: U.S. Department of Health and Human
Services, Centers for Disease Control and Prevention and National
Cancer Institute; https://www.cdc.gov/uscs1–June. 20162013
|
|
2
|
Mandiwanza T, Kaliaperumal C, Khalil A,
Sattar M, Crimmins D and Caird J: Suprasellar pilocytic
astrocytoma: One national center's experience. Childs Nerv Syst.
30:1243–1248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koeller KK and Rushing EJ: From the
archives of the AFIP: Pilocytic astrocytoma: Radiologic-pathologic
correlation. Radiographics. 24:1693–1708. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burkhard C, Di Patre PL, Schüler D,
Schüler G, Yaşargil MG, Yonekawa Y, Lütolf UM, Kleihues P and
Ohgaki H: A population-based study of the incidence and survival
rates in patients with pilocytic astrocytoma. J Neurosurg.
98:1170–1174. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stokland T, Liu JF, Ironside JW, Ellison
DW, Taylor R, Robinson KJ, Picton SV and Walker DA: A multivariate
analysis of factors determining tumor progression in childhood
low-grade glioma: A population-based cohort study (CCLG CNS9702).
Neuro Oncol. 12:1257–1268. 2010.PubMed/NCBI
|
|
6
|
Louis DN, Ohgaki H, Wiestler O and Cavenee
WK: WHO Classification of Tumours of the Central Nervous System.
Fourth Edition. International Agency for Research on Cancer; Lyon,
France: 2007
|
|
7
|
Fisher GP, Tihan T, Goldthwaite PT, Wharam
MD, Carson BS, Weingart JD, Repka MX, Cohen KJ and Burger PC:
Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood
Cancer. 51:245–250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shibahara I, Gawaguchi T, Kanamory M,
Yonezawa S, Takazawa H, Asano K, Ohkuma H, Kaimori M, Sasaki T and
Nishijima M: Pilocytic astrocytoma with histological malignant
without previous radiation therapy-case report. Neurol Med Chir
(Tokyo). 51:144–147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnson DR, Brown PD, Galanis E and
Hammack JE: Pilocytic astrocytoma survival in adults: Analysis of
the surveillance, epidemiology, and end results program of the
national cancer institute. J Neurooncol. 108:187–193. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Korshunov A, Meyer J, Capper D, Christians
A, Remke M, Witt H, Pfister S, von Deimling A and Hartmann C:
Combined molecular analysis of BRAF and IDH1 distinguishes
pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol.
118:401–405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Wu G, Miller CP, Tatevossian RG,
Dalton JD, Tang B, Orisme W, Punchihewa C, Parker M, Qaddoumi I, et
al: Whole-genome sequencing identifies genetic alterations in
pediatric low-grade gliomas. Nat Genet. 45:602–612. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Theeler BJ, Ellezam B, Sadighi ZS, Mehta
V, Tran MD, Adesina AM, Bruner JM and Puduvalli VK: Adult pilocytic
astrocytomas: Clinical features and molecular analysis. Neuro
Oncol. 16:841–847. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wemmert S, Romeike BF, Ketter R, Steudel
WI, Zang KD and Urbschat S: Intratumoral genetic heterogeneity in
pilocytic astrocytomas revealed by CGH-analysis of microdissected
tumor cells and FISH on tumor tissue sections. Int J Oncol.
28:353–360. 2006.PubMed/NCBI
|
|
14
|
Hasselblatt M, Riesmeier B, Lechtape B,
Brentrup A, Stummer W, Albert FK, Sepehrnia A, Ebel H, Gerss J and
Paulus W: BRAF-KIAA1549 fusion transcripts are less frequent in
pilocytic astrocytomas diagnosed in adults. Neuropathol Appl
Neurobiol. 37:803–806. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rodriguez EF, Scheithauer BW, Giannini C,
Rynearson A, Cen L, Hoesley B, Gilmer-Flynn H, Sarkaria JN, Jenkins
S, Long J and Rodriguez FJ: PI3K/AKT pathway alterations are
associated with clinically aggressive and histologically anaplastic
subsets of pilocytic astrocytoma. Acta Neuropathol. 121:407–420.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jones DT, Hutter B, Jäger N, Korshunov A,
Kool M, Warnatz HJ, Zichner T, Lambert SR, Ryzhova M, Quang DA, et
al: Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic
astrocytoma. Nat Genet. 45:927–932. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brokinkel B, Peetz-Dienhart S, Ligges S,
Brentrup A, Stummer W, Paulus W and Hasselblatt M: A comparative
analysis of MAPK pathway hallmark alterations in pilocytic
astrocytomas: Age-related and mutually exclusive [corrected].
Neuropathol Appl Neurobiol. 41:258–261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Medress ZA, Xu LW, Ziskin JL, Lefterova
MI, Vogel H and Li G: Pilocytic astrocytoma with IDH1 mutation in
the cerebellum of an elderly patient. Clin Neuropathol. 34:96–98.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Behling F, Steinhilber J, Tatagiba M,
Bisdas S and Schittenhelm J: IDH1 R132H mutation in a pilocytic
astrocytoma: A case report. Int J Clin Exp Pathol. 8:11809–11813.
2015.PubMed/NCBI
|
|
20
|
Wemmert S, Bettscheider M, Alt S, Ketter
R, Kammers K, Feiden W, Steudel WI, Rahnenführer J and Urbschat S:
p15 promoter methylation - a novel prognostic marker in
glioblastoma patients. Int J Oncol. 34:1743–1748. 2009.PubMed/NCBI
|
|
21
|
Bartek J, Bartkova J and Lukas J: The
retinoblastoma protein pathway and the restriction point. Curr Opin
Cell Biol. 8:805–814. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gil J and Peters G: Regulation of the
INK4b-ARF-INK4a tumor suppressor locus: All for one or one for all.
Nat Rev Mol Cell Biol. 7:667–677. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jen J, Harper JW, Bigner SH, Bigner DD,
Papadopoulos N, Markowitz S, Willson JK, Kinzler KW and Vogelstein
B: Deletion of p16 and p15 genes in brain tumors. Cancer Res.
54:6353–6358. 1994.PubMed/NCBI
|
|
24
|
Schmidt EE, Ichimura K, Reifenberger G and
Collins VP: CDKN2 (p16/MTS1) gene deletion or CDK4 amplification
occurs in the majority of glioblastomas. Cancer Res. 54:6321–6324.
1994.PubMed/NCBI
|
|
25
|
Simon M, Köster G, Menon AG and Schramm J:
Functional evidence for a role of combined CDKN2A
(p16-p14(ARF))/CDKN2B (p15) gene inactivation in malignant gliomas.
Acta Neuropathol. 98:444–452. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rasheed A, Herndon JE, Stenzel TT, Raetz
JG, Kendelhardt J, Friedman HS, Friedman AH, Bigner DD, Bigner SH
and McLendon RE: Molecular markers of prognosis in astrocytic
tumors. Cancer. 94:2688–2697. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakamura M, Watanabe T, Yonekawa Y,
Kleihues P and Ohgaki H: Promoter methylation of the DNA repair
gene MGMT in astrocytomas is frequently associated with G:C->
A:T mutations of the TP53 tumor suppressor gene. Carcinogenesis.
22:1715–1719. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Komine C, Watanabe T, Katayama Y, Yoshino
A, Yokoyama T and Fukushima T: Promoter methylation of the DNA
repair gene O6-methylguanine-DNA methyltransferase is an
independent predictor of shortened progression free survival in
patients with low-grade diffuse astrocytomas. Brain Pathol.
13:176–184. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Munot K, Bell SM, Lane S, Horgan K, Hanby
AM and Speirs V: Pattern of expression of genes linked to
epigenetic silencing in human breast cancer. Hum Pathol.
37:989–999. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jha Chintamani BP, Bhandari V, Bansal A,
Saxena S and Bhatnagar D: The expression of mismatched repair genes
and their correlation with clinicopathological parameters and
response to neo-adjuvant chemotherapy in breast cancer. Int Semin
Surg Oncol. 4:52007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sharma G, Mirza S, Parshad R, Srivastava
A, Gupta SD, Pandya P and Ralhan R: Clinical significance of
promoter hypermethylation of DNA repair genes in tumor and serum
DNA in invasive ductal breast carcinoma patients. Life Sci.
87:83–91. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hegi ME, Liu L, Herman JG, Stupp R, Wick
W, Weller M, Mehta MP and Gilbert MR: Correlation of
O6-methylguanine methyltransferase (MGMT) promoter methylation with
clinical outcomes in glioblastoma and clinical strategies to
modulate MGMT activity. J Clin Oncol. 26:4189–4199. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Herman JG, Graff JR, Myöhänen S, Nelkin BD
and Baylin SB: Methylation-specific PCR: A novel PCR assay for
methylation status of CpG islands. Proc Natl Acad Sci USA.
93:9821–9826. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Felsberg J, Rapp M, Loeser S, Fimmers R,
Stummer W, Goeppert M, Steiger HJ, Friedensdorf B, Reifenberger G
and Sabe MC: Prognostic significance of molecular markers and
extent of resection in primary glioblastoma patients. Clin Cancer
Res. 15:6683–6693. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wong IH, Lo YM, Yeo W, Lau WY and Johnson
PJ: Frequent p15 promoter methylation in tumor and peripheral blood
from hepatocellular carcinoma patients. Clin Cancer Res.
6:3516–3521. 2000.PubMed/NCBI
|
|
36
|
Simpson DJ, Hibberts NA, McNicol AM,
Clayton RN and Farrell WE: Loss of pRb expression in pituitary
adenomas is associated with methylation of the RB1 CpG island.
Cancer Res. 60:1211–1216. 2000.PubMed/NCBI
|
|
37
|
Capper D, Weißert S, Balss J, Habel A,
Meyer J, Jäger D, Ackermann U, Tessmer C, Korshunov A, Zentgraf H,
et al: Characterization of R132H mutation-specific IDH1 antibody
binding in brain tumors. Brain Pathol. 20:245–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Alford R, Gargan L, Bowers DC, Klesse LJ,
Weprin B and Koral K: Postoperative surveillance of pediatric
cerebellar pilocytic astrocytoma. J Neurooncol. 130:149–154. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Klein O, Grignon Y, Civit T, Pinelli C,
Auque J and Marchal JC: Childhood diencephalic pilocytic
astrocytoma. A review of seven observations. Neurochirurgie.
52:3–14. 2006.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Uhlmann K, Rohde K, Zeller C, Szymas J,
Vogel S, Marczinek K, Thiel G, Nürnberg P and Laird PW: Distinct
methylation profiles of glioma subtypes. Int J Cancer. 106:52–59.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gonzalez-Gomez P, Bello J, Lomas J, Arjona
D, Alonso ME, Amiñoso C, De Campos JM, Vaquero J, Sarasa JL,
Casartelli C and Rey JA: Epigenetic changes in pilocytic
astrocytomas and medulloblastomas. Int J Mol Med. 11:655–660.
2003.PubMed/NCBI
|
|
42
|
Wemmert S, Ketter R, Rahnenführer J,
Beerenwinkel N, Strowitzki M, Feiden W, Hartmann C, Lengauer T,
Stockhammer F, Zang KD, et al: Patients with high-grade gliomas
harboring deletions of chromosomes 9p and 10q benefit from
temozolomide treatment. Neoplasia. 7:883–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Skorpen F and Krokan HE: The methylation
status of the gene for O6-methylguanine-DNA methyltransferase in
human Mer+ and Mer− cells. Carcinogenesis.
16:1857–1863. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Esteller M, Hamilton SR, Burger PC, Baylin
SB and Herman JG: Inactivation of the DNA repair gene
O6-methylguanine-DNA methyltransferase by promoter hypermethylation
is a common event in primary human neoplasia. Cancer Res.
59:793–797. 1999.PubMed/NCBI
|
|
45
|
Lissa D and Robles AI: Methylation
analyses in liquid biopsy. Transl Lung Cancer Res. 5:492–504. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mitchell SM, Ho T, Brown GS, Baker RT,
Thomas ML, McEvoy A, Xu ZZ, Ross JP, Lockett TJ, Young GP, et al:
Evaluation of methylation biomarkers for detection of circulating
tumor DNA and application to colorectal cancer. Genes (Basel).
7:pii: E125. 2016. View Article : Google Scholar
|