Introduction
Pancreatic cancer is a type of digestive malignant
tumor, and has a poor prognosis (1).
Patients with pancreatic cancer often experience few symptoms in
the early stages of the disease, and local invasion and distant
metastases typically occur quickly (2). Despite aggressive surgical resection and
advances in systemic treatment methods, the mortality rate of
pancreatic cancer ranks fourth worldwide, with a five-year survival
rate of <5% and a continuously increasing rate of mortality and
morbidity (3,4). As a result, the investigation and
understanding of the mechanisms underlying the invasion and
metastasis of pancreatic cancer is important in the diagnosis and
treatment of this disease.
In a number of previous studies, the interaction of
C-X-C motif chemokine receptor (CXCR) 4 with CXCL12 has been
observed to perform an important role in tumor proliferation,
invasion, angiogenesis, metastasis and migration in numerous types
of cancer (5–7). It is also considered that the
CXCR4/CXCL12 axis is significantly associated with the prognosis of
patients with pancreatic tumors, and may become a novel target for
the treatment of tumors (8). In a
previous study, the regulation of angiogenesis and
lymphangiogenesis was demonstrated to be a possible mechanism by
which CXCR4 influenced the progression of pancreatic cancer using
quantitative-polymerase chain reaction (9). However, this study involved only 30
patients and no critical biomarker was identified.
Therefore, the aim of the present study was to test
the association between the expression of CXCR4 and
clinicopathological factors in a larger sample size. The present
study focused on the association between CXCR4 and vascular
endothelial growth factor-C (VEGF-C), and the effect of CXCR4 on
angiogenesis in pancreatic cancer. Immunohistochemistry was used to
study the association among biomarkers [(CXCR4, VEGF-C, β-catenin,
Ki-67 and matrix metalloproteinase 2 (MMP-2)] in order to
investigate the relevant signaling pathways and corresponding
proteins that are involved in the complex molecular network
underlying the invasion and metastasis of pancreatic cancer.
Materials and methods
Sample collection
Tissue samples were obtained from 60 patients who
were diagnosed with and treated for pancreatic cancer by
pathological section analysis at the Shandong Tumor Hospital
(Jinan, China) between July 2012 and February 2016. The clinical
information for all patients was complete. Informed consent was
obtained from each patient. Patients consisted of 32 males and 28
females, with an average age of 57.5 years (range, 34–74). Samples
of the pancreatic tumor, paracancerous tissues (obtained <2 cm
from the tumor), normal pancreas and the lymph nodes surrounding
the pancreas were obtained through macroscopic curative resection.
Among the 60 samples, 41 were derived from the head of the pancreas
and the remaining 19 originated from alternate areas. A total of 54
tissue samples were defined as being ductal adenocarcinoma, and 6
were other pathological types. In addition, 27 of the samples were
poorly differentiated and 33 cases were well/moderately
differentiated.
There were 23 patients classified as having stage
I–II pancreatic cancer and 37 patients classified as having stage
III–IV pancreatic cancer [according to the 7th edition of
tumor-node-metastasis (TNM) staging system provided by the American
Joint Committee on Cancer] (10). In
total, 35 patients were identified as having lymph nodes metastasis
whilst the remaining 25 cases did not exhibit lymph nodes
metastasis. The present study included 42 cases for which the
maximum lesion diameter was >3 cm, whilst the maximum diameters
for the other 18 cases were ≤3 cm. All tissues were fixed in 4%
neutral buffered formalin for 24 h at 37°C, embedded in paraffin
and sectioned into 4 µm slices. New sections were then stained with
hematoxylin and eosin to verify diagnosis and perform
immunohistochemical analysis.
Immunohistochemical assays
The sections were manipulated to immunohistochemical
staining using the Dako Envision System method (Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA), according to the
manufacturer' protocol. A positive section of company configuration
(Dako; Agilent Technologies, Inc.) was used as a positive control
and PBS was employed as the negative control. The sections were
heated for 1 h at 65°C and dewaxing was performed subsequently.
Sections were then washed with a graded ethanol series (95, 85 and
75%), incubated with ethanol containing 3%
H2O2 for 10 min to block endogenous
peroxidase and microwaved at 98°C for 15 min to retrieve the
antigen. Subsequent to cooling for 10 min at room temperature,
sections were rinsed with PBS and incubated overnight at 4°C with
the following primary antibodies: CXCR4 (dilution, 1:40; cat no.
374606; mouse monoclonal; R&D Systems, Inc., Minneapolis, MN,
USA), CXCL12 (dilution, 1:100; cat no. 79018.111; mouse monoclonal;
R&D Systems, Inc.), cluster of differentiation 34 (dilution,
1:80; cat no. QBEnd/10; mouse monoclonal; Fuzhou Maixin Biotech.
Co., Ltd., Fuzhou, China), VEGF-C (dilution, 1:200; cat no. VG1;
mouse monoclonal; Fujian Gutian Yuanhang Medical Co., , Ltd.),
Ki-67 (dilution, 1:200; cat no. K-2; mouse monoclonal; ZSGB-BIO
Co., Ltd., Beijing, China), β-catenin (dilution, 1:100; cat no.
CAT-5H10; mouse monoclonal; ZSGB-BIO) and MMP-2 (dilution, 1:200;
cat no. ab37150; rabbit polyclonal; Abcam, Cambridge, UK). Sections
were subsequently incubated with a goat anti-mouse horseradish
peroxidase conjugated-immunoglobulin G antibody (dilution, 1:500;
cat no. 111-035-003; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) multimer for 15 min at 37°C. Subsequent to rinsing with PBS,
the sections were visualized using 3,3′diaminobenzidine, and the
slides were counterstained with hematoxylin.
Interpretation of result
The double blind reading method was utilized in the
present study. Cells with a cytomembrane and cytoplasm that was
stained by the CXCL12/CXCR4 antibody were defined as CXCL12/CXCR4
positive. Similarly its corresponding antibody stained the
cytoplasm of VEGF-C positive cells. Ki-67 positive cells expressed
nuclear staining, MMP-2 positive cells exhibited cytoplasmic
staining and β-catenin positive cells demonstrated cytomembrane or
cytoplasmic staining.
Each section should count ≥10 400-fold fields of
view, and cells exhibiting brown granules were considered to be
positive cells (positive for the targeted biomarkers). Slices were
graded based on the percentage of stained cells and the staining
strength in the semi-quantitative integral method detailed below.
The percentage of stained cells was classified as follows: ≤10%, 0
points; >10% and <25%, 1 point; ≥25 and ≤50%, 2 points;
>50%, 3 points. A staining percentage >10% was classified as
being a positive range. Staining intensity was dependent on the
shade of the color: Without staining, 0 points; pale yellow, 1
point; brown, 2 points; dark brown, 3 points. The final score
demonstrated the positive level of the slice and was determined by
the product of the two scores above: A staining score ≤1 was
considered negative (−), 2–3 points indicated positive staining (+)
and ≥4 points demonstrated strong positive staining (++; Fig. 1).
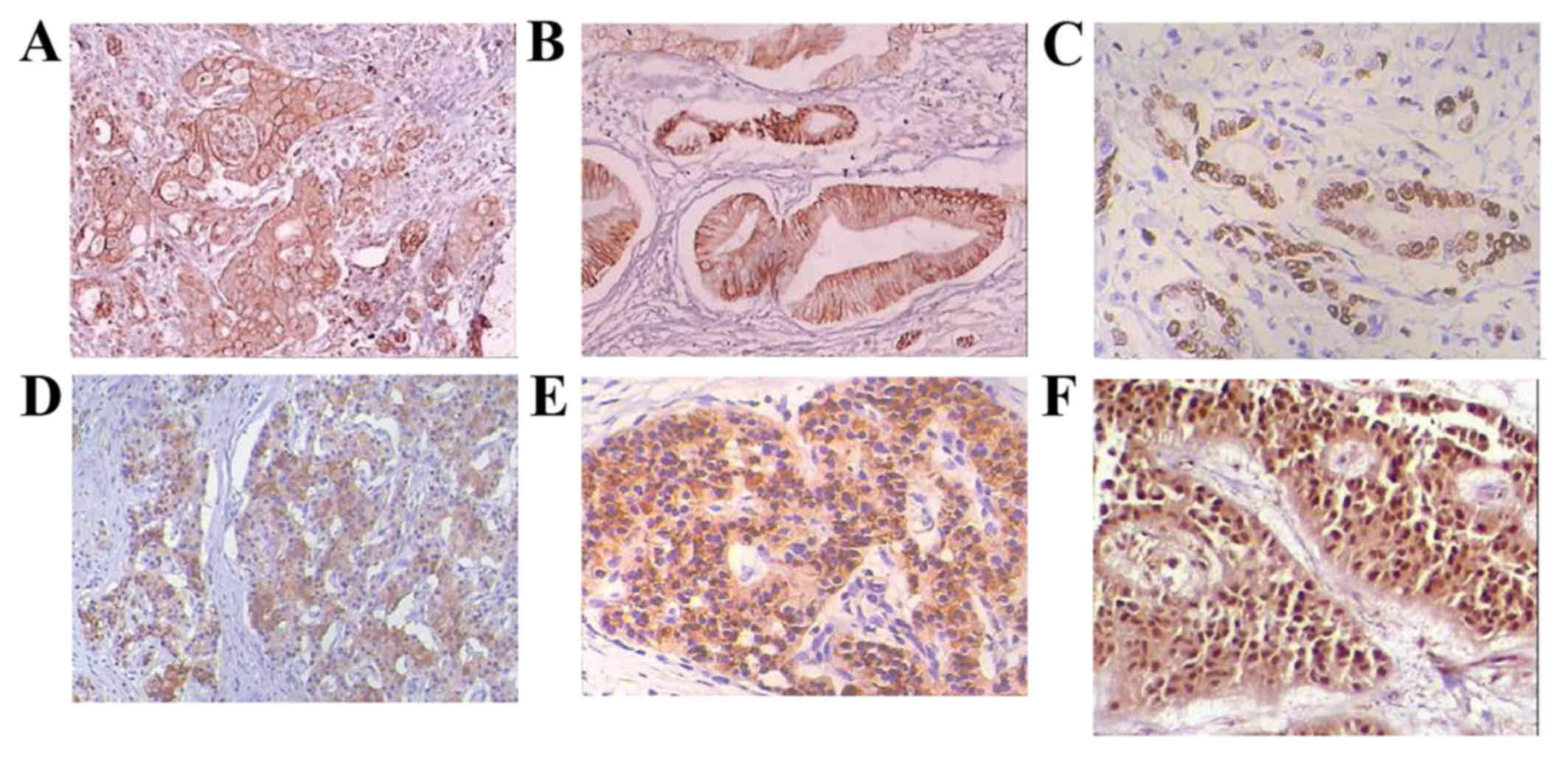 | Figure 1.Immunohistochemical staining of
pancreatic cancer tissue using hematoxylin and eosin staining. (A)
CXCR4 positive expression, magnification ×100; (B) CXCL12 positive
expression, magnification ×100; (C) Ki-67 positive expression,
magnification ×200; (D) MMP-2 positive expression, magnification
×100; (E) β-catenin positive expression, magnification ×200; (F)
VEGF-C positive expression, magnification ×200. CXCR, C-X-C motif
chemokine receptor; CXCR, C-X-C motif chemokine ligand; MMP, matrix
metalloproteinase; VEGF, vascular endothelial growth factor. |
Statistical analysis
Statistical analysis was conducted using SPSS 19.0
(IBM SPSS, Armonk, NY, USA). The χ2-test was used for
univariate analysis to identify the clinicopathological factors
that may affect CXCR4, including gender, age, location,
differentiation, pathological type, tumor size, lymph node
metastasis and TNM stage. Logistic regression and Spearman's rank
test were used for multivariate analysis of CXCR4 with these four
biomarkers. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of CXCR4 in pancreatic
tumors, paracancerous tissues, normal pancreatic tissue and in the
lymph nodes surrounding the pancreas
The level of CXCR4 protein expression is low in
normal pancreatic tissue, and high in pancreatic tumor tissues,
paracancerous tissues and the lymph nodes surrounding the pancreas,
with rates of positive expression of CXCR4 (CXCL12) being 18.3%
(45.0%), 56.7% (86.7%), 50.0% (85.0%) and 53.3% (80.0%),
respectively. No significant differences were identified between
pancreatic tumor tissues, paracancerous tissues and lymph nodes
(P>0.05); however, all three tissue types were significantly
different compared with the normal pancreatic tissue sample
(P<0.05; Table I).
 | Table I.CXCR4/CXCL12 expression in various
tissues. |
Table I.
CXCR4/CXCL12 expression in various
tissues.
|
| CXCR4 expression | CXCL12
expression |
|---|
|
|
|
|
|---|
| Tissue | Negative | Positive | Negative | Positive |
|---|
| Pancreatic tumor | 26 | 34 | 8 | 52 |
| Paracancerous
tissue | 30 | 30 | 9 | 51 |
| Lymph node | 28 | 32 | 12 | 48 |
| Surrounding
tissue | 49 | 11 | 27 | 33 |
The correlation between
clinicopathological factors and CXCR4 expression
In order to illuminate the role of CXCR4 in the
progression of pancreatic cancer, the present study also analyzed
the association between the expression profiles of CXCR4 and the
grade and tumor stage in 60 patients. CXCR4 expression was
significantly associated with tumor lymph node metastasis
(P=0.001), pathological type (P=0.037) and TNM stage (P=0.031).
However, no correlation was observed between CXCR4 expression and
patient age, gender, tumor location, tumor differentiation or tumor
size (Table II).
 | Table II.Correlation of CXCR4 expression with
clinicopathological factors. |
Table II.
Correlation of CXCR4 expression with
clinicopathological factors.
| Characteristics | Case | CXCR4 (−) | CXCR4 (+) | P-value |
|---|
| Age, years |
|
|
| 0.714 |
|
<60 | 33 | 15 | 18 |
|
| ≥60 | 37 | 11 | 16 |
|
| Gender |
|
|
| 0.944 |
|
Female | 28 | 12 | 16 |
|
| Male | 32 | 14 | 18 |
|
| Tumor location |
|
|
| 0.121 |
| Head of
pancreas | 41 | 15 | 26 |
|
|
Others | 19 | 11 | 8 |
|
| Differentiation |
|
|
| 0.228 |
|
Well/moderate | 33 | 12 | 21 |
|
| Poor | 27 | 14 | 13 |
|
| Pathological
type |
|
|
|
0.037a |
|
Adenocarcinoma | 54 | 21 | 33 |
|
|
Others | 6 | 5 | 1 |
|
| Lymph node stage |
|
|
|
0.001a |
|
Negative | 25 | 19 | 6 |
|
|
Positive | 35 | 7 | 28 |
|
| TNM stage |
|
|
|
0.031a |
|
I+II | 23 | 14 | 9 |
|
|
III+IV | 37 | 12 | 25 |
|
| Tumor size, cm |
|
|
| 0.111 |
|
≤3.0 | 18 | 5 | 13 |
|
|
>3.0 | 42 | 21 | 21 |
|
Correlation analysis between CXCR4 and
biomarkers
In order to analyze the correlation of CXCR4 with
VEGF-C, Ki-67, MMP-2 and β-catenin, the present study detected
their expression levels and performed data analysis using the
Spearman's rank test. The results demonstrate that CXCR4 exhibited
a positive correlation with VEGF-C (r=0.417; P=0.001), Ki-67
(r=0.316; P=0.014), MMP-2 (r=0.284; P=0.028) and β-catenin
(r=0.369; P=0.004; Table III).
Furthermore, logistic regression analysis revealed VEGF-C (β=1.722;
P=0.005) and Ki-67 (β=1.196; P=0.047) to be the independent factors
(Table IV).
 | Table III.Analysis of CXCR4 expression levels
with biomarkers using the Spearman's test. |
Table III.
Analysis of CXCR4 expression levels
with biomarkers using the Spearman's test.
|
| CXCR4
expression |
|---|
|
|
|
|---|
| Biomarker | r-value | P-value |
|---|
| VEGF-C | 0.417 |
0.001a |
| β-catenin | 0.368 |
0.004a |
| Ki-67 | 0.316 |
0.014a |
| MMP-2 | 0.284 |
0.028a |
 | Table IV.Analysis of CXCR4 with biomarkers by
logistic regression analysis. |
Table IV.
Analysis of CXCR4 with biomarkers by
logistic regression analysis.
| Biomarker | Coefficient
(β) | P-value | OR |
|---|
| VEGF-C | 1.722 | 0.005a | 5.594 |
| β-catenin | 1.086 | 0.275 | 2.961 |
| Ki-67 | 1.196 | 0.047a | 3.307 |
| MMP-2 | 0.438 | 0.498 | 1.549 |
Discussion
The morbidity of pancreatic cancer is ~10/100,000
and the rate of incidence is continuously increasing worldwide
(11). Pancreatic cancer accounted
for 4–5% of cancer-associated mortality in 2015 worldwide and, as a
result of a high frequency of local or distant metastasis, its
prognosis is poor (3,11–14). Tumor
migration is controlled by its chemokine receptors and the
corresponding chemokine expressed in target organ. When the
chemokine combines with its specific receptor, it may provoke the
aggregation of actin and induce the movement and migration of tumor
cells.
The CXCL12/CXCR4 axis is the typical representation
used to describe the tendency of tumor cells to metastasize; the
expression of CXCL12 and CXCR4 has been observed to increase in
breast cancer, glioma and melanoma (15–18). As
suggested by the present study, the mechanism by which a pancreatic
tumor cell may metastasize to a specify organ could be as follows:
Firstly, tumor cells proliferate in primary lesions, and there is
an overexpression of CXCR4 on the surface of cells. Secondly,
CXCL12, which is expressed on the surface of the target organ, may
promote tumor cells separate from tumor tissue and activate several
adhesion molecules of cells and induce them to secrete more MMP and
VEGF in order to dissolve the extracellular matrix (ECM).
Subsequently, tumor cells may cross the lymph vessel and blood
vessel walls of the tumor into the circulation system, and may then
combine with the vascular wall of the organ that has high
expression levels of CXCL12 (19,8). By this
mechanism, the migrating cells could continue to proliferate in the
new organ and subsequently metastasize to another location.
CXCR4 levels have been detected as overexpressed in
pancreatic cancer tissues and exhibit a close association with the
differentiation, metastasis, growth and prognosis of pancreatic
cancer (8,9,20,21). The results of the present study
demonstrated that the expression levels of CXCR4 in tumor tissues
were increased compared with in normal tissues, indicating that
CXCR4 may be involved in the development of pancreatic cancer. In
addition, the overexpression of CXCR4 in metastatic lymph nodes and
distant metastasis was also associated with the TNM stage.
Therefore, the overexpression of CXCR4 could contribute to the
early diagnosis of patients with pancreatic cancer. The expression
of CXCR4 was low in normal tissues (positive rate=18.3%), while it
was high in pancreatic cancer tissues (56.7%), paracarcinoma
tissues (50.0%) and peripheral lymph nodes (53.3%), indicating that
the deregulation of CXCR4 may be a potential mechanism by which the
growth and migration of cancer is regulated. In addition, high
expression levels of CRXC4 was also closely associated with the
biological effects of pancreatic cancer (pathological type, lymph
node stage, and TNM stage; Table
II).
There are currently few studies investigating CXCL12
and tumor lesion, and studies have identified that there was
abnormal expression of CXCL12 in certain types of cancer, including
ovarian and pancreatic cancer and glioma (22–24).
Several studies have demonstrated that increased expression levels
of CXCL12 in the bone marrow could inhibit tumor cell migration
(23,25), and that the progression of high-grade
glioma with high expression of CXCL12 would be more rapid than
normal (24). In a previous study
investigating CXCL12/CXCR4, the origin of CXCL12 was suggested to
be associated with mesenchymal cells, as a result of the paracrine
mechanism of CXCL12, and mesenchymal cells may be involved in tumor
cell invasion through the CXCR4/CXCL12 axis (25). Another study hypothesized that CXCL12
may restrain the tumor immune response by blocking the maturation
of dendritic cells and by prompting tumor proliferation (26). Therefore, the present study
investigated the correlation between the expression of CXCL12 and
lymph node metastasis, and subsequently demonstrated this
association. In addition, the present study also identified that
the level of CXCL12 expression in the tissues surrounding the tumor
was increased compared with in normal and noncancerous tissues.
These results indicated that CXCL12 performs a vital role in tumor
progression, particularly in the process of lymph node
metastasis.
A previous study on angiogenesis identified that
VEGF was able to promote endothelial cells (EC) to express CXCR4
(27), and a subsequent study
confirmed that CXCL12 could induce ECs to express VEGF (6,28). It may
therefore be considered that CXCL12/CXCR4 and VEGF form a feedback
loop of paracrine signaling in order to coordinate the procedure of
angiogenesis and to enhance their biological function. In the
present study, VEGF-C exhibited higher levels of expression in
tumor-associated tissues compared with in noncancerous pancreatic
tissue, which was correlated with the expression levels of CXCR4
(r=0.556; P<0.001). This result indicates that VEGF-C and CXCR4
collaboratively prompt the invasion and metastasis of pancreatic
cancer. In addition, the present study also investigated the
positive correlation between Ki-67 and CXCR4 (r=0.316; P=0.014),
and suggested that CXCR4 may be able to facilitate tumor cell
growth. It is generally considered that the binding of CXCL12 and
CXCR4 could elevate MMP-2 expression and inhibit the secretion of
tissue inhibitors of MMPs (29).
In addition, it has been revealed that the
combination of CXCL12 and CXCR4 can activate nuclear factor-κB
(NF-κB), which significantly increases the secretion of MMP-2
(30), a result concordant with those
of the present study. The levels of CXCR4 and MMP-2 protein
expression are correlated in pancreatic cancer (r=0.284; P=0.028),
and each were observed to be capable of promoting tumor cell
invasion and metastasis and could be utilized as a criteria for
evaluating the biological behavior of pancreatic tumors. Results of
in vitro experiments in pancreatic cancer indicated that if
CXCR4 was not expressed, the activity of Wnt was suppressed;
therefore, the target gene of Wnt/β-catenin would be downregulated,
ultimately preventing tumor invasion or migration (31). Levels of CXCR4 expression were
determined to be significantly correlated with the expression
levels of β-catenin (r=0.292; P=0.024). Therefore, CXCL12/CXCR4 may
be able to regulate the Wnt/β-catenin signal transduction pathway
in order to alter the performance of corresponding factors. In
addition, the correlation of β-catenin expression with MMP-2 was
observed to be significant (r=0.308; P=0.017), which indicates that
β-catenin may upregulate members of the MMP family's expression to
enhance the decomposition of ECM and vascular basement membrane in
order to provoke the invasion and metastasis of pancreatic
tumors.
The association between the CXCR4/CXCL12 axis and
the biological behavior of pancreatic tumors has been established.
All pancreatic tumor tissues express CXCR4 and CXCL12 at higher
expression levels compared with those in noncancerous tissues. The
CXCL12/CXCR4 axis is involved in the angiogenesis of pancreatic
cancer tumors, promotes tumor cell migration and cellular
proliferation and inhibits apoptosis. It is now believed that
VEGF-C, Ki-67 and CXCR4 can be considered indicators of tumor
therapeutic efficacy and can determine whether tumor metastasis
occurs. The aforementioned biological effects of the CXCR4/CXCL12
axis may be inhibited by VEGF-C antagonists, and may then become a
novel target in the directed therapy of patients with pancreatic
cancer.
The CXCL12/CXCR4 axis performs an essential role in
the behavior of pancreatic cancer. VEGF-C and Ki-67 are two
important biomarkers, through which CXCR4 may induce the metastatic
behavior of pancreatic cancer cells. Therefore, inhibitors of
angiogenesis continue to be effective agents for treating
pancreatic cancer.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim VM and Ahuja N: Early detection of
pancreatic cancer. Chin J Cancer Res. 27:321–331. 2015.PubMed/NCBI
|
|
4
|
Sheffield KM, Crowell KT, Lin YL, Djukom
C, Goodwin JS and Riall TS: Surveillance of pancreatic cancer
patients after surgical resection. Ann Surg Oncol. 19:1670–1677.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vandercappellen J, Van Damme J and Struyf
S: The role of CXC chemokines and their receptors in cancer. Cancer
Lett. 267:226–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chatterjee S, Azad B Behnam and Nimmagadda
S: The intricate role of CXCR4 in cancer. Adv Cancer Res.
124:31–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao H, Guo L, Zhao H, Zhao J, Weng H and
Zhao B: CXCR4 over-expression and survival in cancer: A system
review and meta-analysis. Oncotarget. 6:5022–5040. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu PF, Lu ZP, Cai BB, Tian L, Zou C, Jiang
KR and Miao Y: Role of CXCL12/CXCR4 signaling axis in pancreatic
cancer. Chin Med J (Engl). 126:3371–3374. 2013.PubMed/NCBI
|
|
9
|
Cui K, Zhao W, Wang C, Wang A, Zhang B,
Zhou W, Yu J, Sun Z and Li S: The CXCR4-CXCL12 pathway facilitates
the progression of pancreatic cancer Via induction of angiogenesis
and lymphangiogenesis. J Surg Res. 171:143–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bilimoria KY, Bentrem DJ, Merkow RP,
Tomlinson JS, Stewart AK, Ko CY and Talamonti MS: Application of
the pancreatic adenocarcinoma staging system to pancreatic
neuroendocrine tumors. J Am Coll Surg. 205:558–563. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stathis A and Moore MJ: Advanced
pancreatic carcinoma: Current treatment and future challenges. Nat
Rev Clin Oncol. 7:163–372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharma C, Eltawil KM, Renfrew PD, Walsh MJ
and Molinari M: Advances in diagnosis, treatment and palliation of
pancreatic carcinoma: 1990–2010. World J Gastroenterol. 17:867–897.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang YM, Li G, Sun BC, Zhao XL and Zhou
ZK: Study on the relationship between cxcr4 expression and
perineural invasion in pancreatic cancer. Asian Pac J Cancer Prev.
15:4893–4896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu C, Zhao H, Chen H and Yao Q: CXCR4 in
breast cancer: Oncogenic role and therapeutic targeting. Drug Des
Devel Ther. 9:4953–4964. 2015.PubMed/NCBI
|
|
17
|
Tsai MF, Chang TH, Wu SG, Yang HY, Hsu YC,
Yang PC and Shih JY: EGFR-L858R mutant enhances lung adenocarcinoma
cell invasive ability and promotes malignant pleural effusion
formation through activation of the CXCL12-CXCR4 pathway. Sci Rep.
5:135742015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deng L, Shang Y, Guo S, Liu C, Zhou L, Sun
Y, Nie Y, Fan D, Lu Y and Guo X: Ran GTPase protein promotes
metastasis and invasion in pancreatic cancer by deregulating the
expression of AR and CXCR4. Cancer Biol Ther. 15:1087–1093. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun X, Cheng G, Hao M, Zheng J, Zhou X,
Zhang J, Taichman RS, Pienta KJ and Wang J: CXCL12/CXCR4/CXCR7
chemokine axis and cancer progression. Cancer Metastasis Rev.
29:709–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wehler T, Wolfert F, Schimanski CC, Gockel
I, Herr W, Biesterfeld S, Seifert JK, Adwan H, Berger MR, Junginger
T, et al: Strong expression of chemokine receptor CXCR4 by
pancreatic cancer correlates with advanced disease. Oncol Rep.
16:1159–1164. 2006.PubMed/NCBI
|
|
21
|
Krieg A, Riemer JC, Telan LA, Gabbert HE
and Knoefel WT: CXCR4-A prognostic and clinicopathological
biomarker for pancreatic ductal adenocarcinoma: A Meta-Analysis.
PLoS One. 10:e01301922015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou Y, Larsen PH, Hao C and Yong VW:
CXCR4 is a major chemokine receptor on glioma cells and mediates
their survival. J Brol Chem. 277:49481–49487. 2002.
|
|
23
|
Lapidot T and Kollet O: The essential
roles of the chemokine SDF-1 and its receptor CXCR4 in human stem
cell homing and repopulation of tranaplanted immune-deficient
NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 16:1992–2003. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salmaggi A, Gelati M, Pollo B, Frigerio S,
Eoli M, Silvani A, Broggi G, Ciusani E, Croci D, Boiardi A and De
Rossi M: CXCL12 in malignant glial tumors: A possible role in
angiogenesis and cross-talk between endothelial and tumoral cells.
J Neurooncol. 67:305–317. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhong W, Chen W, Zhang D, Sun J, Li Y,
Zhang J, Gao Y, Zhou W and Li S: CXCL12/CXCR4 axis plays pivotal
roles in the organ-specific metastasis of pancreatic
adenocarcinoma: A clinical study. Exp Ther Med. 4:363–369. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zou W, Machelon V, Coulomb-L'Hermin A,
Borvak J, Nome F, Isaeva T, Wei S, Krzysiek R, Durand-Gasselin I,
Gordon A, et al: Stromal-derived factor-1 in human tumors recruits
and alters the function of plasmacytoid precursor dendritic cells.
Nat Med. 7:1339–1346. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salvucci O, Yao L, Villalba S, Sajewicz A,
Pittaluga S and Tosato G: Regulation of endothelial cell branching
morphogenesis by endogenous chemokine stromal-derived factor-1.
Blood. 99:2703–2711. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Z, Ma Q, Li P, Sha H, Li X and Xu J:
Aberrant expression of CXCR4 and β-catenin in pancreatic cancer.
Anticancer Res. 33:4103–4110. 2013.PubMed/NCBI
|
|
29
|
Lane WJ, Dias S, Hattori K, Heissig B,
Choy M, Rabbany SY, Wood J, Moore MA and Rafii S: Stromal-derived
factor 1-induced megakaryocyte migration and platelet production is
dependent on matrix metalloproteinases. Blood. 96:4152–4159.
2000.PubMed/NCBI
|
|
30
|
Yuecheng Y and Xiaoyan X: Stromal-cell
derived factor-1 regulates epithelial ovarian cancer cell invasion
by activating matrix metalloproteinase-9 and matrix
metalloproteinase-2. Eur J Cancer Prev. 16:430–435. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Z, Ma Q, Liu Q, Yu H, Zhao L, Shen S
and Yao J: Blockade of SDF-1/CXCR4 signalling inhibits pancreatic
cancer progression in vitro via inactivation of canonical Wnt
pathway. Br J Cancer. 99:1695–1703. 2008. View Article : Google Scholar : PubMed/NCBI
|















