Introduction
Pancreatic cancer (PC) has a high mortality rate;
its 5-year survival rate is between 7 and 8% (1). The estimated number of new cases of PC
in China in 2015 was 90,100 and the estimated number of reported
mortalities from the disease was 79,400 (2). The incidence and mortality rates of male
patients with PC has demonstrated an upward trend from 2010 to 2011
(2). PC is primarily treated with
chemotherapeutic drugs, which may result in side effects and
potential drug resistance that can further lead to patient
mortality (3). The current standard
drug for chemotherapy to treat pancreatic cancer is gemcitabine;
however, its efficacy is far from satisfactory (3). To date, there is no other agent that has
promoted a clinically meaningful prolongation of overall survival
(3). The identification of a novel
therapy for this highly aggressive disease is required.
Retrospective studies have suggested that metformin
may reduce the risk and improve the prognosis of patients with PC
(4–7).
It has been demonstrated that metformin activates adenosine
monophosphate (AMP)-activated protein kinase (AMPK), which then
inhibits the mammalian target of rapamycin (mTOR) signaling pathway
(8–11). The mTOR and AMPK signaling pathways
control the mammalian cells' energy status [adenosine triphosphate
(ATP)/AMP ratio] and regulate cell growth (12). Inhibition of mTOR has been
demonstrated to reduce the growth of PC cells (12). Rapamycin specifically inhibits mTOR
and thus inhibits the proliferation and survival of PC cells
(12).
The present study aimed to explore the efficacy of
combined metformin and rapamycin therapy for the treatment of
PC.
Materials and methods
Reagents
L-15 medium and fetal bovine serum (FBS) were
obtained from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Antibodies directed against vascular endothelial growth
factor (VEGF; sc-7269) were obtained from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Antibodies directed against AMPK (#5832),
phosphorylated (p)-AMPKThr172 (#2535), mTOR (#2983),
p-mTORSer2448 (#5536), B-cell lymphoma-2 (Bcl-2;
#15071), epidermal growth factor receptor (EGFR) (#4267), β-actin
(#4970), anti-rabbit immunoglobulin (Ig)G, HRP (horseradish
peroxidase-conjugated)-linked antibody (#7074), and anti-mouse IgG,
HRP-linked antibody (#7076) were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). All other reagents, unless
stated otherwise, were obtained from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany).
Cell culture
The PC cell line SW1990 was acquired from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). It was maintained in L-15 medium with 10% heat-inactivated
FBS and 1% penicillin/streptomycin at 37°C in a 95% humidified
atmosphere.
MTT assay
PC cell viability was measured using a MTT assay.
SW1990 cells were seeded in 96-well plates at a density of
105/ml and allowed to adhere overnight at 37°C and
treated with PBS, metformin (5, 10, 15, and 20 mmol/l), rapamycin
(0.2, 2, 20, and 200 ng/ml) and their combinations: A, metformin (5
mmol/l) + rapamycin (0.2 ng/ml); B, metformin (10 mmol/l) +
rapamycin (2 ng/ml); C, metformin (15 mmol/l) + rapamycin (20
ng/ml); and D, metformin (20 mmol/l) + rapamycin (200 ng/ml))
respectively. A total of 5 mg/ml MTT solution was added to each
well. The cell viability was determined by measuring the absorbance
of formazan which was dissolved in dimethyl sulfoxide at 490 nm
using a microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Western blot analysis
SW1990 cells were treated with PBS (as control),
metformin 20 mmol/l, rapamycin 200 ng/ml or metformin 20 mmol/l +
rapamycin 200 ng/ml at 37°C for 24 h. The cells were then lysed in
a Radioimmunoprecipitation Assay (RIPA) Lysis and Extraction Buffer
(Thermo Fisher Scientific, Inc.). Protein (~15 µg) from each sample
was extracted in RIPA Lysis and Extraction Buffer and quantified
using a Bicinchoninic Acid Protein Assay kit (Thermo Fisher
Scientific, Inc.). Then protein was separated using SDS-PAGE (5–10%
gel) and electro-transferred to polyvinylidene fluoride membranes
(Millipore, Bedford, MA, USA). The membrane was blocked with 5%
nonfat dry milk in Tris-buffered saline Tween-20 at room
temperature for 1 h. The membranes were incubated overnight at 4°C
with primary antibodies directed against EGFR (1:1,000), VEGF
(1:1,000), Bcl-2 (1:1,000), AMPK (1:500), p-AMPK (1:500), mTOR
(1:500) and p-mTOR (1:500), then with secondary antibodies for 1 h
at room temperature. Anti-rabbit IgG, HRP-linked antibody (1:2,000)
was used to test AMPK, p-AMPK, mTOR, p-mTOR, and EGFR, and
anti-mouse IgG, HRP-linked antibody (1:2,000) was used to test
Bcl-2 and VEGF. Then, immunoblot analysis was performed. β-actin
(1:1,000) served as the loading control, and the corresponding
secondary antibody was anti-rabbit IgG, HRP-linked antibody
(1:2,000).
The level of protein expression was examined using a
Novex® enhanced chemiluminescence Chemiluminescent
Substrate Reagent kit (Thermo Fisher Scientific, Inc.) and
performed according to the manufacturer's protocol and quantified
using Image J software (V1.48; National Institutes of Health,
Bethesda, MD, USA). Protein expression was presented as the
percentage of the corresponding control.
Xenograft model
A total of 24 male nu/nu mice aged 4–5 week
old with body weight ranged 15–20 g were supplied by SLAC
Laboratory Animal Co., Ltd. (Shanghai, China). The mice were
maintained in a specific pathogen-free facility, at a temperature
of 23±2°C, 50–60% humidity, a light/dark cycle of 12/12 h and given
free access to water and a standard rodent chow diet.
The new bought mice were housed for 3 days prior to
any medical intervention. SW1990 cells (2×106) were
injected subcutaneously into the right flank of the mice (day 0).
When the mean diameter of the tumors reached 2 mm (at ~1 week), the
animals were randomly divided into four groups: i) The untreated
group (control); ii) the metformin group; iii) the rapamycin group;
and iv) the combination group (n=6/group). Metformin (200 mg/kg
body weight) was administered once daily by oral gavage and
rapamycin (2.0 mg/kg body weight) was delivered by intraperitoneal
injection every other day to the appropriate groups for a total of
3 weeks. The tumor dimensions were measured by calipers a minimum
of twice a week. Tumor volume was calculated according to the
following formula: Tumor volume = length × width2 ×0.5.
Once the tumor volume reached 3 cm3 or significant body
weight loss was observed, the treatment was stopped and the mice
were sacrificed.
The present study was approved by the Animal Ethical
and Welfare Committee of Nanjing Medical University (Nanjing,
China). The experimental procedures all complied with the guide for
the care and use of laboratory animals (13).
Immunohistochemical (IHC)
analysis
The mice were anesthetized and euthanized by
cervical dislocation at day 28 and the xenograft tumors were
surgically removed. Tumors from xenograft cells were fixed in 10%
neutralized buffered formalin for 48 h at room temperature and
embedded in paraffin. Tissue sections were cut 5-µm-thick for
hematoxylin and eosin (H&E) staining and IHC analysis. The
sections were stained with 0.5% H&E for 4 min in each dye, at
room temperature. Light microscopic examination was performed for
histological diagnosis. IHC staining was performed in accordance
with standard protocols (14).
Briefly, non-specific binding was inhibited with Biocare blocking
reagent (Biocare Medical, Concord, CA, USA) for 30 min at room
temperature followed by incubation with anti-human polyclonal IgG
primary antibodies directed against AMPK (1:200), p-AMPK (1:200),
mTOR (1:100), p-mTOR (1:100), VEGF (1:200), EGFR (1:200) and Bcl-2
(1:200) at 4°C overnight, then with secondary antibodies at room
temperature for 30 min. Anti-rabbit IgG, HRP-linked antibody
(1:200) was used to test AMPK, p-AMPK, mTOR, p-mTOR, and EGFR, and
anti-mouse IgG, HRP-linked antibody (1:200) was used to test Bcl-2
and VEGF. Images of all sections were captured using a Nikon MODEL
ECLIPSE Ci-L microscope (Nikon Corporation, Tokyo, Japan). IHC
pictures were captured at magnification, ×100 times and H&E
pictures were captured at magnification, ×200 times.
Statistical analysis
All data are presented as the mean ± standard
deviation (SD) of a minimum of three independent experiments. The
data was analyzed using SPSS software version 22.0 (IBM Corp.,
Armonk, NY, USA). Multiple groups were analyzed using one-way
analysis of variance with the Dunnet post hoc test. P<0.05 was
considered to indicate a statistically significant difference. The
combination index (CI) devised by Chou and Talalay (1984) (15) was calculated using CompuSyn software
version 1.0 (ComboSyn, Inc., Paramus, NJ, USA) (which was updated
online periodically) to ascertain whether the combination of
metformin and rapamycin was additive (CI=1), antagonistic (CI>1)
or synergistic (CI<1). Synergism (CI<1) suggests greater than
expected additive effect, additive effect (CI=1) suggests the
combined effect predicted by the mass-action law principle in the
absence of synergism or antagonism, and antagonism (CI>1)
suggests smaller than expected additive effect (16).
Results
Metformin and rapamycin
synergistically inhibit the proliferation of PC cells in vitro
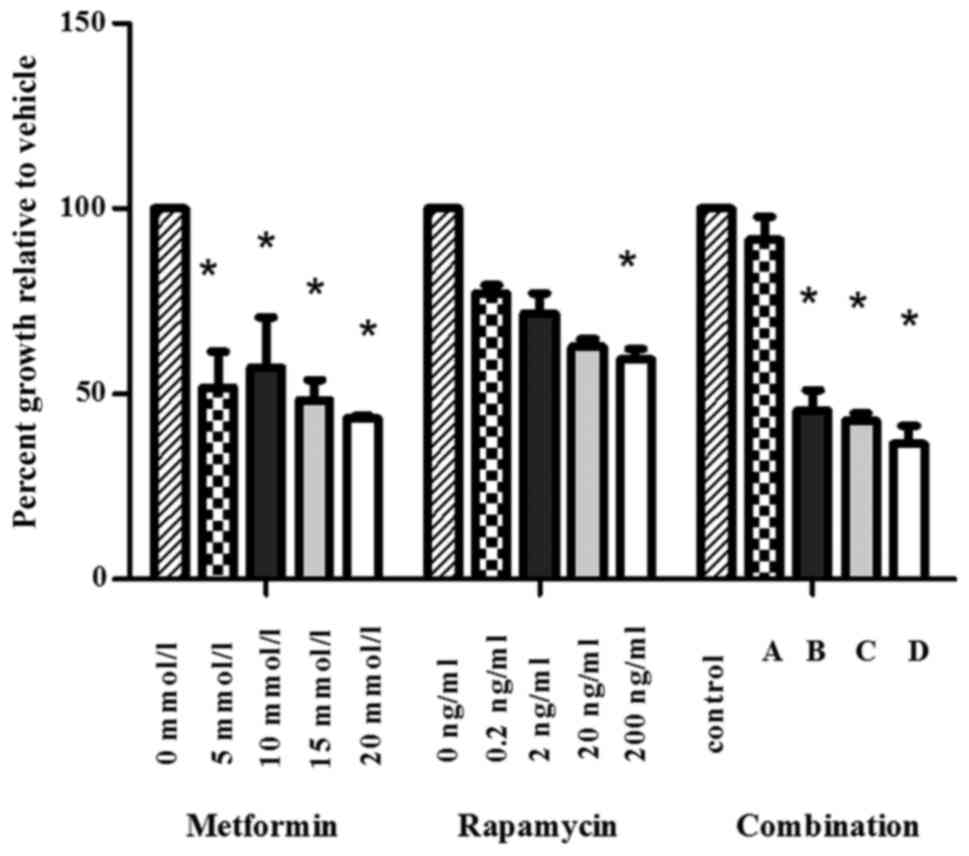
An MTT assay was conducted to analyze the viability
of SW1990 cells following treatment with metformin, rapamycin or a
combination of the two substances at varying concentrations
(Fig. 1). Metformin (5–20 mmol/l)
significantly inhibited the proliferation of SW1990 cells in
comparison with the control group. Low concentrations of rapamycin
(0.2, 2 and 20 ng/ml) did not significantly change cell viability
compared with the control group. The viability of SW1990 cells was
significantly suppressed by rapamycin at the concentration of 200
ng/ml.
To analyze the interaction between metformin and
rapamycin, the Chou and Talalay method (1984), was used (15). The CI values were as follows:
Combination A, 21483.8; combination B, 0.42; combination C, 0.39;
combination D, 0.18. These results suggest that the combination of
metformin and rapamycin synergistically inhibited the growth of
SW1990 cells when administered at higher concentrations (B-D).
In subsequent experiments the concentrations of 20
mmol/l metformin and 200 ng/ml rapamycin were selected for use.
Western blot analysis
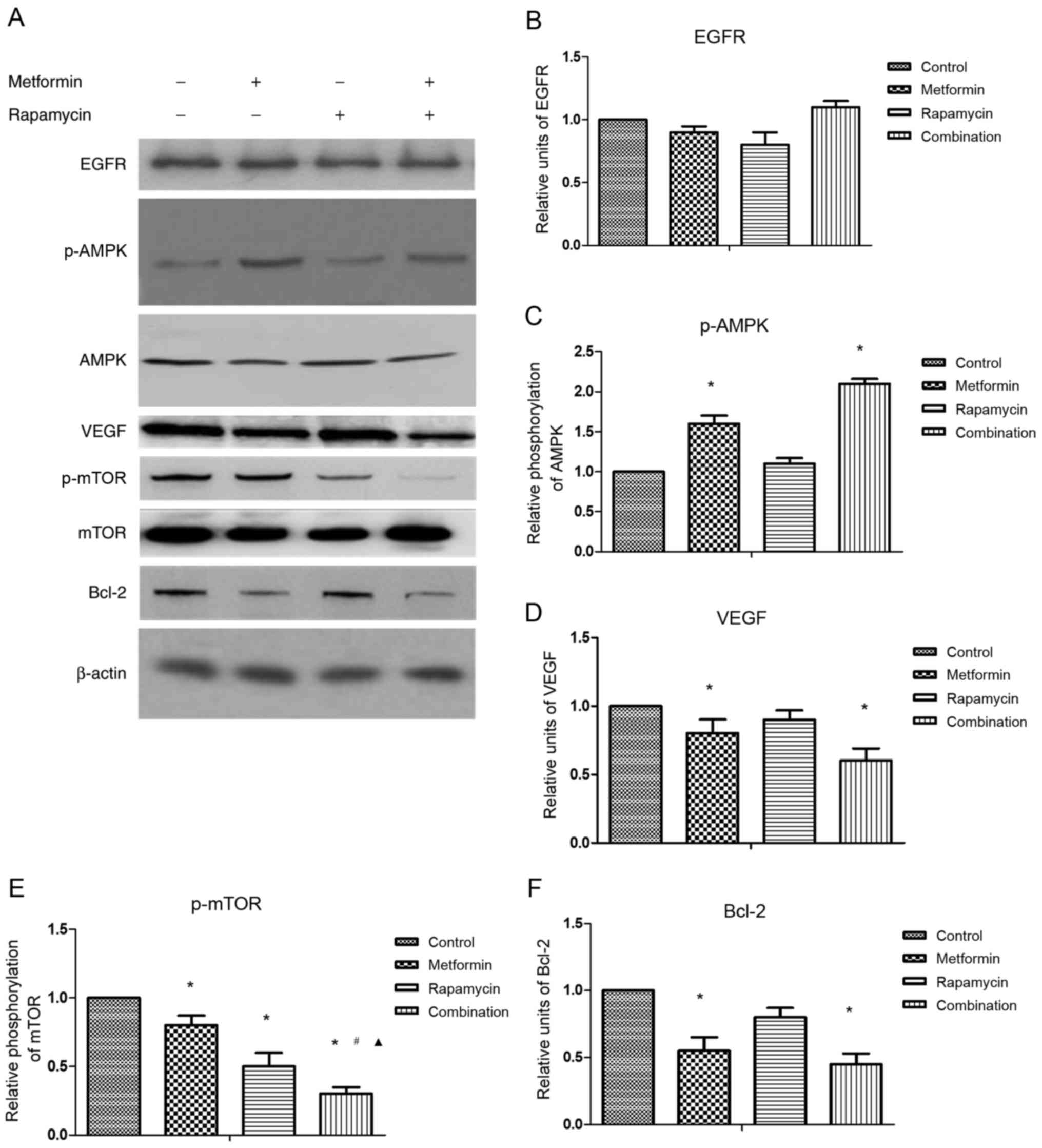
To explore the underlying mechanisms responsible for
the synergy of combination treatment, the effects of metformin and
rapamycin on the expression of selected proteins were investigated
by western blot analysis (Fig.
2A).
No significant differences were observed in the
protein expression of EGFR in any treatment group (Fig. 2B). The results suggested that
metformin treatment alone significantly increased p-AMPK protein
expression (Fig. 2C), but
significantly decreased the protein expression of VEGF (Fig. 2D), p-mTOR (Fig. 2E) and Bcl-2 compared with the control
(Fig. 2F). Rapamycin treatment alone
significantly suppressed p-mTOR protein expression compared with
the control. The combination treatment significantly increased
p-AMPK protein expression, whereas it significantly decreased VEGF,
Bcl-2 and p-mTOR protein expression compared with the control.
Combination treatment also significantly reduced p-mTOR protein
expression compared with metformin or rapamycin treatment alone,
which indicates the synergistic action of metformin and
rapamycin.
Xenograft model
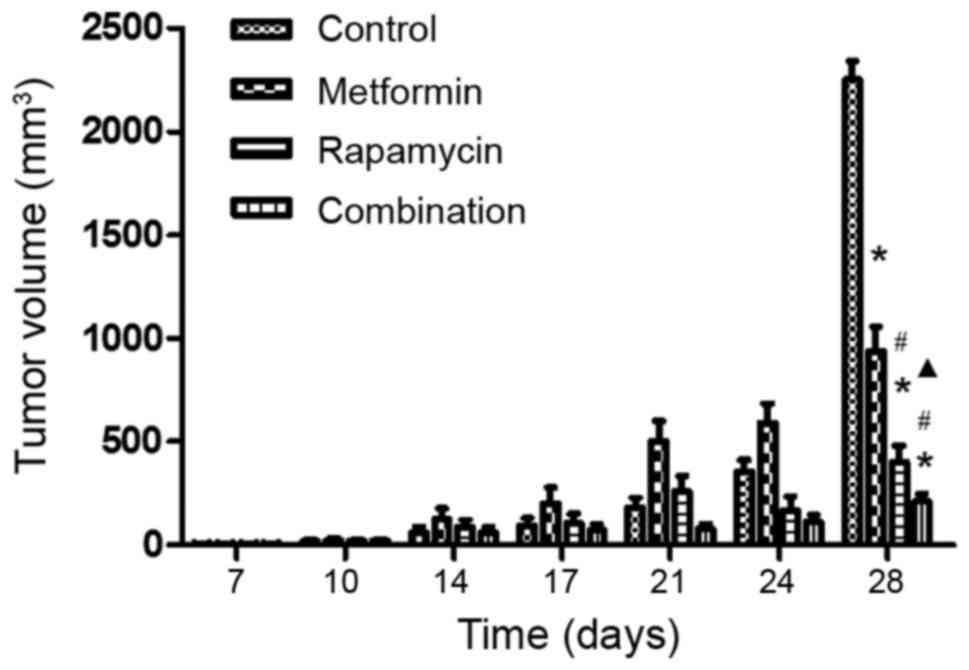
To assess the antitumor efficacy of metformin and
rapamycin upon PC in vivo, a xenograft model was developed
using nu/nu mice. It was observed that at the final time
point (day 28), the tumor volumes differed significantly among the
four groups (Fig. 3). The
administration of metformin alone had a small inhibitory effect and
the injection of rapamycin alone had a moderate inhibitory effect.
However, the combination of metformin and rapamycin exerted a
significantly increased inhibition of tumor growth compared with
the control group, the rapamycin monotherapy group and the
metformin monotherapy group.
No significant loss of body weight was observed in
the mice in the three treatment groups during the 3-week therapy
period, which suggests that the therapy was well tolerated with no
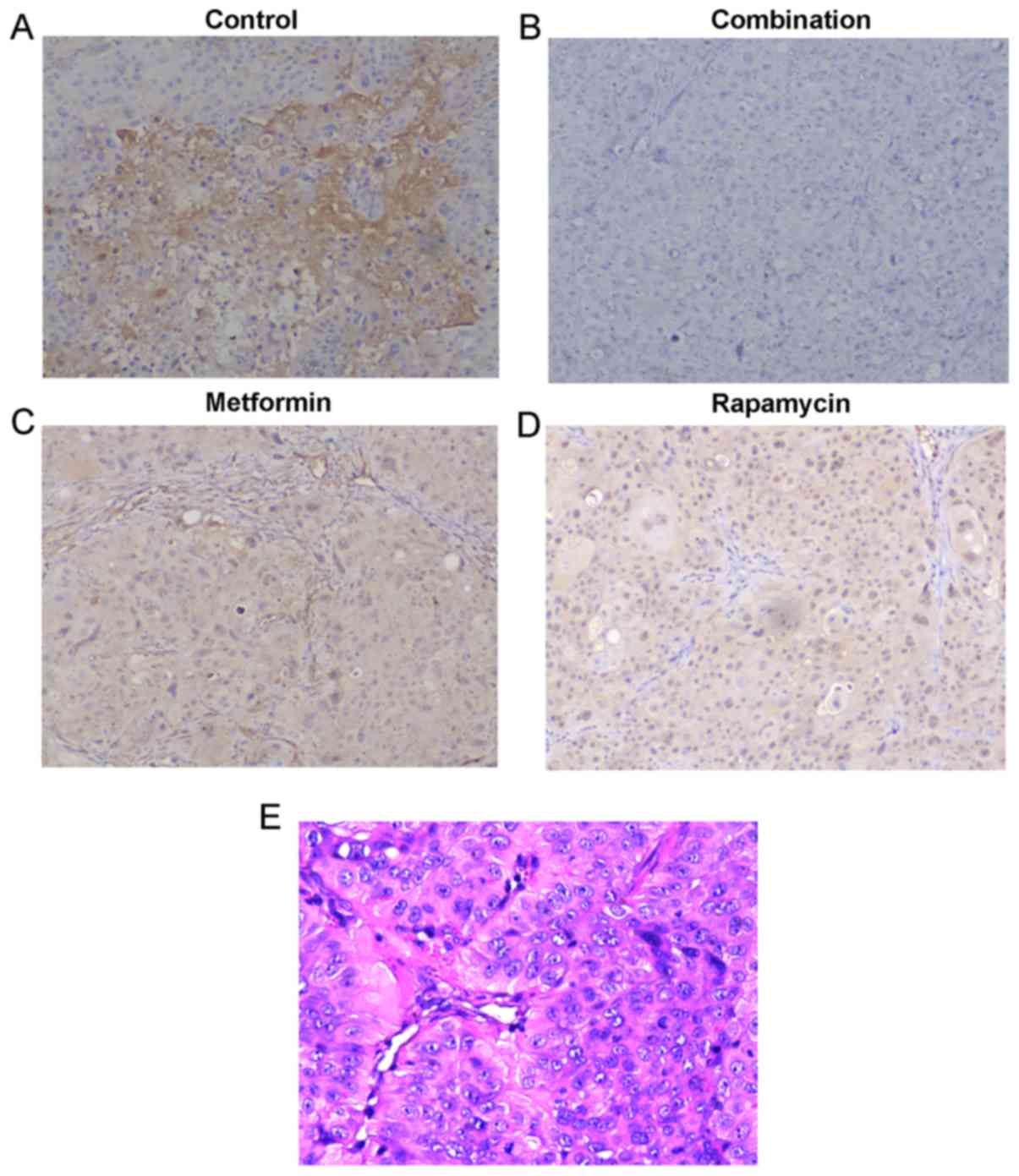
obvious toxicity (data not shown). The protein expression of p-mTOR
in the mouse tumors was detected by IHC analysis (Fig. 4A-D). Fig.
4A demonstrated that p-mTOR was expressed in the tumors in the
control group. It was observed that metformin (Fig. 4C) and rapamycin (Fig. 4D) alone decreased p-mTOR expression,
and, when they were administered in combination (Fig. 4B), they notably decreased p-mTOR
expression to a higher degree. H&E staining (Fig. 4E) of the sections from control group
was performed to ensure the diagnosis of pancreatic cancer of
tumors from xenograft. These results further demonstrate that the
administration of a combination of metformin and rapamycin confers
enhanced anti-PC activity.
Discussion
Multiple types of cancer, including PC, are
characterized by aberrant activation of mTOR (17). Targeting of the mTOR signaling pathway
may be an effective therapeutic strategy to minimize the
progression of PC. Metformin and rapamycin inhibit the growth of PC
cells by suppressing the mTOR signaling pathway (12). It has been previously reported that a
low dose of rapamycin (10−10 mol/l) does not suppress
the growth of PC cells, whereas a high dose (10−8 mol/l)
does (18). In the present study,
rapamycin at lower concentrations (0.2, 2 and 20 ng/ml) did not
significantly inhibit the proliferation of PC cells, whereas 200
ng/ml rapamycin did. The metformin dose of 200 mg/kg body weight
was used in the xenograft experiments. However, millimolar
concentrations of metformin were used in vitro, despite the
maximum concentration used in vivo being at the micromolar
level, which explained why the anti-cancer effect of metformin was
not as notable in vivo as in vitro.
Although rapamycin and metformin suppress the mTOR
signaling pathway (rapamycin directly and metformin by AMPK
signaling), rapamycin is known to activate protein kinase B (AKT),
which may reduce its anti-tumor activity, whereas metformin
treatment decreases AKT activation (19). Metformin and rapamycin affect other
distinct targets, including microRNAs (miRs), which regulate gene
expression following transcription (20). Rapamycin targets are focused on
survival signaling and decreased mTOR-associated growth, through
increasing the expression of cell cycle-regulating miRs and the
let-7b miR precursor (12). Metformin
directly targets Notch, Snail and Slug and reduces the levels of
glucose, insulin and miR-34a expression (12). Metformin and rapamycin inhibit the
growth of PC cells through shared and distinct mechanisms, which
indicates that the integration of metformin into the treatment
regimen of rapamycin may enhance its anti-tumor activity.
In the present study, metformin and rapamycin
exhibited synergic effects at concentrations of metformin (10–20
mmol/l) + rapamycin (2–200 ng/ml). The underlying mechanisms of
their action were further investigated by western blot analysis and
it was revealed that metformin (20 mmol/l) combined with rapamycin
(200 ng/ml) suppressed the phosphorylation of mTOR significantly
compared with monotherapy. Treatment with metformin and rapamycin
alone inhibited the growth of PC in vivo, and treatment with
them in combination consistently exhibited an enhanced effect. IHC
analyses of xenograft tumors demonstrated a stronger suppression of
p-mTOR in the combination treatment group compared with the
monotherapy groups.
The combination of metformin and rapamycin has been
reported to inhibit cancer progression by their combined effects on
the mTORC1 signaling pathway and tissue inflammation (21). When metformin is combined with
everolimus, it may exert a synergistic inhibition of cancer growth
by abrogation of the phosphorylation of S6 and 4E-binding protein 1
(22). Metformin in combination with
dexamethasone has been demonstrated to repress the expression of
myeloid cell leukemia-1 and activate caspase 3, as well as
increasing the number of cells in the G1 phase of the cell cycle
(23). A previous study reported that
metformin combined with vemurafenib may induce apoptosis and result
in the synergistic inhibition of the growth of cancer cells,
suggesting that metformin reverses the resistance of cancer cells
to vemurafenib by altering the cellular energy balance (24). Metformin and salicylate may
synergistically reduce the survival of cancer cells in vitro
by inhibiting de novo lipogenesis and targeting
pro-apoptotic and Bcl-2 family members (25,26).
Metformin also synergizes with chemotherapy to reverse multi-drug
resistance in cancers by targeting the AMPK/mTOR/hypoxia inducible
factor-1α/P-glycoprotein/multidrug resistance-associated protein 1
signaling pathway (27,28). Metformin potentiates the anti-tumor
effect of resveratrol on PC by targeting the VEGF-B signaling
pathway (29).
It has been hypothesized that the combination of
targeted mTOR inhibition by rapamycin with the broader inhibition
provided by metformin may lead to a greater-than-additive
inhibition of growth of PC cells. In the present study, treatment
with metformin alone or in combination with rapamycin significantly
suppressed the expression of VEGF and Bcl-2 compared with the
control, however, no significant differences were detected between
the combination treatment and metformin treatment alone.
Previous studies have revealed that rapamycin
therapy for PC patients was not effective and the efficacy of
metformin in vivo was contradictory in many studies
(30–36). The present study demonstrated the
anti-tumor action of metformin and revealed the synergistic action
of metformin and rapamycin in the treatment of PC cells by the
downregulation of the mTOR signaling pathway. Other pathways may
also be affected by this synergistic treatment, including apoptosis
activation or VEGF inhibition, however this requires further study
to confirm. A combination of metformin and rapamycin may represent
a promising anti-tumor therapy for patients with PC. Additional
studies are required to investigate the mechanisms by which the
synergy functions and to verify its efficacy in the treatment of
patients with PC.
Acknowledgements
The present study was supported by Nanjing Medical
University (grant no. 2015NJMU138).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ding B, Wahid MA, Wang Z, Xie C, Thakkar
A, Prabhu S and Wang J: Triptolide and celastrol loaded silk
fibroin nanoparticles show synergistic effect against human
pancreatic cancer cells. Nanoscale. 9:11739–11753. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soranna D, Scotti L, Zambon A, Bosetti C,
Grassi G, Catapano A, La Vecchia C, Mancia G and Corrao G: Cancer
risk associated with use of metformin and sulfonylurea in type 2
diabetes: A meta-analysis. Oncologist. 17:813–822. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sadeghi N, Abbruzzese JL, Yeung SC, Hassan
M and Li D: Metformin use is associated with better survival of
diabetic patients with pancreatic cancer. Clin Cancer Res.
18:2905–2912. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bodmer M, Becker C, Meier C, Jick SS and
Meier CR: Use of antidiabetic agents and the risk of pancreatic
cancer: A case-control analysis. Am J Gastroenterol. 107:620–626.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee MS, Hsu CC, Wahlqvist ML, Tsai HN,
Chang YH and Huang YC: Type 2 diabetes increases and metformin
reduces total, colorectal, liver and pancreatic cancer incidences
in Taiwanese: A representative population prospective cohort study
of 800,000 individuals. BMC Cancer. 11:202011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mohammed A, Janakiram NB, Brewer M,
Ritchie RL, Marya A, Lightfoot S, Steele VE and Rao CV:
Antidiabetic drug metformin prevents progression of pancreatic
cancer by targeting in part cancer stem cells and mTOR signaling.
Transl Oncol. 6:649–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karnevi E, Said K, Andersso R and
Rosendahl AH: Metformin-mediated growth inhibition involves
suppression of the IGF-I receptor signalling pathway in human
pancreatic cancer cells. BMC Cancer. 13:2352013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soares HP, Ni Y, Kisfalvi K, Sinnett-Smith
J and Rozengurt E: Different patterns of Akt and ERK feedback
activation in response to rapamycin, active-site mTOR inhibitors
and metformin in pancreatic cancer cells. PLoS One. 8:e572892013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vallianou NG, Evangelopoulos A and Kazazis
C3: Metformin and cancer. Rev Diabet Stud. 10:228–235. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cifarelli V, Lashinger LM, Devlin KL,
Dunlap SM, Huang J, Kaaks R, Pollak MN and Hursting SD: Metformin
and rapamycin reduce pancreatic cancer growth in obese prediabetic
mice by distinct microRNA-regulated mechanisms. Diabetes.
64:1632–1642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Research Council, . Guide for the
Care and Use of Laboratory Animals. 8th. National Acadamies Press;
Washington, DC: 2011, PubMed/NCBI
|
|
14
|
Simon R, Mirlacher M and Sauter G:
Immunohistochemical analysis of tissue microarrays. Methods Mol
Biol. 664:113–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou TC and Martin N: CompuSyn for drug
combinations and for general dose-effect analysis. Combosyn, Inc.;
Paramus, NJ: 2005
|
|
17
|
Guertin DA and Sabatini DM: An expanding
role for mTOR in cancer. Trends Mol Med. 11:353–361. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rice S, Pellat L, Ahmetaga A, Bano G,
Mason HD and Whitehead SA: Dual effect of metformin on growth
inhibition and oestradiol productionin breast cancer cells. Int J
Mol Med. 35:1088–1094. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zakikhani M, Blouin MJ, Piura E and Pollak
MN: Metformin and rapamycin have distinct effects on the AKT
pathway and proliferation in breast cancer cells. Breast Cancer Res
Treat. 123:271–279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saha A, Blando J, Tremmel L and DiGiovanni
J: Effect of metformin, rapamycin, and their combination on growth
and progression of prostate tumors in HiMyc mice. Cancer Prev Res
(Phila). 8:597–606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Wei J, Li L, Fan C and Sun Y:
Combined use of metformin and everolimus is synergistic in the
treatment of breast cancer cells. Oncol Res. 22:193–201. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zi FM, He JS, Li Y, Wu C, Yang L, Yang Y,
Wang LJ, He DH, Zhao Y, Wu WJ, et al: Metformin displays
anti-myeloma activity and synergistic effect with dexamethasone in
in vitro and in vivo xenograft models. Cancer Lett. 356:443–453.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hanly EK, Bednarczyk RB, Tuli NY,
Moscatello AL, Halicka HD, Li J, Geliebter J, Darzynkiewicz Z and
Tiwari RK: mTOR inhibitors sensitize thyroid cancer cells to
cytotoxic effect of vemurafenib. Oncotarget. 6:39702–39713. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Brien AJ, Villani LA, Broadfield LA,
Houde VP, Galic S, Blandino G, Kemp BE, Tsakiridis T, Muti P and
Steinberg GR: Salicylate activates AMPK and synergizes with
metformin to reduce the survival of prostate and lung cancer cells
ex vivo through inhibition of de novo lipogenesis. Biochem J.
469:177–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yue W, Zheng X, Lin Y, Yang CS, Xu Q,
Carpizo D, Huang H, DiPaola RS and Tan XL: Metformin combined with
aspirin significantly inhibit pancreatic cancer cell growth in
vitro and in vivo by suppressing anti-apoptotic proteins Mcl-1 and
Bcl-2. Oncotarget. 6:21208–21224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ling S, Feng T, Ke Q, Fan N, Li L, Li Z,
Dong C, Wang C, Xu F, Li Y and Wang L: Metformin inhibits
proliferation and enhances chemosensitivity of intrahepatic
cholangiocarcinoma cell lines. Oncol Rep. 31:2611–2618. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ling S, Tian Y, Zhang H, Jia K, Feng T,
Sun D, Gao Z, Xu F, Hou Z, Li Y and Wang L: Metformin reverses
multidrug resistance in human hepatocellular carcinoma
Bel-7402/5-fluorouracil cells. Mol Med Rep. 10:2891–2897. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu M, Zhang Q, Wang X, Kang L, Yang Y,
Liu Y, Yang L, Li J, Yang L, Liu J, et al: Metformin potentiates
anti-tumor effect of resveratrol on pancreatic cancer by
down-regulation of VEGF-B signaling pathway. Oncotarget.
7:84190–84200. 2016.PubMed/NCBI
|
|
30
|
Driscoll DR, Karim SA, Sano M, Gay DM,
Jacob W, Yu J, Mizukami Y, Gopinathan A, Jodrell DI, Evans TR, et
al: mTORC2 Signaling drives the development and progression of
pancreatic cancer. Cancer Res. 76:6911–6923. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lipner MB, Marayati R, Deng Y, Wang X,
Raftery L, O'Neil BH and Yeh JJ: Metformin treatment does not
inhibit growth of pancreatic cancer patient-derived Xenografts.
PLoS One. 11:e01471132016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei F, Zhang Y, Geng L, Zhang P, Wang G
and Liu Y: mTOR inhibition induces EGFR feedback activation in
association with its resistance to human pancreatic cancer. Int J
Mol Sci. 16:3267–3282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zagouri F, Sergentanis TN, Chrysikos D,
Zografos CG, Papadimitriou CA, Dimopoulos MA, Filipits M and
Bartsch R: Molecularly targeted therapies in metastatic pancreatic
cancer: A systematic review. Pancreas. 42:760–773. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Garrido-Laguna I, Tan AC, Uson M,
Angenendt M, Ma WW, Villaroel MC, Zhao M, Rajeshkumar NV, Jimeno A,
Donehower R, et al: Integrated preclinical and clinical development
of mTOR inhibitors in pancreatic cancer. Br J Cancer. 103:649–655.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Javle MM, Shroff RT, Xiong H, Varadhachary
GA, Fogelman D, Reddy SA, Davis D, Zhang Y, Wolff RA and Abbruzzese
JL: Inhibition of the mammalian target of rapamycin (mTOR) in
advanced pancreatic cancer: Results of two phase II studies. BMC
Cancer. 10:3682010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wolpin BM, Hezel AF, Abrams T, Blaszkowsky
LS, Meyerhardt JA, Chan JA, Enzinger PC, Allen B, Clark JW, Ryan DP
and Fuchs CS: Oral mTOR inhibitor everolimus in patients with
gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol.
27:193–198. 2009. View Article : Google Scholar : PubMed/NCBI
|